Abstract
A serial cross-sectional study of nasopharyngeal carriage among adults aged 60 y or over was conducted in winter-spring 2012 with the aim to describe circulating Streptococcus pneumoniae in an area, Liguria Administrative Region, where the vaccine was implemented for a decade and coverage in pediatric age group reached a value close to 100% for more than 5 y, determining a picture of very high vaccine immunological pressure. The serotype-specific carriage picture in adults was compared with that observed in children by means of a cross-sectional study performed one year before using the same sampling and laboratory methods.
Cluster sampling enrolled 283 adults, representative of the open population. Detection of multi-serotype carriage was performed using, real-time PCR and primer specific PCRs.
Carriage prevalence of participants with at least one positive sample adjusted for age, i.e., period prevalence, was 18.7%, considering the Ligurian population as standard population, showing that the pneumococcal carriage in the elderly is not a rare event as emerged in other surveys. The long-term use of PCV7 has resulted in strong decrease of vaccine types carriage among adults and children. A multivariate analysis showed that age class and contact with children attending day care covariates were strongly associated with Streptococcus pneumoniae carriage.
A strong link between the picture observed in < 5-y-old children and ≥ 60-y-old adults emerged: a strong correlation of specific-serotype prevalence between adults and children and risk factor analysis supported the role played by inter-age-group transmission.
Introduction
Streptococcus pneumoniae is a major cause of respiratory and invasive disease (IPD) worldwide, particularly in children < 5 y of age and in the elderly. Nasopharyngeal colonization by Streptococcus pneumoniae is the key event leading to cross-transmission on one hand and infection and disease on the other.Citation1,Citation2 The addition of the 7-valent pneumococcal conjugate vaccine (Prevenar, Pfizer) to the routine childhood vaccination schedule in the United States, Canada and some European Countries,Citation3 has led to a dramatic decline in vaccine serotype IPD in children, but also to an increase of non-vaccine serotype IPD incidence.Citation4-Citation6 Higher valent vaccines, the 10-valent (Synflorix, GSK) and 13-valent pneumococcal (Prevenar 13, Pfizer) vaccines, were approved between 2008 and 2010 in several Countries.
The decrease in rates of vaccine-serotype IPD among vaccinated children has been accompanied by a decrease in the rates of vaccine-serotype IPD among adults in some Countries, by reducing vaccine-serotype colonization in both age group.Citation7-Citation9
Pneumococcal carriage has proven to be a useful tool for monitoring how vaccination affects circulating pneumococcal serotypes in pediatric age group, but the epidemiological picture of serotypes carried in adults has been poorly investigated.
The growing immunological pressure due to the increasing and more-and-more lasting vaccine coverage in children and its effects in adults, the recent approval of 13-valent conjugate vaccine for prevention of IPD in adults aged 50 y or over, the availability of 23-valent polysaccharide vaccine make the definition of pneumococcal carriage in adults of great interest to anticipate and evaluate the effect of preventive strategies.
A serial cross-sectional study of nasopharyngeal carriage among adults aged 60 y or over was conducted in winter-spring 2012 with the aim to describe circulating Streptococcus pneumoniae in an area, Liguria Administrative Region, where the vaccine was implemented for a decade and coverage in pediatric age group reached a value close to 100% for more than 5 y, determining a picture of very high vaccine immunological pressure.Citation10
In a previous study, a representative sample of pre-school children showed that, 97.8% of the children were compliant with the schedule and almost all (98.6%) received at least 2 doses pneumococcal vaccine since the 12th month of life.Citation11 The cross-sectional study was specifically addressed (1) to better characterize the indirect effect of very high pediatric pneumococcal vaccine coverage on the rate of carriage and the prevalence of serotypes carried in the naso-pharynx of adults and (2) to evaluate demographic, behavioral and risk factors for carriage in this specific population in the post-vaccination era. The serotype-specific carriage picture in adults was compared with that observed in children by means a cross-sectional study performed one year before using the same sampling and laboratory methods.Citation11
Results
During the period of recruitment between February 16th and March 1st, 2012, a total of 283 adults aged 60 y or over were enrolled. One participant in excess with respect to the protocol was recruited by 3 GPs and the 3 adults were included in the study population. Two hundred 48 (87.6%), 238 (84.1%) and 221 (78.1%) participants returned on March, April and May, respectively. Two, 3 and 4 samples were collected from 22 (7.8%), 47 (16.6%) and 197 (69.6%) individuals, respectively. Seventeen (6%) adults did not return after the first visit. The median of interval between visits was 29 d (interquartile range 28–36 d). The demographic and behavioral characteristics and risk factors of participants were reported in .
