Abstract
The increased worldwide awareness of seasonal and pandemic influenza, including pandemic H1N1 virus, has stimulated interest in the development of economic platforms for rapid, large-scale production of safe and effective subunit vaccines. In recent years, plants have demonstrated their utility as such a platform and have been used to produce vaccine antigens against various infectious diseases. Previously, we have produced in our transient plant expression system a recombinant monomeric hemagglutinin (HA) protein (HAC1) derived from A/California/04/09 (H1N1) strain of influenza virus and demonstrated its immunogenicity and safety in animal models and human volunteers. In the current study, to mimic the authentic HA structure presented on the virus surface and to improve stability and immunogenicity of the HA antigen, we generated trimeric HA by introducing a trimerization motif from a heterologous protein into the HA sequence. Here, we describe the engineering, production in Nicotiana benthamiana plants, and characterization of the highly purified recombinant trimeric HA protein (tHA-BC) from A/California/04/09 (H1N1) strain of influenza virus. The results demonstrate the induction of serum hemagglutination inhibition antibodies by tHA-BC and its protective efficacy in mice against a lethal viral challenge. In addition, the immunogenic and protective doses of tHA-BC were much lower compared with monomeric HAC1. Further investigation into the optimum vaccine dose and/or regimen as well as the stability of trimerized HA is necessary to determine whether trimeric HA is a more potent vaccine antigen than monomeric HA.
Introduction
Influenza presents one of the most significant threats to human populations, and its prevention is one of the world’s greatest public health challenges because of antigenic variation of influenza viruses caused by mutations in hemagglutinin (HA).Citation1 From 1976 to 2007 in the US, the estimated annual average of excess deaths resulting from respiratory and circulatory causes associated with seasonal influenza was about 23,600 (range 3,300 to 48,600).Citation2 The recent emergence of H1N1 swine influenzaCitation3-Citation5 is testimony to the unpredictability and threat imposed by influenza viruses on the health and well-being of global populations and underscores the urgent need for rapid and robust influenza vaccine production methods to better prevent and mitigate the potential effects of pandemics. The increased worldwide awareness of seasonal and pandemic influenza has stimulated interest in the development of economic platforms for large-scale production of safe and effective subunit vaccines. The use of recombinant DNA techniques to generate vaccine antigens is an attractive alternative that avoids dependence on chicken egg supply and live viruses for production, and also allows for complete control over the sequence during production processes.
Influenza HA, one of the major viral glycoproteins, exists as a homotrimeric type I transmembrane protein on the surface of virions.Citation6 During the virus life cycle, HA plays a key role in viral infectivity and pathogenicity by binding to the host cell surface receptors enabling the virus to enter host cells.Citation6 A large body of evidence has revealed that protection provided by influenza vaccines is mediated primarily by an anti-HA neutralizing antibody.Citation7 Therefore, HA has been a major target antigen for influenza vaccine development. Progress in recombinant DNA technology allows for rapid cloning of influenza virus HA genes, expression of correctly folded and biologically active HA in eukaryotic systems and high levels of recombinant HA production. The immunogenicity and safety of recombinant HA expressed in a baculovirus-insect cell system (FluBlok® trivalent seasonal influenza vaccine, Protein Sciences Corp.) has been evaluated in a number of clinical trials.Citation8-Citation10 FluBlok has been shown to be well tolerated and immunogenic in adults older than 18 y, and has demonstrated clinical efficacy in the prevention of influenza in two field trials.Citation9,Citation10
Recently, plant production systems have emerged as very promising alternatives for vaccine manufacturing, since they are economic biomass generators that can be rapidly scaled, possess eukaryotic post-translational protein modification machinery broadly similar to that of mammals and do not harbor human pathogens.Citation11-Citation13 We have demonstrated that plant-derived HA from the A/Wyoming/03/03 (H3N2) strain of influenza has authentic antigenicity and is immunogenic in mice inducing significant serum hemagglutination inhibition (HAI) and virus neutralizing (VN) antibody titers.Citation14 Furthermore, HA from A/Indonesia/05/05 (H5N1) has also been cloned and expressed in N. benthamiana and shown to induce HAI antibodies in mice and ferrets with exhibited protective efficacy against challenge infection in ferrets.Citation15 Similarly produced HAs from a novel A/California/04/09 (H1N1) swine influenza virus strain as well as from A/Brisbane/59/07, A/Brisbane/10/07 and B/Florida/04/06 seasonal influenza strains were shown to induce serum anti-HA IgG and HAI antibody responses in mice.Citation16 Clinical development of several plant-derived HA-based influenza candidate vaccines is currently ongoing. A plant-produced soluble HA from an A/California/04/09 (H1N1) swine influenza virus strain engineered by Fraunhofer USA Center for Molecular Biotechnology (FhCMB, Newark, DE)Citation17 has been evaluated in a Phase 1 clinical trial and was demonstrated to be safe and immunogenic in healthy human volunteers.Citation18 Another vaccine product derived from A/California/04/09 (H1N1) influenza virus, H1 HA-based virus-like particles (VLPs) produced by Medicago, Inc. has been also evaluated in a Phase 1 trial and demonstrated to be safe and immunogenic in healthy adults.Citation19 Furthermore, researchers at Medicago, Inc. have also produced an HA-based VLP vaccine candidate from an A/Indonesia/05/05 (H5N1) influenza virus strain and demonstrated its safety and immunogenicity in a Phase 2 clinical trial.Citation20,Citation21
