Abstract
Purpose: Multi-drug resistant tuberculosis (MDR-TB) and extremely drug resistant (XDR) TB are big problems in the world. We have developed novel TB therapeutic vaccines, HVJ-Envelope/HSP65 + IL-12 DNA vaccine (HSP65-vaccine), granulysin vaccine and killer specific secretory protein of 37kDa (Ksp37) vaccine.
Methods and Results: HSP65 vaccine showed strong therapeutic effect against both MDR-TB and XDR-TB in mice. Intradermal immunization of HSP65-vaccine showed stronger therapeutic effect against TB than intramuscular or subcutaneous immunization. Furthermore, the synergistic therapeutic effect was observed when the vaccine was administrated in combination with Isoniazid (INH), which is a first line drug for chemotherapy. The combination of types of vaccines (HSP65- and granulysin- vaccines) also showed synergistic therapeutic effect. In the monkey model, granulysin-vaccine prolonged the survival period after the infection of TB and long-term survival was observed in vaccine-treated group. We examined the potential of two kinds of novel DNA vaccines (Ksp37-vaccine and granulysin-vaccine). Both vaccines augmented in vivo differentiation of CTL against TB. We measured the amount of Ksp37 protein in human serum and revealed that the level of Ksp37 protein of patients with tuberculosis was lower than that of healthy volunteers. Therefore, we established Ksp37 transgenic mice as well as granulysin transgenic mice to elucidate the function of those proteins. Both transgenic mice were resistant to TB infection.
Conclusion: These data indicate the potential of combinational therapy; the combination of two DNA vaccines or combination of DNA vaccine with antibiotic drug. Thus, it will provide a novel strategy for the treatment of MDR-TB.
Introduction
Tuberculosis is a major global threat to human health, with about 2 million people dying every year from Mycobacterium tuberculosis (TB) infection. The only tuberculosis vaccine currently available is an attenuated strain of Mycobacterium bovis BCG (BCG), although its efficacy against adult TB disease remains controversial. Furthermore, multi-drug resistant tuberculosis (MDR-TB) and extremely drug resistant TB (XDR-TB) are becoming big problems in the world. About 500,000 of people around the world are affected by MDR-TB every year. However, there are a small number of effective drugs against MDR-TB. In such circumstances, the development of therapeutic as well as prophylactic vaccines against TB is required.
Cynomolgus monkey model is the best animal TB model to evaluate the potential of newly developed vaccines as reported by Walsh and Tan.Citation1 Onset and progress after TB infection in the cynomolgus monkey is very similar to human TB disease.Citation1–Citation3 SCID-PBL/hu is an IL-2 receptor γ-chain disrupted mouse (NOD-SCID) transplanted with human PBL. It is an in vivo humanized immune model and provides an useful tool for investigating human immune responses to the administration of vaccines.Citation4,Citation5 Transgenic mice that express the components of vaccine also provide a lot of information about novel TB vaccines. Therefore, we used three animal models (cynomolgus monkey model of TB, SCID-PBL/hu mice and transgenic mice) to develop three kinds of novel vaccines against TB.
We have previously evaluated a novel therapeutic TB vaccine which consists of plasmid DNA encoding HSP65 +IL-12 and HVJ-Envelope (HSP65-vaccine) and revealed the efficacy of the vaccine in these animal models.Citation6–Citation9 However, it is very important to treat the patients with TB completely within a short period of time. Therefore, we improved the regimen of the vaccination. We first examined a combination therapy of vaccination and chemotherapy. In the present study, we used a HSP65-vaccine for the vaccination and first line TB drugs (INH or RFP) for the chemotherapy. We next identified a suitable administration route to increase the efficacy of the DNA vaccine. The result suggested that intradermal vaccination is more suitable for the DNA vaccine than intramuscular or subcutaneous vaccination.
Finally, we developed plasmid-based DNA vaccines encoding the granulysin or Ksp37. Granulysin is a member of the saposin-like protein family and colocalizes with perforin and granzymes in the cytolytic granules of human CTL and NK cells. In the presence of perforin, it has a cytolytic activity against intracellular pathogens in the cytoplasm of infected cells. It also has a cytotoxic effect on tumor cells.Citation10–Citation12 Granulysin is present in cytoplasm of human CD8 positive cytotoxic T cells and NK cells. It has been suggested that granulysin has a cytolytic activity against M. tuberculosis outside macrophages and reduces the number of M. tuberculosis in the macrophage dependent on the presence of perforin in vitro.Citation11 However, the precise role of granulysin in vivo has not been elucidated yet. Therefore, we have established granulysin transgenic mice to elucidate in vivo role of granulysin and obtain the information to develop novel vaccines against M. tuberculosis. Transgenic mice of 15K and 9K granulysins were resistant to TB infection in vivo. This is the first report indicating an in vivo role of granulysin in the defense against the infection of TB. In addition, we demonstrated the efficacy of granulysin-vaccine in the monkey model of TB.
Ksp37 is also produced from CTL and NK cells.Citation13 However, immunological function of Ksp37 has not been elucidated yet. In the first step, we first examined the amount of Ksp37 in the serum of patients with TB. The result indicated that Ksp37 in the serum of patients with TB was lower than that of healthy volunteer. In the next step, we have established Ksp37 transgenic mice to elucidate the in vivo role on Ksp37 in the immunity to the infection of M. tuberculosis. Ksp37 transgenic mouse was resistant to the infection of TB, suggesting the anti-TB effect of Ksp37 in vivo.
To increase the efficacy of the vaccine, we examined the synergistic effect of the combination therapy, in which HSP65-vaccine and first line chemotherapy drug (INH) were administrated. We also examined the synergistic effect of the combination of two kinds of DNA vaccines. HSP65- and granulysin-vaccine were simultaneously administrated in the murine models of TB and efficacy of the vaccines was evaluated in vivo.