Table 1. Characteristics of the study population
Carriage prevalence of participants with at least one positive sample adjusted for age, i.e., period prevalence, was 18.7% (95% C.I. 13.2–24.4%), considering the Ligurian population as standard population. It was significantly higher in 70–79 y age-group showing a proportion of positive individuals more than twice than that observed in older adults ().
The point prevalence of carriage adjusted for age was stable between the first and the fourth time point: it was 9.5% (95% C.I. 5.3–13.7%), 10.7% (95% C.I. 6–15.4%), 7.6% (95% C.I. 3.5–11.7%) and 10.9% (95% C.I. 5.9–15.9%), in February, March, April and May, respectively. Eighty-two per cent of carriers were colonized by more than one serotype.
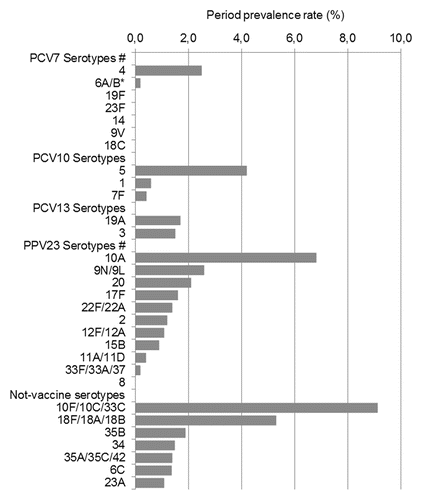
The proportion of adults colonized by different serotypes, after adjustment for age, is illustrated in . The age-adjusted coverage offered by PCV7, PCV10, PCV13 and PPV23 was 2.7% (95% C.I. 0.4–5%), 7.4% (95% C.I. 3.7–11.2%), 9.1% (95% C.I. 5–13.2%) and 15% (95% C.I. 10–20%), respectively. Among PCV7 serotypes, serotype 4 was carried by 2.5% (age-weighted carriage prevalence, 95% C.I. 0.3–4.7%) of the population and accounted for 89% of all PCV7 isolates. Among serotypes included in recently available conjugate vaccines, serotypes 5, present in PCV10 and PCV13 vaccine composition, showed the highest prevalence, being carried by 4.2% (95% C.I. 1.3–7.1%) of the population, while among PPV23 serotypes, 10A (age-adjusted carriage prevalence 6.8%, 95% C.I. 3.2–10.4%) and 9N/9L (2.6%, 95% C.I. 0.3–4.9%) were the most prevalent (). Among serotypes not included in vaccine composition, serotypes 10F (age-adjusted carriage prevalence 9.1%, 95% C.I. 5–13.2%) and 18F/18A/18B (5.3%, 95% C.I. 2.1–8.5%) showed the highest prevalence. Serotype10F primers also detected the rare serotypes 10C and 33C.
Figure 1. Proportion of children colonized with different serotypes, after adjustment for age. *The serotype 6B primer set used in the study and recommended by CDC was also cross-reactive with the common serotype 6A cps operon sequence. #PCV7 and PPV23 do not include serotype 6A
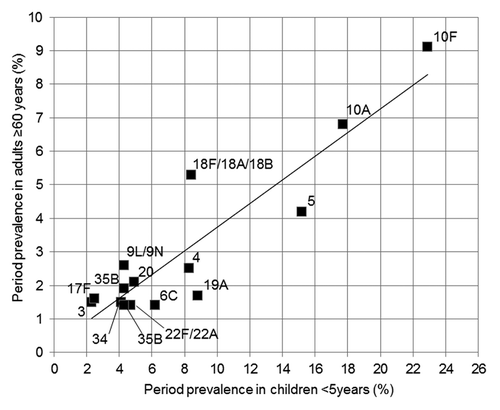
In is shown the bivariate fit of prevalence of different serotypes observed in adults aged 60 y or over by that observed in children younger than 5 y emerged in previous study performed in the 2010 autumn in the same area and the same sampling and laboratory methods. In the Figure are reported data regarding 15 serotypes showing prevalence in adults ≥ 1.2%. Strong monotonic correlation exists between the two variables (Spearman’s rho = 0.66, p-value = 0.008) and a very strong linear dependence between two variables was shown by Pearson’s product-moment coefficient (r = 0.912, p-value < 0.001).