Although the development of recombinant subunit HA-based influenza vaccines has shown encouraging progress, there are still some challenges to be overcome, such as stability of vaccine antigens and immunogenicity comparable to the currently licensed inactivated virus vaccines.Citation22 Unlike the HA molecules on the viral surface that exist as homotrimers, recombinant HA molecules tend to be expressed as either aggregates or monomers depending on the expression system and target antigen strain.Citation23 Mimicking the authentic trimeric HA (tHA) structure presented on the virus surface may provide a way to improve the immunogenicity of recombinant HA-based vaccines. One approach to address this is to generate VLPs using recombinant DNA technology. Using this approach, functional H5N1 HA-based VLPs have been produced in plants and insect cells.Citation24-Citation26 Another approach to generate recombinant tHA is to enforce and stabilize the trimerization of recombinant HA using trimerization motifs.Citation22,Citation27-Citation29 Trimerization of viral HA is due to specific interactions along heptad repeat (HR) regions of the HA2 domain. These HR regions undergo significant structural changes, which leads to viral infection of the host cell. Trimerization and structural changes are driven by the coiled-coil nature of the HR regions. Due to the structural dominance of these coiled-coil motifs, the engineering efforts employed here sought to adapt versions of these motifs to induce trimerization in our HAC1, monomeric antigen.
In this study, we have designed, produced in plants, purified and characterized an engineered tHA antigen (tHA-BC) from A/California/04/09 strain of influenza virus, and compared immunogenicity and protective efficacy of this new subunit influenza vaccine candidate with plant-produced monomeric HAC1 in mice.
Results
Engineering and screening of plant-derived recombinant trimeric HA antigens
To induce in vivo trimerization of HAC1, three trimerization motifs were used: a motif from coronin, an actin binding protein,Citation30 the foldon domain from the bacteriophage T4 fibritin protein,Citation31,Citation32 or an HR domain of HA from A/Wyoming/03/03 strain of influenza virus that forms a trimeric subunit protein when expressed in plants (R. M. Jones, unpublished observation). These trimerization motifs were engineered at the C-terminus of HA from A/California/04/09 (H1N1) strain of influenza virus, which, when expressed and purified independent of trimerization motifs, produces monomeric HAC1. Constructs of each were made with or without the 15 amino acids (E516-I530) of the bromelain cleavage site (constructs with “B” are without) and prior to the transmembrane span (). Two additional constructs were engineered to introduce HR from HA of A/Brisbane/59/07 strain of influenza virus (HAB1(H1)): the first by introducing mutations in the second HR region of HAC1 to resemble that of HAB1(H1) and the second by replacing the globular domain of HAB1(H1) with that of HAC1 (residues C59 through C292), thus utilizing the stalk region of the HAB1(H1) (). All constructs were engineered to have a poly-histidine (6 × His) tag for purification and the KDEL sequence for the endoplasmic reticulum (ER) retention at the C-terminus.
Table 1. Construct description
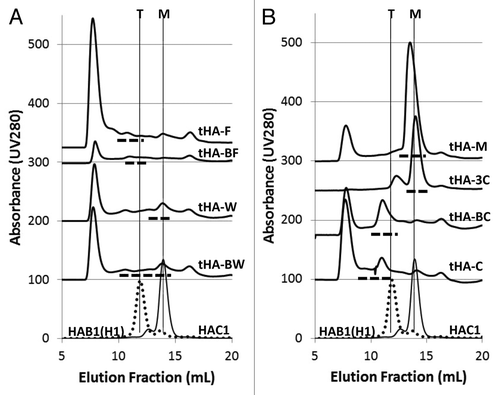
To determine which construct would result in tHA assembly, the recombinant HA was recovered by immobilized metal affinity chromatography (IMAC) capture for each target from clarified extract of plant tissue inoculated with each construct followed by size exclusion chromatography (SEC). SEC eluates were analyzed by western blotting using an anti-4 × His monoclonal antibody (mAb). SEC retention values of targets were compared with those of HAC1 (control monomeric HA) or HAB1(H1) (control tHA). The SEC elution profiles are shown in and B. The dashed line under each elution profile indicates the area where target was detected by western blotting. The bottom two profiles in each panel represent purified HAB1(H1) (trimer) and HAC1 (monomer). The analysis of tHA-C and tHA-BC () and tHA-F and tHA-BF () suggests expression of tHA for these four constructs. However, tHA-BC that was engineered by including a 32-aa trimerization motif from coronin was selected for further evaluation based on its relatively higher expression level. The construct tHA-M, modified with point mutations to resemble HAB1(H1) HR regions, produced monomeric HA (), as well as placing the globular head of HAC1 on the stalk region of HAB1(H1) as in tHA-3C. For the test constructs which used a trimerization domain from HA of A/Wyoming/03/03 for forced trimerization, target product was identified in fractions associated with monomeric HA for tHA-W and in a range of fractions that eluted as potentially greater than trimeric all the way to monomeric sizes for tHA-BW ().
