Abstract
Rubella is usually a mild disease with nonspecific symptoms, but can cause congenital rubella syndrome (CRS) when infection occurs during pregnancy. The objective of this study was to evaluate the sensitivity and positive predictive value of different data sources used for surveillance purposes in the Rubella Elimination Program of Catalonia between 2002 and 2011. The Urgent Notification to the Statutory Disease Reporting System, the Individualized Disease Reporting System, screening for other viruses included in the Measles Elimination Program, the Microbiological Reporting System and the Minimum Hospital Discharge Data were evaluated. 100 suspected cases of postnatal rubella and 6 suspected cases of CRS were detected. For postnatal rubella, Urgent Notification had the highest sensitivity (32.5%; 95%CI 18.6–49.1), followed by the Virus screening in Measles Elimination Program (25%; 95%CI 12.7-41.2). Virus screening in the Measles Elimination Program had the highest PPV (76.9%; 95%CI 46.1–94.9), followed by the Individualized Disease Reporting System (57.1%; 95%CI 28.9–82.3). For CRS cases, the Individualized Disease Reporting System had the highest sensitivity (100%, 95%CI 29.2–100) and the highest PPV (60%; 95%CI 14.7–100). Most confirmed postnatal cases (25 cases, 48.1%) were in the 25–44 y age group followed by the 15–24 y age group (11 cases, 21.2%). The highest values of sensitivity and PPV for the detection of confirmed cases corresponded to activities that were specifically introduced in the measles and rubella elimination programs.
Introduction
The Rubella virus is an enveloped RNA virus that belongs to the genera Rubivirus in the family Togaviridae. Postnatal rubella is usually a mild disease with nonspecific symptoms and is often difficult to diagnose clinically as it may be confused with other infections.Citation1,Citation2 Although many infections are subclinical, rubella virus infection is an important public health problem due to the teratogenic effects that may result from congenital infection, particularly when the infection occurs during the first trimester of pregnancy. Infection during the first month of pregnancy may cause miscarriage, fetal death, premature delivery and congenital rubella syndrome (CRS), which occurs in up to 85% of infants born to women infected during the first two months of pregnancy.Citation3,Citation4 About 112,000 cases of CRS occur each year worldwide.Citation5 The prognosis of affected children varies and, therefore, the community and family consequences must be taken into account, because children may require significant special education, both for single and multiple handicaps.Citation2 Birth defects characteristics of CRS can also occur for other reasons and correct classification of suspected CRS is based on laboratory results rather than the clinical presentation. The rubella virus is antigenically stable and, therefore, antigenic variation does not pose a risk in the use of the rubella vaccine for serological diagnoses.Citation6
Because rubella and measles are vaccine-preventable diseases with an exclusively human reservoir, the viruses cannot survive in the environment and there are specific and sensitive techniques to diagnose cases. In 1998, the WHO European Region approved the aims of eliminating indigenous measles and controlling congenital rubella.Citation7 In 2003, a plan focused on achieving these objectives by 2010 was approved. In 2005, most European countries had included the rubella vaccine in their immunization schedules and, because the prevention of rubella and measles depends on similar activities, a strategic plan for 2005–2010 was approved with the aims of eliminating endemic measles and endemic rubella and preventing CRS (< 1 case per 100,000 live births) by 2010. In July 2008 in Spain this strategic plan was approved as an extension to the measles elimination plan.Citation8 In September 2010, the aims of the WHO European Region were postponed to 2015.Citation9,Citation10 In Catalonia (Spain) rubella vaccination at age 11 began in 1978, with a policy of double vaccination with the measles, mumps and rubella vaccine (MMR) at 12 mo with the rubella vaccine at 11 y for girls added from 1980. MMR vaccination at 15 mo and 11 y was introduced in 1988. To reduce the number of cohorts vaccinated with a single dose, the second dose of MMR was advanced to 4 y in 1998.Citation11 Finally, in 2008, after the appearance of an outbreak of measles that involved 381 cases between August 2006 and June 2007, mainly in children aged < 15 mo,Citation12 the first dose of MMR was changed back to 12 mo, because protection of babies due to passive immunity is shorter lasting in vaccinated mothers not exposed to measles.Citation13 Therefore, people born after 1981 should have received two doses of MMR (one at 12 or 15 mo and another later in life), with an estimated coverage of 98% for the first doseCitation14 and nearly 90% for the second dose.Citation15 The Autonomous Government of Catalonia launched a program to eliminate indigenous measles in 1991 and, given the good results obtained, established a program for the elimination of rubella in 2002.Citation16 The objective of this study was to evaluate the sensitivity and positive predictive value of different data sources used for surveillance of postnatal rubella and CRS in the context of THE Rubella Elimination Program.