Figure 2. Bivariate fit of prevalence of the 15 most prevalent serotypes observed in adults aged 60 y or over by that observed in children younger than 5 y, which emerged in previous study performed in the autumn of 2010.
A univariate analysis showed that the proportion of adults with at least one positive sample for S. pneumoniae was significantly higher in individuals who had contacts with children (p-value = 0.003) or who had contact with children attending day care (p-value = 0.01) (). Carriage rate was also significantly different in the 3 age groups (p-value = 0.042): in particular, adults aged 70–79 y showed significantly higher period prevalence respect with older individuals (≥ 80 y)(p-value = 0.012). No other variables were significant in univariate analysis ().
The percentage of adults who had contact with children attending day care was stable in the 3 age-groups ranging between 19.7 and 25.6%; carriage rate was similar in individuals with and without contact with children attending day care in 60–69 y age-group, but it was the double in the “contacts” aged 70–79 (42.2% vs 19.8%) and ≥ 80 y (20% vs 8.2%).
A multivariate analysis showed that age class and contact with children attending day care covariates were strongly associated with S. pneumoniae carriage and were included in the final model. In particular, contact with children attending day care doubled the carriage prevalence (prevalence ratio 2.16, 95% C.I. 1.23–3.63, p-value = 0.008), and belong to 70–79 y age group increased the carriage prevalence by 2.5 fold (prevalence ratio 2.5, 95% C.I. 1.17–5.85, p-value = 0.017) respect with ≥ 80 y subjects. On contrary, prevalence ratio between 60–69 y and ≥ 80 (reference) years age group was very close to 1 (prevalence ratio 1.11, 95% C.I. 0.33–3.08, p-value = 0.849).
Discussion
Although the picture of pneumococcal carriage in adults has been less studied than that in children, some surveys estimated the prevalence of carriage in adults both in epidemiological picture characterized by high compliance for conjugate vaccine in children and before PCV7 implementation. Carriage prevalence in adults widely ranged in the different surveys, according several factors such as age distribution, behavioral issues, composition of household, contact with children, antibiotic use and vaccine coverage in the first years of life. Before PCV7 introduction, the proportion of study adult participants colonized by S. pneumoniae in Western Countries, such as, USA, UK and Finland ranged between 1.5 and 13%, reaching 26% and 30% in some developing areas such as Nigeria and Thailand.Citation2,Citation12-Citation17 The impact of pneumococcal vaccine use in the first years of life on carriage in adults was different, determining different epidemiological pictures: the prevalence was > 30% in Australian aboriginal population aged 35–44, 45–54 and ≥ 55 y and it was < 1% in ≥ 60 y subjects living in Sydney, one year after PCV7 introduction;Citation18,Citation19 it was about 20% in age-matched parents (mean age 35 y),Citation20 and in 35–44 and ≥ 45 y adults in the Netherland and Alaska,Citation2 respectively, after PCV7 vaccination had been conducted for 3 y. After more a decade of pneumococcal conjugate vaccine implementation, it ranged between 8.9 and 13.8% in native American adults aged 17–39, 40–64 and ≥ 65 y.Citation21 The majority of the above mentioned surveys were not specifically addressed to evaluate carriage in the elderly, the age class presenting the highest morbidity and mortality for streptococcus diseases, and data were usually aggregated not allowing to evaluate carriage in the different elderly age groups. Their adult study population often included parents of new born, infants and young children introducing relevant bias in terms of age distribution and exposition to pediatric reservoir. Furthermore, the study population aged > 60 or > 65 y was very limited with exception of a couple of ad hoc studies,Citation12,Citation18 reducing the power of estimates in the elderly.
In this study we characterized the carriage picture in individuals aged 60 y or over in an Italian Administrative Region where vaccine was introduced in the pediatric immunization program and reached very high vaccine coverage for 10 and 5 y, respectively. The study was designed to have population as representative as possible of the elderly population in our area and no potential bias was knowingly introduced. Furthermore, the use of improved and more sensitive techniques for detection of multiserotype carriage, i.e., broth enrichment and real-time PCR and sequential PCRs for detection and typing, respectively, as recently introduced by CDC, allows to better understand the replacement phenomena compared with the single-colony methods routinely applied also in recent surveys.