Figure 1. Evaluation of trimeric HA vaccine candidates by SEC. Monomeric HAC1 and trimeric HAB1(H1) elution profiles are shown at the bottom of each panel. The elution volumes for monomeric HA (14 mL) and tHA (12 mL) are indicated by the vertical lines marked M and T, respectively. tHAs were captured over IMAC and separated over analytical SEC. Fractions were collected and HA identified by western blot; the dashed lines under each profile indicate the fractions where trimeric candidates were identified. Oligomerization state of each candidate as monomeric or trimeric was based on their relative elution volume to the standard retentions of HAC1 or HAB1(H1).
Production and characterization of the plant-derived recombinant tHA-BC antigen
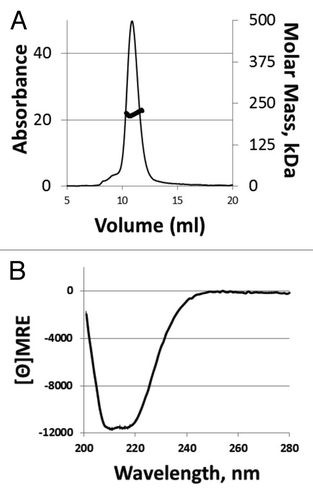
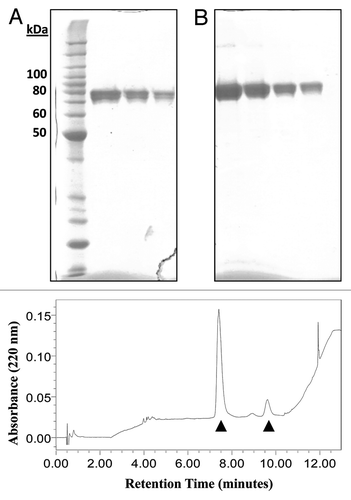
tHA-BC was produced in N. benthamiana and purified by detergent extraction of homogenized aerial plant tissues, IMAC capture of the target from clarified extract, ammonium precipitation of contaminants followed by buffer exchange and final polishing in two ion exchange chromatography (IEX) steps. Purified tHA-BC was characterized by physicochemical and immunochemical methods. Analyses including SDS-PAGE and reverse-phase ultra-performance liquid chromatography (RP-UPLC) demonstrated > 90% purity of the tHA-BC antigen (). The RP-UPLC profile showed three main peaks () and tHA-BC identity was confirmed by western blotting using an anti-H1 polyclonal antiserum (data not shown) in the peaks indicated by the arrowheads. Notably, fractionation of the purified tHA-BC antigen over a SEC column followed by detection with UV and multi-angle laser light scattering (SEC-MALLS) demonstrated the presence of a dominant trimeric solution species, with a calculated molar weight of 218 kDa (). Circular dichroism (CD) spectroscopy analysis of tHA-BC spectra indicated 26% helical content (), which agrees with published H1 crystal structures from A/California/04/09 HA that show approximately 23% helical structure in the HA molecule.Citation32 The addition of the coronin helix would theoretically add 4–5% to the helical nature of the molecule. The HA content in the tHA-BC preparation was evaluated in a single-radial immunodiffusion (SRID) assay, using standard antiserum and antigen generated for the egg-produced influenza vaccine. Results showed equivalent concentration values for tHA-BC to those measured by absorbance at 280 nm (A280). In contrast, measurements of HA content in monomeric HAC1 preparations by SRID tend to be significantly higher than the concentration determined by A280 (data not shown).
Figure 2. Electrophoretic mobility and RP-UPLC analysis of tHA-BC. Coomassie-stained SDS-PAGE of (A) HAC1 and (B) tHA-BC. Molecular weight markers (lane 1), HAC1 monomer (lanes 2–4) or tHA-BC (lanes 5–8) at various loads. tHA-BC loaded at approximately 1 µg indicates > 90% purity. (C) RP-UPLC profile for tHA-BC. The elution profile shows three main peaks at 7.5, 9.0 and 9.75 min. Western blot analysis of these peaks revealed that tHA-BC is present in peaks at 7.5 and 9.75 min, resulting in a combined purity of > 90%. Elution absorbance was recorded at 220 nm from a C4 column with a 1%/min gradient across the main peak.