In parallel, we investigated the immunological effects of granulysin and Ksp37 and revealed the synergistic effect of two molecules on the induction of TB-specific CTL (manuscript submitted).
These findings demonstrated that granulysin-vaccine and Ksp37-vaccine might provide very useful weapon as TB vaccines, and those efficacy will be enhanced in combination with other DNA vaccine (including HSP65 vaccine) and INH.
Results
Novel therapeutic vaccine of HVJ-Envelope/HSP65 DNA + IL-12 DNA vaccine
Therapeutic effect of the vaccine in murine models of TB
Therapeutic efficacy of HSP65 DNA + IL-12 DNA vaccine (HSP65-vaccine) was evaluated in murine models. At 30 d after intravenous challenge of MDR-TB, the CFUs of TB in the lungs, spleen, and liver were counted and therapeutic efficacy of HVJ-Envelope DNA vaccine was evaluated.
shows the feature of novel TB vaccines; the vaccine consists of a plasmid DNA vaccine expressing mycobacterial heat shock protein 65 (HSP65)+interleukin-12 (IL-12) and a vector (hemagglutinating virus of Japan (HVJ)-liposome or HVJ-envelope). We used the mouse model in our previous study.Citation6,Citation7 This vaccine showed therapeutic effect against TB in the mouse: (1) This vaccine significantly decrease the number of drug sensitive H37Rv TB in the spleen and the lung of mice. (data not shown) This vaccine also decreased the number of MDR-TB in the lungs and spleen of mice, indicating the efficacy of the vaccine in the mouse model of TB. (2) Significant prolongation of survival was observed in the XDR-TB infected mice. The survival period of the mouse treated with this vaccine was prolonged compared with non-vaccinated mice. Those results demonstrated that this vaccine have strong therapeutic effect against XDR-TB. (3) We have established the mouse model of chronic TB disease using an inhalation system (aerosol chamber) for the infection of TB. By using this model, therapeutic efficacy of this vaccine was examined. The vaccine was administered 5 weeks after aerosol infection of TB. Treatment with this vaccine decreased the number of TB in the lung of the mice (data not shown).
Table 1. Therapeutic efficacy of HVJ-Envelope/HSP65 DNA + IL-12 DNA vaccine on TB infection in murine models
Thus, we demonstrated that this vaccine had a therapeutic effect against XDR-TB and MDR-TB as well as drug-sensitive TB.
Synergistic effect of HVJ-Envelop/HSP65 DNA + IL-12 DNA vaccine in combination with INH against TB infection
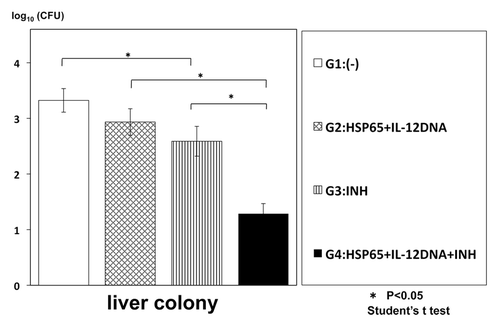
To enhance the efficacy, we examined synergistic effect of combinational therapy of this vaccine and INH (isoniazid) on the infection of TB. The numbers of TB in the mouse spleen and liver were significantly decreased compared with the monotherapy, indicating the synergistic effect of the combinational therapy ( and ).
Figure 1. Synergistic therapeutic efficacy of HVJ-Envelope/HSP65 DNA+IL-12 DNA vaccine and INH on TB infection of mice. BALB/c mice were infected with H37Rv TB by using intratracheal aerosol challenge using aerosol chamber. One week after challenge of TB, the vaccine and INH(0.03mg/mouse) were administered 6 times for 3 weeks. Five weeks after TB challenge, mice were sacrified, and CFU of TB in the spleen were evaluated. G1 vs. G2; p < 0.05; G1 vs. G3; p < 0.05; G3 vs. G5; p < 0.05; G2 vs. G5 p < 0.05; Student’s test
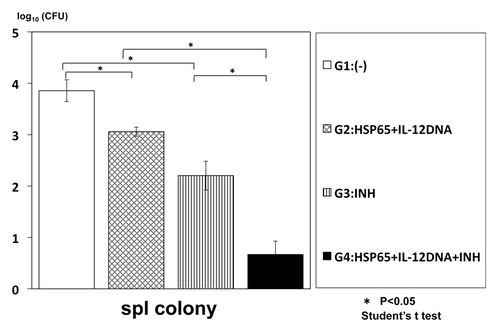
Figure 2. Synergistic therapeutic efficacy of HVJ-Envelope/HSP65 DNA+IL-12 DNA vaccine and INH on TB infection of mice. BALB/c mice were infected with H37Rv TB by using intratracheal aerosol challenge using aerosol chamber. One week after challenge of TB, the vaccine and INH(0.03mg/mouse) were administered 6 times for 3 weeks. Five weeks after TB challenge, mice were sacrified, and CFU of TB in the liver were evaluated. G2 vs. G4; p < 0.05; G3 vs. G4; p < 0.05; Student’s test
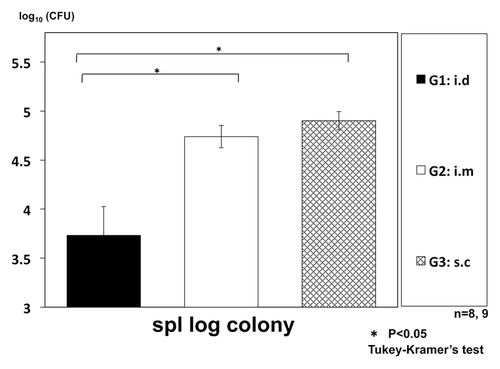
We also compared the administration route of the vaccine to improve the regimen of the therapy. We selected three routes (intradermal, intramuscular and subcutaneous injections) for our experiment. The efficacy of intradermal administration (i.d.) was highest among three administration routes ().