Results
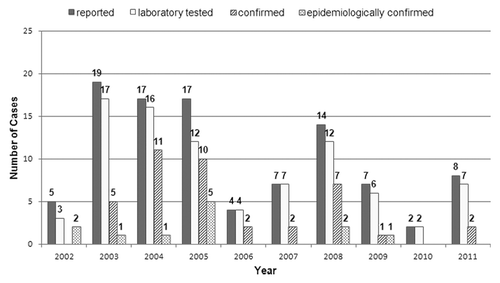
Between 2002 and 2012, 100 suspected cases of postnatal rubella and 6 suspected cases of CRS were detected (). For postnatal rubella, the source that detected the greatest number of suspected cases was the Urgent Notification to the Statutory Disease Reporting System (46 cases, 46%), followed by the Microbiological Reporting System (26 cases, 26%). The sensitivity for these two data sources to detect confirmed cases was 32.5% (18.6–49.1) and 22.5% (10.8–38.5), respectively (). Virus screening in the Measles Elimination Program had the highest PPV (76.9%; 95%CI 46.1–94.9), followed by the Individualized Disease Reporting System (57.1%; 95%CI 28.9–82.3).
Figure 1. Reported cases of postnatal rubella and congenital rubella syndrome. Catalonia, 2002–2011. *Diagnosis was Streptococcus agalactiae infection. **Diagnosis was cytomegalovirus infection. ***IgM and RT-PCR for rubella virus were negative; unknown etiology
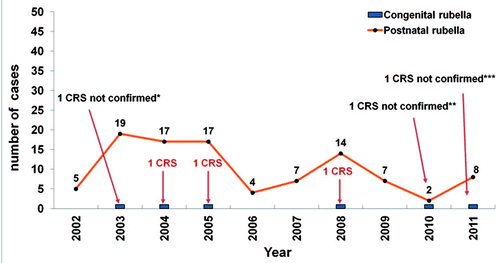
Table 1. Postnatal and congenital rubella cases reported by different sources
For CRS, the source which detected the greatest number of suspected cases was the Individualized Disease Reporting System (5 cases, 83%), with a sensitivity of 100% (95%CI 29.2–100). The Minimum Hospital Discharge Data detected one suspected case (17%), with a sensitivity of 0% (0–70.7). The highest PPV (60%; 95%CI 14.7–100) corresponded to the Statutory Disease Reporting System.
During the whole study period, 86% (86/100) of reported cases were laboratory tested, but after 2005 this proportion rose to 90.5% (38/42) ().
For all confirmed cases (laboratory confirmed cases and epidemiologically linked to a laboratory confirmed case), the greatest number of cases was in the 25–44 y age group (25 cases, 48.1%), followed by the 15–24 y age group (11 cases, 21.2%). One confirmed case was a pregnant women and 8 were related to contact with pregnant women ().
Table 2. Distribution of reported postnatal rubella cases by age groups
In 24 of the 52 postnatal confirmed rubella cases (46.2%), the source of infection was related to an imported origin, specifically Brazil (9 cases), Morocco (5 cases), Ecuador (3 cases), Portugal (2 cases) and Angola, Australia and Romania with 1 case each (). All these cases occurred in unvaccinated persons. In the other 28 confirmed cases, the origin could not be determined, but all were unrelated to any indigenous transmission chain; only 8 cases had received the vaccine (7 cases received one dose and 1 case received two doses).
Table 3. Distribution of confirmed cases of postnatal rubella and congenital rubella syndrome according to country of origin, age and immunization status
Of the confirmed cases of CRS, 2 were related to temporary stays in Morocco and Poland, respectively, during pregnancy, but it was not possible to determine the country of origin in the third; in all three cases, the mother was unvaccinated.
Of the 86 cases of postnatal rubella with laboratory study, in 40 cases (46.5%) the diagnosis was rubella infection. Other etiologies were parvovirus B19 (2 cases), herpesvirus 6 (1 case) and cytomegalovirus (1 case); in 42 cases, the etiology was unknown .
Discussion
The main results of this study show that the rate of laboratory investigation of suspected cases of postnatal rubella and CRS is high in Catalonia and that it is important to combine different sources of data for surveillance of these diseases in the era of elimination.
The proportion of reported cases that were laboratory tested was 86% in the whole period and > 90% during the last six years of the program. These rates are higher than the 80% fixed as an indicator of quality for rubella surveillance.Citation17
Although nearly 50% of suspected cases were detected by only one specific source (Urgent Notification to the Statutory Disease Reporting System) Rubella Elimination Program Urgent Notification) and 60% by both this source and the Individualized Disease Reporting System, these percentages must be considered clearly insufficient to achieve elimination of the disease, and shows that a comprehensive strategy is needed to achieve this goal, as no single data source is likely to be sufficient to convincingly document progress to rubella elimination.Citation18-Citation20
For postnatal rubella, the highest PPV values corresponded to virus screening in the Measles Elimination Program (77%), suggesting this system is very efficient, because the majority of cases detected were confirmed cases. However, if we had considered only this system, we would have missed 30 confirmed cases of postnatal rubella.