Immunogenicity of tHA-BC in mice
Immunogenicity studies were conducted in mice to evaluate the minimal effective dose that generated protective correlates of immunity. Assessment of sera of individual mice immunized with tHA-BC plus Alhydrogel indicated that mean serum hemagglutination inhibition (HAI) antibody titers in all dose groups increased by study day 42 (3 weeks post 2nd vaccination) and showed a further increase in the response through study day 70 (). Following the primary dose, on study day 21, serum HAI titers of individual mice immunized with tHA-BC or monomeric HAC1 plus Alhydrogel were undetectable except for a mouse in the group immunized with 0.25 µg of tHA-BC (). Average HAI titers on study day 42, following a booster dose (day 21), were higher in groups immunized with tHA-BC when compared with the groups immunized with monomeric HAC1, although the differences were not significant except between groups immunized with 0.25 µg of tHA-BC or HAC1 (p < 0.05). These HAI titers were further increased on study day 70 with more than 75% of mice immunized with tHA-BC generating an HAI titer of ≥ 1:40 (87.5, 75 and 75% at doses of 1, 0.5 and 0.25 µg, respectively). The group of animals immunized with a licensed H1N1 vaccine elicited higher HAI antibody responses than those observed in the other groups throughout the study. However, significant differences were not observed (p > 0.1) between groups immunized with the H1N1 vaccine and 0.25 µg of tHA-BC plus Alhydrogel on both study days 42 and 70.
Table 2. Serum HAI antibody responses and percent responders
Protection of mice against lethal virus challenge
To evaluate the protective efficacy of tHA-BC, mice were immunized on study days 0 and 21 with tHA-BC with or without Alhydrogel and challenged intranasally with 50 lethal dose 50% (LD50) of mouse-adapted A/California/04/09 (MA-CA/04/09) virus. The protective efficacy of tHA-BC was compared with that of monomeric HAC1 plus Alhydrogel. All mice from groups immunized with tHA-BC in the absence of Alhydrogel along with the phosphate buffered saline (PBS) control succumbed to the infection by 6 d post challenge except for a mouse in the group immunized with 0.625 µg without adjuvant (). The average body weight loss of mice immunized with tHA-BC alone was approximately 20% and was at a comparable rate to the PBS control group (). Conversely, groups immunized with tHA-BC plus Alhydrogel at all doses tested maintained their body weight throughout the observation period (), and 100% of mice survived the challenge infection. In the group immunized with 5 µg of monomeric HAC1 plus Alhydrogel, 100% of animals maintained their initial body weight and survived the challenge infection ( and B). Four days following challenge, lungs were collected from 4 mice per group and viral titers were measured. Viral titers in the lung tissue from groups immunized with tHA-BC plus Alhydrogel and monomeric HAC1 plus Alhydrogel were significantly lower than those obtained from the PBS control group (). Viral titers in the lung tissues from groups immunized with tHA-BC alone were at levels equivalent to the PBS control group ().
Figure 4. Protection of mice against the lethal virus challenge infection. Mice immunized with either HAC1 or tHA-BC were challenged on study day 45 with the MA-CA/04/09 virus. (A) Body weights were measured up to 19 d after challenge infection on days shown in the figure. (B) Mice were monitored daily up to 14 d after challenge infection. Open circles, triangles, squares and asterisks represent groups immunized with 5, 2.5, 1.25 and 0.625 µg/dose of tHA-BC, respectively. Diamonds: 5 µg/dose of monomeric HAC1. Closed bars: PBS control. Dashed lines: Groups immunized without Alhydrogel. The body weight change of a mouse in the group immunized with 0.625 µg of tHA-BC alone that recovered from infection 6 d post challenge was not included in this chart.
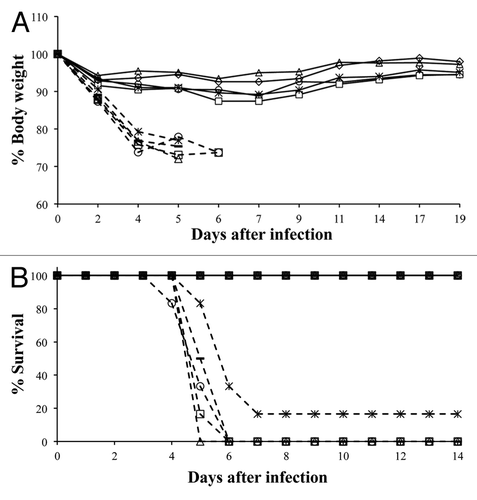
Table 3. Virus titers in the lung tissue following challenge infection
Discussion
The emergence of a novel A/California/04/09 strain of influenza A virus stimulated the ongoing development of new economic platforms for rapid, large-scale production of subunit influenza vaccines. In this study, we have utilized our launch vector-based transient expression technology to rapidly produce an engineered trimeric form of recombinant HA antigen of the indicated strain of influenza A virus, in N. benthamiana plants, and confirmed the identity and trimeric state of the protein. Using a chromatography-based purification scheme, we were able to obtain a highly pure (> 90%) preparation of recombinant tHA-BC. Physicochemical methods showed that it formed a stable solution trimer with an expected folding based on helical nature. In addition, SRID data suggests that tHA-BC produced in plants behaved in a similar manner to the egg-produced influenza vaccine while maintaining authentic antigenicity of influenza HA.