Figure 3. Therapeutic efficacy of intradermal (i.d.) vaccination of HVJ-Envelope/HSP65 DNA+IL-12 DNA, compared with intramuscular (i.m.) or subcutaneous (s.c.) vaccination using intratracheally aerosol infected DBA/1 mice. DBA/1 mice were infected with H37Rv TB by using intratracheal aerosol challenge using aerosol chamber. One week after challenge of TB, 100μg of HVJ-Envelope/HSP65 DNA+IL-12 DNA were administered 6 times for 3 weeks by i.d, i.m, or s.c administration. Four weeks after TB challenge, mice were sacrified, and CFUs of TB in the spleen were evaluated. G1 vs. G2; p < 0.05; G1 vs. G3; p < 0.05; Student’s test
Therefore, in the monkey model we plan to study the efficacy of intradermal injection of this vaccine, in comparison to i.m administration.
IL-2 receptor γ-chain gene disrupted SCID-PBL/hu
We have very important humanized immune model (SCID-PBL/hu) to study the human T cell immune response in vivo as reported first in Cancer Research 1997.Citation4 We evaluated the efficacy of novel vaccines in vivo using this humanized immune model.
We used IL-2 receptor γ-chain gene knockout mouse-based model (NOD SCID-PBL/hu) to analyze the human T cell responses to the vaccine ().
Table 2. Therapeutic efficacy and Immune responses using IL-2 receptor γ-chain gene disrupted SCID-PBL/hu models
The efficacy of HSP65 + IL-12 DNA vaccine was examined in this IL-2 receptor γ-chain gene disrupted SCID-PBL/hu-model and significant decrease of the number of TB in the liver was observed as shown in . This model shows stronger human CTL induction and proliferation than conventional SCID mouse-based model (CB17-SCID-PBL-hu) ().
Therapeutic efficacy of this vaccine on monkey model
We are developing a GMP level of DNA vaccine that contains two expression units in one plasmid vector for future clinical trial. In this study, we used a GMP level of DNA vaccine for the evaluation of the potency in the monkey model of TB. Monkeys were intratracheally instillated with 5 × 102 CFU of human TB (Erdman strain). After TB infection, 9 times intramuscular injection of this vaccine (total 400 μg/monkey) was conducted. Therapeutic efficacy was evaluated on the basis of survival, ESR, body weight, immune responses, chest X-ray and PPD skin test. The monkey group treated with HVJ-Envelope/HSP65 DNA + IL-12 DNA vaccine showed 100% survival (data not shown). In contrast, the monkey group of control saline showed 60% survival. Thus, the therapeutic DNA vaccine improved the survival rate of TB-infected monkeys, compared with the saline (control).
These data indicated the therapeutic efficacy of a GMP-level of DNA vaccine in TB-infected monkeys.
Efficacy of granulysin-vaccines
Efficacy of granulysin in transgenic mice
We noticed the in vivo function of granulysin, since it shows a cytolytic activity against Mycobacterium tuberculosis. The features of granulysin are as follows:
(1) Granulysin is cytolytic molecules expressed by human CTL and NK cells and show the cytolytic activity against a variety of tumors and microbes, including Mycobacterium tuberculosis. Granulysin belongs to the saposin-like protein family that includes amoebapores and NK lysine. Recent studies show that granulysin also has chemoattractant and proinflammatory activities. However, in vivo anti-microbe activity and anti-tuberculosis activity of granulysin has not been elucidated yet.
(2) It has been reported that the granulysin has the function of in vitro cytotoxic activity against M. tuberculosis outside the macrophage cells, and contributes the in vitro reduction of M. tuberculosis in the macrophage in the presence of perforin.
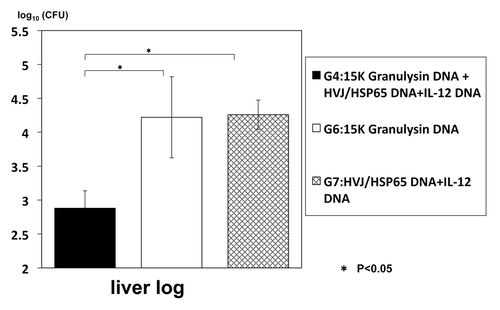
(3) However, the precise role of granulysin in the in vivo against the tuberculosis infection has not been elucidated yet. Therefore, we have established granulysin transgenic mice to elucidate mechanism of granulysin in vivo. We have established two kinds of transgenic mice by using usual microinjection method: 15K granulysin transgenic mice and 9K granulysin transgenic mice. We measured CFU number of M. tuberculosis in the lung four weeks after intravenous injection of TB (5 × 105 /mouse). As shown in , reduction of CFU number was observed in 15K granulysin transgenic mice compared with the normal C57BL/6 mice, indicating the in vivo anti-TB effect of 15K granulysin. As summarized in , augmentation of immune responses were also observed in 15K granulysin transgenic mice: in vivo induction of cytotoxic T cells against TB, enhanced proliferation of T cells stimulated with TB antigen and augmentation of cytokine production. Furthermore, we examined synergistic effects of the combination of two vaccines. As shown in , the combination of HSP65-vaccine and granulysin-vaccine showed synergistic effects and 10 times reduction of the CFU number in the liver of TB-infected mice was observed. The number of TB in the liver was significantly reduced by the combination of two vaccines. Efficacy of granulysin-vaccine in monkey models
Table 3. In vivo anti-TB effect of 15kDa Granulysin Transgenic mouse
Figure 4. The establishments of 15K granulysin transgenic mice and 9K granulysin transgenic mice. The efficacies of 15K granulysin transgenic mice and 9K granulysin transgenic mice on TB infection were summarized in this Figure. An anti-TB effect, the induction of CTL against TB, the proliferation of T cells against TB and γ-IFN production were augmented in these transgenic mice, compared with wild type C57BL/6 mice.