Period prevalence we estimated in > 60 y adults (18.7%) was higher respect with those observed in adults > 40 and > 65 y in the USA (9.6% and 13%, respectively), in condition of very high vaccine immunological pressure close to that experienced in Liguria, although the carriage in < 5 y children was slightly higher in American Community (55.4%) than in Liguria (50.1%) and all American adults lived with children. The prevalence was also higher to that registered in Australian and Finish elderly adults (< 1 and 1.5%, respectively) one year after and before PCV7 introduction. The higher prevalence we observe could be due to the study design, i.e., serial prevalence vs point prevalence, as well as the high sensitivity of pneumococcus detection methods used. Lack of data on picture before PCV7 implementation do not allow to bring to light changes in prevalence in Ligurian children and adults, but only to photograph the prevalence after PCV7 implementation.
The risk factor analysis supports the assumption that the exposure to colonized children are associated with an increasing risk of carriage in adults. In particular, multivariate analysis showed that contact with children attending day care doubled the carriage prevalence in adults and day care attendance was shown in a previous study to increase the carriage prevalence by 51% in Ligurian children.Citation22 The role played by pediatric reservoir and the possible exchange between grandparents and grandchildren emerged by relationship between prevalence of different serotypes in adults aged 60 y or over and that observed in children younger than 5 y. Strong correlation exists between the serotype-specific prevalence in the two age groups ().
The long-term use of PCV7 has resulted in strong decrease near elimination of vaccine types carriage among adults: carriage prevalence of adults colonized by at least one serotypes covered by PCV7 was less than 3% and almost due to serotype 4. The almost complete replacement and the relevant circulation of serotype 4 that was carried by 8.2% of children was highlighted in the pediatric carriage study.Citation11
But the relevant circulation of serotype 4 results in different effects in children and adults: data from IPD and non-IPD surveillance following PCV7 introduction showed that in adults, 12.1% and 18.2% out of IPD and non-IPD were due to serotype 4. No cases of serotype 4 IPD and non-IPD were detected by surveillance system among children highlighting the different pattern in children and adults and high vaccine effectiveness in preventing both IPD and non-IPD due to this PCV7 serotype.
The proportions of adults carriers of any PCV7, PCV10 and PCV13 serotypes reflects the vaccine serotype prevalence observed among children: the total and vaccine-serotype carriage prevalence were about one third of that registered in pediatric age group.
The serotypes picture among Ligurian adults was different than that observed in other Countries after PCV7 implementation: the most prevalent serotypes, i.e., 10F, 10A, 5, 18A/18F/18C and 4, were poorly represented among serotypes carried among adults in the USA, Netherland and in an Australian Aboriginal population.Citation2,Citation20,Citation21
These differences are probably attributable to the demographic, social and behavioral differences and different selective pressure induced by antibiotics and vaccines.
There are several limitation of this study. Risk factor data could be subject to reporting or memory bias, particularly for smoking or antibiotics use. A total of 990 nasopharyngeal swabs were collected, representing 88% of all samples foreseen by the protocol, considering 100% compliance.
Regarding the comparison between the serotype picture in the two age groups, samples from children and ≥ 60 y adults were collected at a distance of one year, but previous studies showed that carriage changes are usually progressive and slow.
Moreover, in the light of recent evidences about the possible presence of non-pneumococcal homologs as confounding of PCR based determination of pneumococcal serotype from NP specimens, serotyping results should be carefully evaluated. Further insight that goes beyond the scope of this study will be necessary.Citation23
In conclusion, the present population-based serial cross-sectional study showed that, in a Western Country with very high vaccine immunological pressure in pediatric age group, the pneumococcal carriage in the elderly is not a rare event as emerged in other surveys, as about one fifth of adults carried Streptococcus pneumoniae during winter or spring. A cross-sectional study performed one year before using the same sampling and laboratory methods in pediatric age group allowed to highlight a strong link between the picture observed in < 5-y-old children and ≥ 60-y-old adults: a strong correlation of specific-serotype prevalence between adults and children and risk factor analysis supported the role played by inter-age-group transmission. The long-term use of PCV7 has resulted in strong decrease of vaccine types carriage among adults and children and as PCV13 was introduced in routine childhood vaccination schedule and proposed for > 50 y adults, we expect to observe a continued decrease in carriage of 1, 3, 5, 19A serotypes characterized by high invasive potential. Serotypes not included in PCV13 composition and circulating both in children and adults, i.e., 10A, 10F, 18F, 9L/9N, presenting a low or intermediate invasive ability, will be of ongoing importance in Liguria population. These results highlight the need for continued intensive surveillance of carried Streptococcus pneumoniae as well as strains causing invasive and non-invasive disease in all age groups.