tHA-BC delivered via an intramuscular (IM) route with Alhydrogel induced HAI serum antibody titers in mice. In the presence of Alhydrogel, the dose could be reduced down to 0.25 µg and still elicit HAI titers of ≥ 1:40 in over 70% of immunized animals at levels approaching those obtained from mice immunized with a licensed H1N1 vaccine produced in eggs. Furthermore, tHA-BC at doses down to 0.625 µg adjuvanted with Alhydrogel conferred protection to mice against a lethal challenge with live influenza virus, increasing animal survival while reducing weight loss and virus titers in the lungs. Other studies that explored trimerization of recombinant HA used the trimerization motif from the leucin zipper GCN4pII.Citation28,Citation29 Lin et al. demonstrated the immunogenicity of this trimeric HA produced in a baculovirus-insect cell expression system in mice and evaluated the potential of this trimeric HA as an influenza vaccine using a prime-boost strategy with recombinant adenovirus vector. However, a direct comparison of the immunogenicity of the monomeric vs. the trimeric HA was not present in their study.Citation28 On the other hand, Weldon et al. developed trimeric HA using the motif from GCN4pII expressed in a baculovirus-insect cell system and demonstrated its enhanced immunogenicity compared with monomeric HA.Citation29 In our study described here, significant differences in the protective efficacy of the plant-produced tHA-BC antigen compared with monomeric HAC1 was not demonstrated. However, immunogenicity, in the presence of Alhydrogel adjuvant, was demonstrated at much lower target protein doses for tHA-BC as compared with HAC1. At the doses tested in these studies, Alhydrogel was required to induce protective immune responses against tHA-BC. Therefore, further investigation into the optimum vaccine dose, formulation and regimen as well as the stability of the trimerized HA is necessary to draw definitive conclusions regarding which vaccine design is superior.
In summary, we engineered and produced recombinant tHA from A/California/04/09 strain of influenza virus using a trimerization motif from an actin binding protein, coronin. The resulting HA antigen consists of a dominant, tHA species and small proportions of possibly hexamer or higher oligomers as determined by SEC-MALLS. Immunization of mice with the tHA-BC antigen in the presence of aluminum adjuvant elicited robust HAI antibody responses and protected the animals from the lethal challenge infection.
Materials and Methods
Design, cloning, expression and purification of influenza HA trimers
The HAC1 nucleotide sequence, encompassing amino acids 18–530 of A/California/04/09 (NCBI accession number ACQ76318.1) strain of influenza virus was optimized for expression in plants and synthesized by GENEART AG (Regensburg, Germany) as described previously.Citation15 The optimized HAC1 sequences, amino acids D18-E515 or D18-I530, were used to test various trimerization schemes. Residues of HAC1 were changed to match residues from A/Brisbane/59/2007 (NCBI accession number ACA28844) suspected to be involved in trimer formation of the A/Brisbane/59/2007 HA. Specifically, the following mutations were made to HAC1: H416K, K419R, I421M and L433I, which resulted in the clone tHA-M. A second, chimeric, HAC1/HAB1(H1) construct fused the globular domain of HAC1 (residues C59 through C292) with the stalk region of HAB1(H1) (residues D18-L58 and D292-I529), resulting in the clone tHA-3C (). The use of three different trimerization motif sequences was also employed to effect trimeric HA: amino acids V430 to K461 from coronin (NP_034028),Citation29 amino acids G458 to L484 from the bacteriophage T4 fibritin protein (NCBI accession number ADJ39878),Citation30,Citation31 or amino acids L397 to L447 from the A/Wyoming/03/03 HA(NCBI accession number AAT08000) were fused to the C-terminus of HAC1, resulting in the constructs tHA-C, tHA-F and tHA-W, respectively (). With each of these trimerization motif fusions, additional sets of constructs were made by deleting the C-terminus of HAC1 at the bromelain cleavage site, resulting in the constructs tHA-BC, tHA-BF and tHA-BW ().
All constructs were engineered to contain a Tobacco Etch Virus cleavage siteCitation34 directly after the HA sequence and prior to the trimerization motifs. A 6 × His affinity purification tagCitation35 and the ER retention signal (KDEL)Citation36 were included at the C-terminus of each construct. The resulting sequences were inserted into the launch vector pGRD4Citation15,Citation37 to obtain the final constructs for tHA screening. The constructs were transformed into A. tumefaciens strain GV3101 that was then introduced into hydroponically grown N. benthamiana plants by vacuum infiltration, as described previously.Citation15,Citation38
At seven days post infiltration, leaf tissue of the infiltrated plants was harvested, frozen at -80°C, homogenized and extracted in a phosphate-based buffer containing 0.5% Triton X-100. The extract was clarified by centrifugation (78,000 × g for 30 min) and microfiltration. The recombinant HAs were captured by IMAC (Ni-Sepharose; GE Healthcare, Pittsburgh, PA) and eluted with a phosphate-based buffer containing 300 mM imidazole. These IMAC-captured trimeric candidates were subsequently screened by passage over a Superdex S200 10–300 GL column to evaluate the trimeric solution properties for each construct. Fractions collected over the elution profile were examined by western blot probing with an anti-4 × His mAb (Qiagen, Valencia, CA) as the primary antibody.