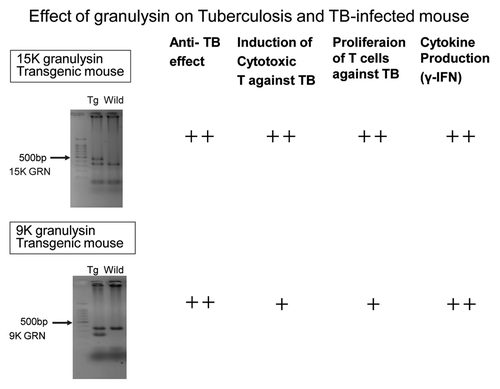
Figure 5. Therapeutic effect of granulysin DNA vaccine on TB-infected (DBA/1) mice DBA/1 mice were infected with H37Rv TB using intratracheal aerosol challenge One week after challenge of TB, 100μg of HVJ-Envelope/HSP65 DNA+IL-12 DNA and/or 100 μg of HVJ-Envelope/granulysin DNA were injected i.m. into mice 6 times for 3 weeks. Four weeks after TB challenge, mice were sacrified, and CFUs of TB in the liver were evaluated. G1 vs. G2; p < 0.05; G1 vs. G3; p < 0.05; Student’s test
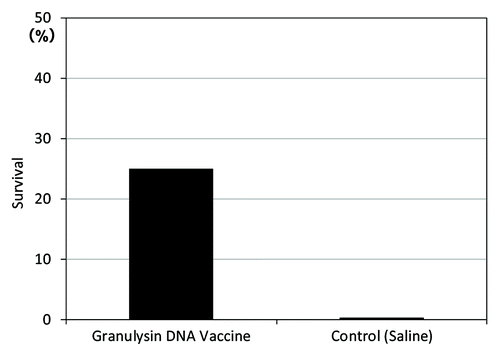
We examined the efficacy of granulysin-vaccine in the therapeutic model of TB. The survival rate of granulysin-vaccine (HVJ-Envelope/15K granulysin DNA vaccine)-treated group was 25% (1/4) at 1 y after TB infection (). In contrast, all monkeys in saline group were died within 200 d after TB challenge. Thus, survival rate at one year after TB infection was 0% (0/4).
Figure 6. Therapeutic efficacy (survival) of HVJ-Envelope/15K granulysin DNA vaccine, 365 d after TB infection using cynomolgus monkey models. Five × 102 M. tuberculosis (Erdman strain) were intratracheally into cynomolgus monkeys as described in Materials and Methods. Four weeks after challenge of TB, 400μg of HVJ-Envelope/15K granulysin were injected i.m. Six times every two weeks. Survival of monkeys treated with this vaccine were evaluated for 1 y (365 d).
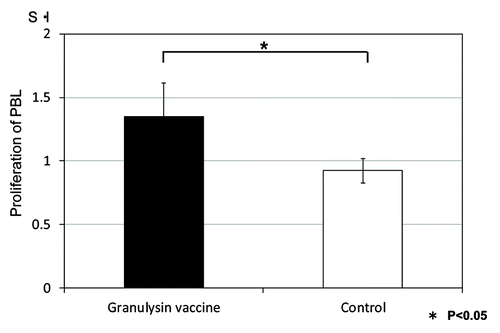
The proliferation of PBL from the monkeys treated with granulysin-vaccine was augmented compared with that of control (saline treated) monkeys (). These results indicated the efficacy of granulysin-vaccine in therapeutic models using monkey. Thus, granulysin-vaccine is effective in the monkey as well as the mouse model of TB.
Figure 7. Proliferation of PBL from monkeys vaccinated with HVJ-Envelope/15K granulysin DNA by the stimulation with HSP65 antigen. Five × 102 M. tuberculosis (Erdman strain) were intratracheally into cynomolgus monkeys as described in Materials and Methods. Four weeks after challenge of TB, 400μg of HVJ-Envelope/15K granulysin were injected i.m. Six times every two weeks. The proliferation of PBL from monkeys vaccinated with HVJ-Envelope/15K granulysin on 13 weeks after TB challenge were assessed by the 3H-TdR uptake of lymphocyte for 3 d culture.
Efficacy of Ksp37-Vaccine in Therapeutic models
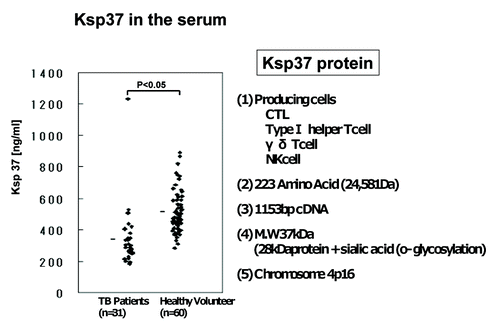
Ksp37 protein is produced from CTL, TypeI helper T cell, γ/δ T cell and NK cell. Ksp37 is composed of 223 amino acids. We analyzed the concentration of Ksp37 in the serum of patients with TB by ELISA. The level of Ksp37 protein in the serum of patients with TB (n = 31) was significantly lower than that of healthy volunteers (n = 60) (p < 0.05) (). This is first report suggesting the relation between the serum level of Ksp37 and TB disease ().