Materials and Methods
Study design, data source and variables
The study is population-based serial cross-sectional analysis of nasopharyngeal pneumococcal carriage among adults aged 60 y or over.
Surveillance sampling was performed in clusters: each cluster was made up of subjects monitored by a General Practitioner (GP). Fourteen GPs in the Genoa metropolitan area were randomly selected, each of which sampled 20 adults. Adults were consecutively sampled by GPs during routine visit examinations due to signs and symptoms not potentially related to S. pneumoniae disease on February 2012. Participants were invited to return at 1-mo interval on March, April and May 2012.
Exclusion criteria included participation in clinical trials or other epidemiological studies and the unwillingness to present themselves for examination every month. At the enrolment visit, physician (1) checked the inclusion and exclusion criteria; (2) administered a standardized questionnaire to collect demographic data, contact with < 5 y children and with children attending day care, co-morbidities, respiratory tract infections in the last month, antimicrobial and steroid use in the previous 3 mo, admission to hospital in the last year and data on possible behavior risk factors for colonization with Streptococcus pneumoniae and vaccination history; and (3) collected the biological specimen for detection and typing of Streptococcus pneumoniae. At the following visits, the inclusion and exclusion criteria, the demographic and risk factors data were re-checked and the nasopharyngeal swabs collected. Questionnaires were signed by the participants and were anonymous but labeled to match the respective samples per adults. The research protocol was approved by the Ethics Committee of I.R.C.C.S. “San Martino-IST,” Genoa.
Participants and study size
The study protocol was expected to enroll 280 adults ≥ 60 y of age. The period of recruitment was limited to between February 16th and March 1st, 2012 and the expected interval between visits ranged between 15 and 45 d. Survey sample size was estimated based on the results for expected carriage rate of 1%, considering the design effect of the study due to cluster sampling.Citation12,Citation18 The estimates of design effect for sample size calculation were 1.5 for cluster sampling. Overall sample size necessary to achieve a precision for carriage prevalence, the main outcome measure of the study, of ± 1.5% for the 95% confidence interval were calculated as 254 adults; the final sample size chosen was 280 participants. The software used for sample size calculation was Open Epi, Version 2.0, freely available on http://www.openepi.com/OE2.3/Menu/OpenEpiMenu.htm.
Nasopharyngeal swabs and laboratory assays
Carriage samples were collected by trained physicians by using calcium alginate swab specimens (Fisherbrand, catalog number 14–959–78; Fisher Scientific, Pittsburg, PA) and transported in 1.0 ml skim milk-tryptone-glucose-glycerol (STGG) medium. These nasopharyngeal swabs in STGG medium were preserved on wet ice and frozen at -70°C within 4 h. Before freezing them at -70°C, the NP-STGG specimens were vortexed for 10 to 20 sec to disperse the organisms from the swab. The detection of specific sequences of S. pneumoniae in biological specimens was performed using Real Time PCR preceded by broth enrichment for enhanced pneumococcal growth, as recommended by CDC.Citation13,Citation14
DNA was extracted from Supplemented Todd-Hewitt Broth (STHB) culture by QIAamp DNA Minikit, (Qiagen); the extracted genetic material was used in lytA gene-specific realtime PCR for S. pneumoniae detection and in sequential multiplex PCR assay for serotype deduction.Citation13 Positive samples were defined as those with cycle threshold (Ct) values lower than 30. A human gene sequence for β-globin was detected by the test as a positive extraction control.