Further purification of the selected trimeric candidate included precipitation of contaminants with 1.4 M ammonium sulfate, passage of the target over Capto Q, buffer exchange and polishing over diethylaminoethyl resin with a final trimer separation over Superdex S200 prep grade. All chromatography resins were from GE Healthcare. The purified tHA preparation was filter-sterilized using a 0.2 µm membrane (Millipore, Billerica, MA), aliquoted and stored at -70°C.
tHA-BC characterization
Purity of the purified tHA-BC protein was determined by separation on a 10% SDS-PAGE gel followed by staining with Coomassie Gel Code blue (Pierce, Rochford, IL), as well as by RP-UPLC using a Waters Acquity system (Waters, Milford, MA) with a BEH C4 column and water/acetonitrile (both containing 0.1% trifluoroacetic acid) mobile phases. The yield of tHA-BC was estimated by measuring optical density of the preparations at 280 nm in a denaturing buffer with absorptivity coefficient calculated based on the recombinant sequence. Identity of the tHA-BC protein was established by reactivity in immunoblots using a polyclonal anti-H1 antibody (04/260, National Institute for Biological Standards and Control, Hertfordshire, UK) and an anti-HA mAb specific for HAC1.Citation17 Secondary structure of the tHA-BC preparation was accessed by CD spectroscopy on a Jasco model 815 CD spectropolarimeter. Solution state molar mass was determined by SEC-MALLS (Treos 3 angle detector, Wyatt Technology Corp., Santa Barbara, CA).
tHA-BC immunogenicity study
tHA-BC was evaluated for immunogenicity in mice and compared with HAC1 monomer. For this purpose, six-week–old BALB/c mice (8 per group) were immunized IM with tHA-BC or monomeric HAC1 at doses of 0.25, 0.5 or 1 µg, with 0.3% Alhydrogel (Accurate Chemicals, Westbury, NY) containing 1.5 mg/mL aluminum, on study days 0 and 21. Animals in control groups received either 0.5 µg of an H1N1 licensed monovalent vaccine without Alhydrogel or saline with 0.3% Alhydrogel on study days 0 and 21. Serum samples were collected on days -1 (pre-bleed), 21 (prior to 2nd vaccination), 42 (post 2nd vaccination) and 70, and evaluated for HAI antibody titers against A/California/7/2009 (NYMC X-179A) strain of influenza virus as described below.
tHA-BC virus challenge study
The protective efficacy of tHA-BC was assessed in mice against a lethal challenge with live influenza virus. In these experiments, six- to eight-week–old BALB/c mice (10 per group) were immunized IM with tHA-BC at doses of 0.625, 1.25, 2.5 or 5 µg with or without 0.3% Alhydrogel (1.5 mg/mL aluminum), or with HAC1 monomer at a dose of 5 µg plus Alhydrogel, on study days 0 and 21. Animals in the control group received PBS alone, on study days 0 and 21. On day 45, vaccinated mice were challenged intranasally with 50 LD50 of mouse-adapted A/California/04/09 (MA-CA/04/09)Citation39 (generously provided by Richard Webby, St. Jude Children's Hospital, Memphis, TN) influenza virus and monitored for weight loss and survival for 14 d. Four days post challenge, 4 mice from each group were euthanized and virus titers in whole lungs were examined. Here, lung tissues were homogenized in sterile PBS, and tissue homogenates were clarified by low-speed centrifugation and titrated for virus infectivity in a standard plaque assay using MDCK cells (ATCC, Manassas, VA). Viral titers are expressed as log10 plaque-forming unit (PFU)/mL.
Hemagglutination inhibition assay
The HAI assay protocol was adapted from the World Health Organization Manual on Animal Influenza Diagnosis and SurveillanceCitation40 and Noah and colleages.Citation41 HAI antibody responses were assessed per group as HAI geometric mean titers (GMTs) with standard error of mean (SEM) along with the percentage of animals with HAI titers ≥ 1:40 (% responders).
Acknowledgments
The authors would like to thank Jennifer Jaje, Shama Satinover and Rebecca Snow for technical support and Dr. Natasha Kushnir for editorial assistance. This project was supported by a grant from the Defense Advanced Research Projects Agency. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency.
Submitted
11/29/12
Accepted
12/03/12
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Gerhard W, Yewdell J, Frankel ME, Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature 1981; 290:713 - 7; http://dx.doi.org/10.1038/290713a0; PMID: 6163993
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057 - 62; PMID: 20798667
- Centers for Disease Control and Prevention (CDC). Swine-origin influenza A (H1N1) virus infections in a school - New York City, April 2009. MMWR Morb Mortal Wkly Rep 2009; 58:470 - 2; PMID: 19444151
- Domínguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009; 302:1880 - 7; http://dx.doi.org/10.1001/jama.2009.1536; PMID: 19822626
- Centers for Disease Control and Prevention. Updated estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April 2009 – April 10, 2010. May 14, 2010. http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm (accessed 16 September 2012).