Figure 8. Killer specific secretory protein of 37kDa (Ksp37 protein) in the serum of patients with tuberculosis. Ksp37 protein in the serum of 31 patients with TB and 60 healthy volunteers were assessed by ELISA.
Therefore, we tried to elucidate the in vivo function of Ksp37 protein, especially function as an anti-TB agent in vivo.
In the first step, we have established a Ksp transgenic mouse for the analysis of function in vivo. We measured the CFU number of M. tuberculosis in the lung 3 weeks after TB aerosol infection. In Ksp transgenic mice, the CFU number of M. tuberculosis was decreased compared with that of wild type control mice. () This result indicated the anti-TB effect of Ksp37 in vivo.
Figure 9. In vivo anti-TB effect of Ksp37 transgenic mice. Ksp37 Tg mice were established as described in Materials and Methods. Ksp37 Tg mice, 15K granulysin Tg mice, 9K graunlysin Tg mice and wild type C57BL/6 mice were infected with H37Rv TB by using intratracheal aerosol challenge using aerosol chamber.
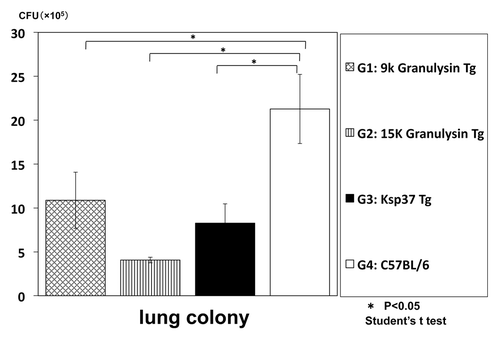
These finding suggested that Ksp37 produced from CTL and NK cell functions as an important anti- TB factor in humans and mice.
Discussion
In the present study, we evaluated the potential of three kinds of novel therapeutic vaccines (HSP65-, granulysin- and Ksp37-vaccines) in mouse and monkey models of TB. All of the vaccines showed anti-TB effects in therapeutic models. It is noteworthy that efficacy of novel therapeutic vaccines were demonstrated in monkey models as well as murine models. Thus, this is the leading report of new vaccine against TB. According to our knowledge, only a few therapeutic vaccines against TB have been reportedCitation14,Citation15 HSP65-vaccine as well as 15K granulysin-vaccine delivered by HVJ-Envelope vector prolonged the survival and augmented the immune responses in the cynomolgus monkey model which closely mimics human TB disease. Thus, we are taking advantage of the availability of multiple animal models and are accumulating essential data on the DNA vaccine/HVJ-envelope in anticipation of initiating a phase I clinical trial.
It is very important to evaluate the long-term survival in a monkey model, as human TB is a chronic infection disease. Thus, it is necessary for the development of effective vaccine to evaluate the long-term survival of monkey.Citation2,Citation3,Citation7–Citation9 In this study, increase in the survival rate was also observed in HVJ-Envelope/15K granulysin vaccine-treated group, compared with saline-treated group (control group). In addition, it is noteworthy that histopathological improvement was observed in the lung of vaccine-treated monkey (365 d after TB infection). A lot of granulomatous lesions were observed in lung of survived monkey, while a little or no such lesions were observed in lung of saline-treated monkey, which had died of TB within 200 d after TB challenge. Histology of granulomatous lesions observed in this experiment was very similar to human lung TB granuloma by histopathological examinations.
Efficacy of 15K granulysin-vaccine was studied in murine models of TB. We used therapeutic models in this experiment. Furthermore, we examined the synergistic effect of two vaccines (the combination of HSP65- and granulysin-vaccines) in the same therapeutic model. The results indicated the synergistic effect of the combinational vaccination. Therefore, the combination of these therapeutic vaccines might be useful for the development of vaccines against human TB infectious disease. In summary, it was demonstrated that granulysin-vaccine had a therapeutic effect against TB in the mouse and monkey models of TB.
We also elucidated the in vivo function of Ksp37. Ksp37 is expressed in cytotoxic lymphocytes, selectively in the effector subset of CD8+ T cells, CD16+ NK cells and γ/δT cells.Citation13 Expression of Ksp37 mRNA was closely correlated with good prognosis in ovarian cancer cells and gliomas.Citation16,Citation17 However, detailed immunological function has not been elucidated yet. We first revealed that the level of Ksp37 protein in the serum of patients with TB was lower than that of healthy volunteer. The result suggested the relation between the serum level of Ksp37 and TB disease. Next, we have established Ksp37 transgenic mice to elucidate the in vivo role of Ksp37 in the defense against the infection of M. tuberculosis. Ksp37 transgenic mice showed in vivo anti TB effect. Thus it was demonstrated that Ksp37 played an important role in anti-TB function in human as well as mice bodies. Finally, we examined the efficacy of Ksp37-vaccine in the mouse model of TB. Similar to granulysin-vaccine, Ksp37-vaccine augmented in vivo differentiation of CTL against TB (data not shown). In addition, simultaneous administration of Ksp37- and granulysin-vaccines induced CTL generation synergistically (data not shown). Therefore, these findings indicate that granulysin- and Ksp37-vaccine might provide very useful weapon as a novel TB vaccine, in the monotherapy or combination therapy.
The HSP65 vaccine showed a significant therapeutic effect against TB, as described previously: (1) Prolongation of survival of mice infected with XDR-TB; (2) Decrease in the CFU of TB in lung, liver and spleen of mice infected with MDR-TB as well as drug-sensitive TB (H37Rv); (3) Decrease in the CFU of TB in organs of mice challenged with TB in the in vivo humanized immune model of SCID-PBL/hu.