Molecular typing was performed on lytA positive samples, using a sequential algorithm with 8 PCR multiplexes, each comprising an amplification reaction with 4 pairs of type-specific primers, modified for the detection and typing of the bacteria in the colonized subject. The algorithm enables 40 of the most common types to be identified. Another pair of primers specific for the CPS operon, a highly-conserved region in all pneumococcal types, could confirm the presence of specific sequences of S. pneumoniae.Citation13,Citation14
Statistical analysis
Adjustmentments for age was used to estimate prevalence rates and was calculated with the Ligurian model population of 2009. Confidence intervals for prevalence rates were calculated considering the design of the study, assuming a design effect for confidence interval calculation of 1.5 for cluster sampling. Design effect for sampling was calculated assuming the interclass correlation equal to 0.025 and the number of elements in the cluster equal to 20. Differences in observed prevalence rates were statistically tested by using a 2-sided χ2 test. Binary regression model was used to calculate prevalence ratios and their corresponding 95% confidence intervals by using generalized linear model with log link. JMP software was used for the analysis.
Relationship between period prevalence of different serotypes observed in adults aged 60 y or over and that observed in children younger than 5 y emerged in the present and in a previous study performed in the 2010 autumn in the same area and using the same sampling and laboratory methods, respectively, was explored.Citation11 For the correlation analysis were considered only serotypes showing prevalence in adults ≥ 1.2%. The non-parametric measure of dependence between the two variables was expressed as Spearman’s rank correlation coefficient or Spearman’s rho. The linear dependence between period prevalence of different serotypes in the two age-groups was assessed by Pearson’s product-moment coefficient. R and Rho values ranging between 0.8 and 1 and 0.6 to 0.8 were considered to define a very strong and strong correlation between variables, respectively.
| Abbreviations: | ||
| CDC | = | Centers for disease control and prevention |
| CPS | = | capsular polysaccharide synthesis locus |
| Ct | = | cycle threshold |
| GP | = | general practitioner |
| IPD | = | invasive pneumococcal disease |
| NP | = | nasopharyngeal |
| PCV7 | = | 7-valent pneumococcal conjugate vaccine |
| PCV10 | = | 10-valent pneumococcal conjugate vaccine |
| PCV13 | = | 13-valent pneumococcal conjugate vaccine |
| PPV23 | = | pneumococcal polysaccharide vaccine |
| STGG | = | skim milk-tryptone-glucose-glycerol medium |
| STHB | = | supplemented Todd-Hewitt broth |
Acknowledgments
Authors would like to thank Pfizer for Financial Support.
Disclosure of Potential Conflicts of Interest
F.A., G.I. and P.D. have previously participated at speaker’s bureaus and advisory board meetings sponsored by GSK, Novartis, Pfizer and Sanofi Pasteur and have received research funding as principal investigators or co-investigators from Crucell Berna, Novartis, GSK, Pfizer and Sanofi Pasteur. D.dF., P.C., A.C., E.R., R.I., M.M., G.B. and A.O. have no conflict of interest. No other relationships/conditions/circumstances that present a potential conflict of interest exist.
References
- Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 2004; 4:144 - 54; http://dx.doi.org/10.1016/S1473-3099(04)00938-7; PMID: 14998500
- Hammitt LL, Bruden DL, Butler JC, Baggett HC, Hurlburt DA, Reasonover A, et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis 2006; 193:1487 - 94; http://dx.doi.org/10.1086/503805; PMID: 16652275
- De Carvalho Gomes H, Muscat M, Monnet DL, Giesecke J, Lopalco PL. Use of seven-valent pneumococcal conjugate vaccine (PCV7) in Europe, 2001-2007. Euro Surveill 2009; 14:19159; PMID: 19341605
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al, Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737 - 46; http://dx.doi.org/10.1056/NEJMoa022823; PMID: 12724479
- Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis 2007; 196:1346 - 54; http://dx.doi.org/10.1086/521626; PMID: 17922399