- Wilson IA, Cox NJ. Structural basis of immune recognition of influenza virus hemagglutinin. Annu Rev Immunol 1990; 8:737 - 71; http://dx.doi.org/10.1146/annurev.iy.08.040190.003513; PMID: 2188678
- Han T, Marasco WA. Structural basis of influenza virus neutralization. Ann N Y Acad Sci 2011; 1217:178 - 90; http://dx.doi.org/10.1111/j.1749-6632.2010.05829.x; PMID: 21251008
- Treanor JJ, Schiff GM, Couch RB, Cate TR, Brady RC, Hay CM, et al. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis 2006; 193:1223 - 8; http://dx.doi.org/10.1086/503050; PMID: 16586358
- Cox MM, Patriarca PA, Treanor J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respi Viruses 2008; 2:211 - 9; http://dx.doi.org/10.1111/j.1750-2659.2008.00053.x; PMID: 19453397
- Keitel WA, Treanor JJ, El Sahly HM, Gilbert A, Meyer AL, Patriarca PA, et al. Comparative immunogenicity of recombinant influenza hemagglutinin (rHA) and trivalent inactivated vaccine (TIV) among persons > or =65 years old. Vaccine 2009; 28:379 - 85; http://dx.doi.org/10.1016/j.vaccine.2009.10.037; PMID: 19879222
- Chichester JA, Yusibov V. Plants as alternative systems for production of vaccines. Hum Vaccin 2007; 3:146 - 8; http://dx.doi.org/10.4161/hv.3.4.4148; PMID: 17643065
- Mett V, Farrance CE, Green BJ, Yusibov V. Plants as biofactories. Biologicals 2008; 36:354 - 8; http://dx.doi.org/10.1016/j.biologicals.2008.09.001; PMID: 18938088
- Rybicki EP. Plant-made vaccines for humans and animals. Plant Biotechnol J 2010; 8:620 - 37; http://dx.doi.org/10.1111/j.1467-7652.2010.00507.x; PMID: 20233333
- Shoji Y, Chichester JA, Bi H, Musiychuk K, de la Rosa P, Goldschmidt L, et al. Plant-expressed HA as a seasonal influenza vaccine candidate. Vaccine 2008; 26:2930 - 4; http://dx.doi.org/10.1016/j.vaccine.2008.03.045; PMID: 18440103
- Shoji Y, Bi H, Musiychuk K, Rhee A, Horsey A, Roy G, et al. Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine 2009; 27:1087 - 92; http://dx.doi.org/10.1016/j.vaccine.2008.11.108; PMID: 19100806
- Shoji Y, Farrance CE, Bautista J, Bi H, Musiychuk K, Horsey A, et al. A plant-based system for rapid production of influenza vaccine antigens. Influenza Other Respi Viruses 2012; 6:204 - 10; http://dx.doi.org/10.1111/j.1750-2659.2011.00295.x; PMID: 21974811
- Shoji Y, Chichester JA, Jones M, Manceva SD, Damon E, Mett V, et al. Plant-based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum Vaccin 2011; 7:Suppl 41 - 50; http://dx.doi.org/10.4161/hv.7.0.14561; PMID: 21266846
- Fraunhofer USA Center for Molecular Biotechnology. Fraunhofer USA CMB Confirms Interim Results for Its Plant-Produced H1N1 Influenza Vaccine. March 21, 2012. http://www.businesswire.com/news/home/20120321005221/en/Fraunhofer-USA-CMB-Confirms-Interim-Results-Plant-Produced (accessed 15 October 2012).
- Medicago Inc. Medicago reports positive U.S. clinical trial results for its H1N1 / seasonal influenza vaccine. June 8, 2011. http://www.medicago.com/English/news/News-Releases/News-ReleaseDetails/2011/Medicago-reports-positive-US-clinical-trial-results-for-its-H1N1–seasonal-influenza-vaccine1125593/default.aspx (accessed 15 October 2012).
- Landry N, Ward BJ, Trépanier S, Montomoli E, Dargis M, Lapini G, et al. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS One 2010; 5:e15559; http://dx.doi.org/10.1371/journal.pone.0015559; PMID: 21203523
- Medicago Inc. Medicago reports positive Phase II final results for its avian flu pandemic vaccine. June 30, 2011. http://www.medicago.com/English/news/News-Releases/News-ReleaseDetails/2011/Medicago-reports-positive-Phase-II-final-results-for-its-avian-flu-pandemic-vaccine1125833/default.aspx (accessed 15 October 2012).