Here, we revealed the synergistic effects of the combination therapy of HSP65-vaccine and a first line chemotherapy drug Isoniazid (INH). It is very important to make a suitable regimen, which enables the treatment of the patient with TB to complete within a shorter period. In such circumstances, our data demonstrating the synergistic effect of the combinational therapy using a DNA vaccine and a chemotherapy drug will provide a new strategy for the treatment of TB.
We also revealed the importance of administration route of DNA vaccine. Generally, vaccines are administrated either intradermally (i.d.), intramuscullary (i.m.) or subcutaneously (s.c.). Our data suggested that the intradermal injection is suitable for the administration of our DNA vaccines. Therefore, in the monkey model we plan to conduct the efficacy study of intradermal injection of this vaccine. We will compare the efficacy of intradermal administration to conventional i.m administration.
In the recent study using cynomologus monkeys, it is suggested that i.d. vaccination of HSP65-vaccine showed stronger therapeutic effects against TB than i.m. vaccination on the basis of the prolongation of survival and ESR (Erythrocyte Sedimentation Rate).
DNA vaccine is a relatively new approach of immunization for infectious diseases.Citation3,Citation4,Citation18–Citation21 We have developed a hemagglutinating virus of Japan envelope (HVJ-Envelope) using inactivated Sendai virus, as a nonviral vector for drug delivery.Citation22–Citation24 It can efficiently deliver DNAs, siRNAs, proteins and anti-cancer drugs into cells both in vitro and in vivo.Citation25–Citation27 Therefore, HVJ-Envelope was suitable as an efficient and safe vector for DNA vaccines.
The priority of development of vaccine(s) to prevent reactivation of TB will be increased, since large proportion of the world is latently infected with TB. The combination of HSP65-vaccine with conventional vaccine (BCG) showed synergistic effects in the mouse and monkey models of TB and prolonged the survival of animals. Therefore, it will be important to evaluate the current vaccines as post-exposure vaccines. Combination of several vaccines or combination of vaccines with drugs for chemotherapy might provide a new insight for the prevention of the reactivation of TB.
In conclusion, our data indicated the synergistic therapeutic effect of combination of HSP65-, granulysin- and Ksp37-vaccines or combination of these DNA vaccines and first line chemotherapy drug(s). Combinational therapy using vaccines and antibiotics might provide novel rationale against MDR-TB therapy. Furthermore, the efficacies of HSP65 vaccine and granulysin vaccine were confirmed in the murine therapeutic model for XDR-TB and cynomolgus monkey therapeutic model. These data will provide a rationale for moving this vaccine into clinical trial. HSP65-, granulysin- and Ksp37-vaccines might be useful vaccines against TB including XDR-TB and MDR-TB after the clinical trials.
Materials and Methods
Methods for the evaluation of the therapeutic efficacy of the vaccine on the M. tuberculosis-infected monkeys
Cynomolgus monkeys were housed in a BL 3 animal facility of the Leonard Wood Memorial Research Center. The animals were vaccinated nine times with the HVJ-envelope with expression plasmid of both HSP65 and human IL-12 (HSP65 + hIL-12/HVJ: 400 ug i.m.), one week after the challenge with the M. tuberculosis Erdman strain (5 × 102) by intratracheal instillation. Immune responses and survival were examined as described in our previous studies.Citation2,Citation6,Citation7
The animals were vaccinated with HVJ-Envelope/15K granulysin DNA vaccine 6 times. Four weeks after challenge of TB, 400 μg of HVJ-Envelope/15K granulysin were injected i.m. six times every two weeks. Survival of monkeys treated with this vaccine were evaluated. All animal experiments were approved by the Leonard Wood Memorial Animal Care and Use Committee and the National Hospital Organization Kinki-chuo Chest Medical Center Animal Care and Use Committee.
Methods for the evaluation of the efficacy of vaccines on the M. tuberculosis-infected mice
DNA vaccines encoding M. tuberculosis HSP65 and IL-12 were encapsulated into HVJ-Envelope.Citation3,Citation6,Citation8,Citation28 HVJ-Envelope were prepared as described previously1.Citation4,Citation29 The HVJ-Envelope complex was aliquoted and stored at –70°C until use. Groups of mice were vaccinated three times with 100 μl of HVJ-Envelope solution containing 50 μg of pcDNA-IgHSP65 and 50 μg of pcDNA-mIL12p40p35-F in the tibia both anterior muscles after TB challenge.Citation28,Citation29 At 30 d after intravenous challenge of M. tuberculosis H37Rv and MDR-TB, the number of CFU in the lungs, spleen, and liver were counted and therapeutic efficacy of HVJ-Envelope DNA vaccines was evaluated.Citation28,Citation29 DBA/1 mice were treated with HVJ-Envelope/HSP65 DNA+IL-12 DNA vaccine three times i.m. at 1, 8 and 15 d after the challenge of 5 × 105 CFU MDR-TB i.v. Therapeutic efficacy was also evaluated by chronic TB infection model of mice using aerosol challenge of TB (15CFU/mouse: Madison aerosol exposure chamber, University of Wisconsin). Mice were maintained in isolator cages, manipulated in laminar flow hoods and used between 8–10 weeks of age. All vaccinations and experiments on isolate tissue of animal were done under anesthetic state with sevoflurane. Infected animals were housed in individual micro-isolator cages in a Biosafety Level (BL) 3 animal facility of the NHO Kinki-chuo Chest Medical Center. All animal experiments were approved by the National Hospital Organization Kinki-chuo Chest Medical Center Animal Care and Use Committee.