- Rose M, Zielen S. Impact of infant immunization programs with pneumococcal conjugate vaccine in Europe. Expert Rev Vaccines 2009; 8:1351 - 64; http://dx.doi.org/10.1586/erv.09.78; PMID: 19803758
- Rodenburg GD, de Greeff SC, Jansen AG, de Melker HE, Schouls LM, Hak E, et al. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis 2010; 16:816 - 23; http://dx.doi.org/10.3201/eid1605.091223; PMID: 20409372
- Black S, Shinefield H, Baxter R, Austrian R, Bracken L, Hansen J, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J 2004; 23:485 - 9; http://dx.doi.org/10.1097/01.inf.0000129685.04847.94; PMID: 15194827
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al, Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737 - 46; http://dx.doi.org/10.1056/NEJMoa022823; PMID: 12724479
- Durando P, Crovari P, Ansaldi F, Sticchi L, Sticchi C, Turello V, et al, Collaborative Group for Pneumococcal Vaccination in Liguria. Universal childhood immunisation against Streptococcus pneumoniae: the five-year experience of Liguria Region, Italy. Vaccine 2009; 27:3459 - 62; http://dx.doi.org/10.1016/j.vaccine.2009.01.052; PMID: 19200823
- Ansaldi F, de Florentiis D, Canepa P, Zancolli M, Martini M, Orsi A, et al. Carriage of Streptoccoccus pneumoniae 7 years after implementation of vaccination program in a population with very high and long-lasting coverage, Italy. Vaccine 2012; 30:2288 - 94; http://dx.doi.org/10.1016/j.vaccine.2012.01.067; PMID: 22306795
- Palmu AA, Kaijalainen T, Saukkoriipi A, Leinonen M, Kilpi TM. Nasopharyngeal carriage of Streptococcus pneumoniae and pneumococcal urine antigen test in healthy elderly subjects. Scand J Infect Dis 2012; 44:433 - 8; http://dx.doi.org/10.3109/00365548.2011.652162; PMID: 22263905
- da Gloria Carvalho M, Pimenta FC, Jackson D, Roundtree A, Ahmad Y, Millar EV, et al. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J Clin Microbiol 2010; 48:1611 - 8; http://dx.doi.org/10.1128/JCM.02243-09; PMID: 20220175
- Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol 2006; 44:124 - 31; http://dx.doi.org/10.1128/JCM.44.1.124-131.2006; PMID: 16390959
- Adetifa IM, Antonio M, Okoromah CA, Ebruke C, Inem V, Nsekpong D, et al. Pre-vaccination nasopharyngeal pneumococcal carriage in a Nigerian population: epidemiology and population biology. PLoS One 2012; 7:e30548; http://dx.doi.org/10.1371/journal.pone.0030548; PMID: 22291984
- Turner P, Turner C, Jankhot A, Helen N, Lee SJ, Day NP, et al. A longitudinal study of Streptococcus pneumoniae carriage in a cohort of infants and their mothers on the Thailand-Myanmar border. PLoS One 2012; 7:e38271; http://dx.doi.org/10.1371/journal.pone.0038271; PMID: 22693610
- Flasche S, Van Hoek AJ, Sheasby E, Waight P, Andrews N, Sheppard C, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med 2011; 8:e1001017; http://dx.doi.org/10.1371/journal.pmed.1001017; PMID: 21483718
- Ridda I, Macintyre CR, Lindley R, McIntyre PB, Brown M, Oftadeh S, et al. Lack of pneumococcal carriage in the hospitalised elderly. Vaccine 2010; 28:3902 - 4; http://dx.doi.org/10.1016/j.vaccine.2010.03.073; PMID: 20398618
- Mackenzie GA, Leach AJ, Carapetis JR, Fisher J, Morris PS. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis 2010; 10:304; http://dx.doi.org/10.1186/1471-2334-10-304; PMID: 20969800
- Spijkerman J, van Gils EJ, Veenhoven RH, Hak E, Yzerman EP, van der Ende A, et al. Carriage of Streptococcus pneumoniae 3 years after start of vaccination program, the Netherlands. Emerg Infect Dis 2011; 17:584 - 91; PMID: 21470445
- Scott JR, Millar EV, Lipsitch M, Moulton LH, Weatherholtz R, Perilla MJ, et al. Impact of more than a decade of pneumococcal conjugate vaccine use on carriage and invasive potential in Native American communities. J Infect Dis 2012; 205:280 - 8; http://dx.doi.org/10.1093/infdis/jir730; PMID: 22128315
- Ansaldi F, de Florentis D, Canepa P, Bandettini R, Diana MC, Martini M, et al. Epidemiological changes after PCV7 implementation in Italy: perspective for new vaccines. Hum Vaccin 2011; 7:Suppl 211 - 6; http://dx.doi.org/10.4161/hv.7.0.14602; PMID: 21546795
- Carvalho MdaG, Bigogo GM, Junghae M, Pimenta FC, Moura I, Roundtree A, et al. Potential nonpneumococcal confounding of PCR-based determination of serotype in carriage. J Clin Microbiol 2012; 50:3146 - 7; http://dx.doi.org/10.1128/JCM.01505-12; PMID: 22760044