- Wei CJ, Xu L, Kong WP, Shi W, Canis K, Stevens J, et al. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J Virol 2008; 82:6200 - 8; http://dx.doi.org/10.1128/JVI.00187-08; PMID: 18417563
- Vanlandschoot P, Beirnaert E, Neirynck S, Saelens X, Jou WM, Fiers W. Molecular and immunological characterization of soluble aggregated A/Victoria/3/75 (H3N2) influenza haemagglutinin expressed in insect cells. Arch Virol 1996; 141:1715 - 26; http://dx.doi.org/10.1007/BF01718294; PMID: 8893793
- D’Aoust MA, Lavoie PO, Couture MM, Trépanier S, Guay JM, Dargis M, et al. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol J 2008; 6:930 - 40; http://dx.doi.org/10.1111/j.1467-7652.2008.00384.x; PMID: 19076615
- D’Aoust MA, Couture MM, Charland N, Trépanier S, Landry N, Ors F, et al. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J 2010; 8:607 - 19; http://dx.doi.org/10.1111/j.1467-7652.2009.00496.x; PMID: 20199612
- Khurana S, Wu J, Verma N, Verma S, Raghunandan R, Manischewitz J, et al. H5N1 virus-like particle vaccine elicits cross-reactive neutralizing antibodies that preferentially bind to the oligomeric form of influenza virus hemagglutinin in humans. J Virol 2011; 85:10945 - 54; http://dx.doi.org/10.1128/JVI.05406-11; PMID: 21865396
- Cornelissen LA, de Vries RP, de Boer-Luijtze EA, Rigter A, Rottier PJ, de Haan CA. A single immunization with soluble recombinant trimeric hemagglutinin protects chickens against highly pathogenic avian influenza virus H5N1. PLoS One 2010; 5:e10645; http://dx.doi.org/10.1371/journal.pone.0010645; PMID: 20498717
- Lin SC, Huang MH, Tsou PC, Huang LM, Chong P, Wu SC. Recombinant trimeric HA protein immunogenicity of H5N1 avian influenza viruses and their combined use with inactivated or adenovirus vaccines. PLoS One 2011; 6:e20052; http://dx.doi.org/10.1371/journal.pone.0020052; PMID: 21655326
- Weldon WC, Wang BZ, Martin MP, Koutsonanos DG, Skountzou I, Compans RW. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS One 2010; 5:pii:e12466; http://dx.doi.org/10.1371/journal.pone.0012466; PMID: 20824188
- Kammerer RA, Kostrewa D, Progias P, Honnappa S, Avila D, Lustig A, et al. A conserved trimerization motif controls the topology of short coiled coils. Proc Natl Acad Sci U S A 2005; 102:13891 - 6; http://dx.doi.org/10.1073/pnas.0502390102; PMID: 16172398
- Tao Y, Strelkov SV, Mesyanzhinov VV, Rossmann MG. Structure of bacteriophage T4 fibritin: a segmented coiled coil and the role of the C-terminal domain. Structure 1997; 5:789 - 98; http://dx.doi.org/10.1016/S0969-2126(97)00233-5; PMID: 9261070
- Yang X, Lee J, Mahony EM, Kwong PD, Wyatt R, Sodroski J. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J Virol 2002; 76:4634 - 42; http://dx.doi.org/10.1128/JVI.76.9.4634-4642.2002; PMID: 11932429
- Xu R, McBride R, Nycholat CM, Paulson JC, Wilson IA. Structural characterization of the hemagglutinin receptor specificity from the 2009 H1N1 influenza pandemic. J Virol 2012; 86:982 - 90; http://dx.doi.org/10.1128/JVI.06322-11; PMID: 22072785
- Kapust RB, Tözsér J, Copeland TD, Waugh DS. The P1′ specificity of tobacco etch virus protease. Biochem Biophys Res Commun 2002; 294:949 - 55; http://dx.doi.org/10.1016/S0006-291X(02)00574-0; PMID: 12074568
- Porath J. Immobilized metal ion affinity chromatography. Protein Expr Purif 1992; 3:263 - 81; http://dx.doi.org/10.1016/1046-5928(92)90001-D; PMID: 1422221
- Herman EM, Tague BW, Hoffman LM, Kjemtrupp SE, Chrispeels MJ. Retention of phytohemagglutinin with carbohyterminal tetrapeptide KDEL in the nuclear envelope and the endoplasmic reticulum. Planta 1990; 182:302 - 12; http://dx.doi.org/10.1007/BF00197126
- Musiychuk K, Stephenson N, Bi H, Farrance CE, Orozovic G, Brodelius M, et al. A launch vector for the production of vaccine antigens in plants. Influenza Other Respi Viruses 2007; 1:19 - 25; http://dx.doi.org/10.1111/j.1750-2659.2006.00005.x; PMID: 19453476
- Green BJ, Fujiki M, Mett V, Kaczmarczyk J, Shamloul M, Musiychuk K, et al. Transient protein expression in three Pisum sativum (green pea) varieties. Biotechnol J 2009; 4:230 - 7; http://dx.doi.org/10.1002/biot.200800256; PMID: 19156736
- Ilyushina NA, Khalenkov AM, Seiler JP, Forrest HL, Bovin NV, Marjuki H, et al. Adaptation of pandemic H1N1 influenza viruses in mice. J Virol 2010; 84:8607 - 16; http://dx.doi.org/10.1128/JVI.00159-10; PMID: 20592084
- World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance. 2002.
- Noah DL, Hill H, Hines D, White EL, Wolff MC. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol 2009; 16:558 - 66; http://dx.doi.org/10.1128/CVI.00368-08; PMID: 19225073