Methods for the establishment of SCID-PBL/hu model
IL-2 receptor γ-chain disrupted NOD-SCID-PBL/hu was constructed as described in our previous study.Citation4,Citation5 CTL activity was assessed using the method as described previously.Citation30–Citation32
Methods for the establishment of granulysin transgenic mouse
Either 15K granulysin gene, 9K granulysin gene or secreted 9K granulysin DNA (15K Gra secretory signal DNA was fused into N-terminal of 9K granulysin DNA) were transferred to expressing plasmid DNA (pCAGGS) having CAG promoter. DNA fragment was injected to pronuclei embryo and grafted to 200 foster parents. Two types of 15K granulysin Tg mice, 3 types of 9K granulysin Tg mice and 6 types of secreted 9K granulysin Tg mice were made. Granulysin activity was assessed by monoclonal antibody targeting 15K granulysin and 9K granulysin. Mycobacterium tuberculosis H37Rv 5 × 105 CFU was intravenously injected to 15K granulysin Tg mice, 9K granulysin Tg mice, wild type (control) mice and normal C57BL/6 mice (8~12weeks).Citation3,Citation7 From 2 to 12 weeks after injection, these mice were sacrificed. The lungs, the liver and the spleen of these mice were removed, homogenized and cultivated for 14 d on 7H11 agar medium. Then, the number of colony of Mycobacterium tuberculosis was measured.Citation28,Citation29
Method for the establishment of Ksp37 transgenic mouse
Ksp37 gene were transferred to expressing plasmid DNA (pCAGGS) having CAG promoter. DNA fragment was injected to pronuclei embryo and grafted to 200 foster parents. Two types of Ksp Tg mice (#13, #14) were made. Ksp activity was assessed by monoclonal antibody targeting Ksp 37.
Reagents
Isoniazid (INH) was obtained from Sigma Co. Ltd (lot No. 117K0712). Rifampicin (RFP) was obtained from Sigma Co. Ltd (lot No. 087K18753). An amount of 0.03 mg/mouse of INH and 0.1 mg/mouse of RFP were administered to mice per os.
Statistical analysis
Student’s t tests and Tukey-Krumer’s test were used to compare log 10 value of CFU between groups following challenge of TB. Student’s t tests were also performed to compare immune responses between groups in T cell proliferation assay. A P-value of < 0.05 was considered significant.
| Abbreviations: | ||
| HVJ | = | hemagglutinating virus of Japan |
| 15K granulysin | = | 15 kilodalton granulysin |
| Ksp37 | = | killer specific secretory protein of 37kDa |
| MDR-TB | = | multi-drug registant tuberculosis |
| XDR-TB | = | extremely drug resistant tuberculosis |
| Tg | = | transgenic |
Acknowledgments
This study was supported by Health and Labour Science Research Grants from MHLW, Research on Publicly Essential Drugs and Medical Devices, Japan Health Science Foundation, The Association for Preventive Medicine of Japan and Grant-in-Aid for Scientific Research (B,C) from the Ministry of Education, Culture, Sports, Science and Technology Japan, and Grant of Osaka Tuberculosis Foundation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Walsh GP, Tan EV, dela Cruz EC, Abalos RM, Villahermosa LG, Young LJ, et al. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat Med 1996; 2:430 - 6; http://dx.doi.org/10.1038/nm0496-430; PMID: 8597953
- Kita Y, Tanaka T, Yoshida S, Ohara N, Kaneda Y, Kuwayama S, et al. Novel recombinant BCG and DNA-vaccination against tuberculosis in a cynomolgus monkey model. Vaccine 2005; 23:2132 - 5; http://dx.doi.org/10.1016/j.vaccine.2005.01.057; PMID: 15755583
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Evaluation of a novel vaccine (HVJ-liposome/HSP65 DNA+IL-12 DNA) against tuberculosis using the cynomolgus monkey model of TB. Vaccine 2007; 25:2990 - 3; http://dx.doi.org/10.1016/j.vaccine.2007.01.014; PMID: 17280753
- Tanaka F, Abe M, Akiyoshi T, Nomura T, Sugimachi K, Kishimoto T, et al. The anti-human tumor effect and generation of human cytotoxic T cells in SCID mice given human peripheral blood lymphocytes by the in vivo transfer of the Interleukin-6 gene using adenovirus vector. Cancer Res 1997; 57:1335 - 43; PMID: 9102222
- Okada M, Okuno Y, Hashimoto S, Kita Y, Kanamaru N, Nishida Y, et al. Development of vaccines and passive immunotherapy against SARS corona virus using SCID-PBL/hu mouse models. Vaccine 2007; 25:3038 - 40; http://dx.doi.org/10.1016/j.vaccine.2007.01.032; PMID: 17289225
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Novel prophylactic and therapeutic vaccine against tuberculosis. Vaccine 2009; 27:3267 - 70; http://dx.doi.org/10.1016/j.vaccine.2009.01.064; PMID: 19200841
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Novel therapeutic vaccine: granulysin and new DNA vaccine against Tuberculosis. Hum Vaccin 2011; 7:Suppl 60 - 7; http://dx.doi.org/10.4161/hv.7.0.14563; PMID: 21546794
- Kita Y, Okada M, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Development of therapeutic and prophylactic vaccine against Tuberculosis using monkey and transgenic mice models. Hum Vaccin 2011; 7:Suppl 108 - 14; http://dx.doi.org/10.4161/hv.7.0.14571; PMID: 21263229
- Okada M, Kita Y. Tuberculosis vaccine development: The development of novel (preclinical) DNA vaccine. Hum Vaccin 2010; 6:297 - 308; http://dx.doi.org/10.4161/hv.6.4.10172; PMID: 20372079
- Peña SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J Immunol 1997; 158:2680 - 8; PMID: 9058801
- Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998; 282:121 - 5; http://dx.doi.org/10.1126/science.282.5386.121; PMID: 9756476
- Huang LP, Lyu SC, Clayberger C, Krensky AM. Granulysin-mediated tumor rejection in transgenic mice. J Immunol 2007; 178:77 - 84; PMID: 17182542
- Ogawa K, Tanaka K, Ishii A, Nakamura Y, Kondo S, Sugamura K, et al. A novel serum protein that is selectively produced by cytotoxic lymphocytes. J Immunol 2001; 166:6404 - 12; PMID: 11342666
- Rook GAW, Lowrie DB, Hernàndez-Pando R. Immunotherapeutics for tuberculosis in experimental animals: is there a common pathway activated by effective protocols?. J Infect Dis 2007; 196:191 - 8; http://dx.doi.org/10.1086/518937; PMID: 17570105
- Kaufmann SH, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet 2010; 375:2110 - 9; http://dx.doi.org/10.1016/S0140-6736(10)60393-5; PMID: 20488515
- Elgaaen BV, Haug KB, Wang J, Olstad OK, Fortunati D, Onsrud M, et al. POLD2 and KSP37 (FGFBP2) correlate strongly with histology, stage and outcome in ovarian carcinomas. PLoS One 2010; 5:e13837; http://dx.doi.org/10.1371/journal.pone.0013837; PMID: 21079801
- Yamanaka R, Arao T, Yajima N, Tsuchiya N, Homma J, Tanaka R, et al. Identification of expressed genes characterizing long-term survival in malignant glioma patients. Oncogene 2006; 25:5994 - 6002; http://dx.doi.org/10.1038/sj.onc.1209585; PMID: 16652150
- Huygen K. DNA vaccines: application to tuberculosis. Int J Tuberc Lung Dis 1998; 2:971 - 8; PMID: 9869111
- Lowrie DB. DNA vaccines against tuberculosis. Curr Opin Mol Ther 1999; 1:30 - 3; PMID: 11249680
- Hoft DF. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet 2008; 372:164 - 75; http://dx.doi.org/10.1016/S0140-6736(08)61036-3; PMID: 18620952
- Gupta UD, Katoch VM, McMurray DN. Current status of TB vaccines. Vaccine 2007; 25:3742 - 51; http://dx.doi.org/10.1016/j.vaccine.2007.01.112; PMID: 17321015
- Saeki Y, Matsumoto N, Nakano Y, Mori M, Awai K, Kaneda Y. Development and characterization of cationic liposomes conjugated with HVJ (Sendai virus): reciprocal effect of cationic lipid for in vitro and in vivo gene transfer. Hum Gene Ther 1997; 8:2133 - 41; http://dx.doi.org/10.1089/hum.1997.8.17-2133; PMID: 9414261
- Kaneda Y, Nakajima T, Nishikawa T, Yamamoto S, Ikegami H, Suzuki N, et al. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther 2002; 6:219 - 26; http://dx.doi.org/10.1006/mthe.2002.0647; PMID: 12161188
- Kaneda Y. New vector innovation for drug delivery: development of fusigenic non-viral particles. Curr Drug Targets 2003; 4:599 - 602; http://dx.doi.org/10.2174/1389450033490740; PMID: 14577648
- Kaneda Y, Yamamoto S, Nakajima T. Development of HVJ envelope vector and its application to gene therapy. Adv Genet 2005; 53:307 - 32; http://dx.doi.org/10.1016/S0065-2660(05)53012-8; PMID: 16240999
- Ito M, Yamamoto S, Nimura K, Hiraoka K, Tamai K, Kaneda Y. Rad51 siRNA delivered by HVJ envelope vector enhances the anti-cancer effect of cisplatin. J Gene Med 2005; 7:1044 - 52; http://dx.doi.org/10.1002/jgm.753; PMID: 15756713
- Mima H, Yamamoto S, Ito M, Tomoshige R, Tabata Y, Tamai K, et al. Targeted chemotherapy against intraperitoneally disseminated colon carcinoma using a cationized gelatin-conjugated HVJ envelope vector. Mol Cancer Ther 2006; 5:1021 - 8; http://dx.doi.org/10.1158/1535-7163.MCT-05-0352; PMID: 16648574
- Yoshida S, Tanaka T, Kita Y, Kuwayama S, Kanamaru N, Muraki Y, et al. DNA vaccine using hemagglutinating virus of Japan-liposome encapsulating combination encoding mycobacterial heat shock protein 65 and interleukin-12 confers protection against Mycobacterium tuberculosis by T cell activation. Vaccine 2006; 24:1191 - 204; http://dx.doi.org/10.1016/j.vaccine.2005.08.103; PMID: 16216394
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Novel prophylactic vaccine using a prime-boost method and hemagglutinating virus of Japan-envelope against tuberculosis. Clin Dev Immunol 2011; 2011:549281; http://dx.doi.org/10.1155/2011/549281; PMID: 21437226
- Okada M, Yoshimura N, Kaieda T, Yamamura Y, Kishimoto T. Establishment and characterization of human T hybrid cells secreting immunoregulatory molecules. Proc Natl Acad Sci U S A 1981; 78:7717 - 21; http://dx.doi.org/10.1073/pnas.78.12.7717; PMID: 6801660
- Okada M, Sakaguchi N, Yoshimura N, Hara H, Shimizu K, Yoshida N, et al. B cell growth factors and B cell differentiation factor from human T hybridomas. Two distinct kinds of B cell growth factor and their synergism in B cell proliferation. J Exp Med 1983; 157:583 - 90; http://dx.doi.org/10.1084/jem.157.2.583; PMID: 6600487
- Okada M, Kitahara M, Kishimoto S, Matsuda T, Hirano T, Kishimoto T. IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J Immunol 1988; 141:1543 - 9; PMID: 3261754