For CRS, the Individualized Statutory Disease Reporting System had a high PPV (60%), and therefore this source should be considered as very efficient in detecting true cases. In contrast, the Minimum Hospital Discharge Data had a PPV of 0%. Unlike postnatal rubella surveillance, which can easily be integrated with measles surveillance, CRS surveillance requires a specific system that can identify suspected cases in patients < 12 mo of age and therefore the Minimum Hospital Discharge Data must be included in the CRS control program.Citation5 Even though this source was reviewed twice yearly, no confirmed cases were detected. We searched only for the codes 056.0 and 771.0 of the 9th International Classification of Diseases (congenital rubella and rubella with neurological complications). It would probably be a good idea to review other codes, such 743.30 (congenital cataracts) and 389.1 (hearing impairment) to improve the utility of this data source.
Another important finding was that the 15–44 y age group had the highest frequency of confirmed cases (71.2%). There were more males than females in this age group and this may reflect the fact that the rubella vaccine was introduced earlier in females than in males in Catalonia,Citation21 although males infected by the rubella virus probably had contacts with females of a similar age and, therefore, a susceptible pregnant woman might be exposed to the virus. Thus, to reduce the number of susceptible subjects and the continuing circulation of the virus, it is important not only that any contact with the health system should be used to vaccinate females of childbearing age,Citation22 but also that adult males should receive the MMR vaccine when evidence of immunity against rubella is not provided.Citation23
The origin of imported cases was determined in only 47% of cases, a rate clearly lower than the target of 80% established as an indicator of quality for rubella surveillance activities in the context of an elimination program.Citation17 Efforts are needed to improve this rate, and sequencing of nucleic acid amplified directly from samples or from tissue culture might provide useful information on the origin of imported cases.Citation24-Citation26
Confirmed cases where the country of origin was known mainly came from Brazil and Morocco, in agreement with the results obtained in a previous seroepidemiological study in a representative sample of pregnant women in Catalonia which showed that, in immigrant women, the prevalence of women susceptible to rubella was higher than that of indigenous women and that the majority of immigrant women susceptible to rubella infection came from South America and Africa.Citation27 The results are also similar to those obtained by Vargas-Leguás et al. in a seroepidemiological study in patients attended in a Catalan hospital.Citation28 Most confirmed cases of postnatal rubella and all CRS cases occurred in unvaccinated people, as shown in other European studies.Citation20,Citation29-Citation31
Laboratory confirmation is a very important component of any elimination program.Citation32-Citation34 Each suspected vaccine failure requires laboratory confirmation to maintain reliable surveillance and control and establish the specific etiology of the disease. However, unfortunately, this is not always possible. Serological studies were not possible in 12 confirmed cases of postnatal rubella and epidemiological investigation of the cases demonstrated their link to laboratory- confirmed cases, and therefore, showed they were confirmed cases. Surveillance of each suspected case is essential to provide evidence that no endemic transmission of rubella virus has occurred.Citation25,Citation35
Because the clinical signs of rubella are highly non-specific, etiological agents others than rubella virus were detected in suspected cases, as reported by other authors.Citation36
The number of rubella cases (both postnatal and CRS) in Catalonia during the study period was low and limited to undervaccinated subjects. The fact that these outbreaks have not resulted in the spread of sustained transmission outside the unvaccinated groups suggests that immunization coverage in the general population has been sufficient to prevent spread.Citation37 However, to maintain adequate vaccine coverage and avoid pockets of susceptible individuals, health care providers and public health professionals should make efforts to ensure that all people without documented evidence of rubella immunization receive at least one dose (and preferably two doses separated by ≥ 28 d) of MMR vaccine.Citation16 Because pockets of endemic transmission pose risk beyond national borders,Citation37-Citation39 special efforts should be made in immigrants and travelers, as currently recommended in Catalonia and by Spanish experts.Citation16-Citation40
Although large measles outbreaks have occurred in Catalonia,Citation12,Citation41 no large rubella outbreaks have been detected and no endemic transmission has occurred. The fact that the herd immunity threshold needed to interrupt rubella transmission is 83–85%, compared with 92–94% for measles, may explain the different behavior observed for the two diseases.Citation42
In conclusion, this study shows that Urgent Notification to the Statutory Disease Reporting System introduced when the Rubella Elimination Program of Catalonia was launched, was the most important source of data in detecting true cases of postnatal rubella and that the highest PPV was obtained by the virus screening in the Measles Elimination Program. Other data sources that were not specifically included in the rubella or measles elimination programs had lower sensitivities and PPV. Because postnatal rubella is usually a mild disease with non-specific symptoms, specific surveillance activities must be performed to maintain adequate surveillance of the disease.
Material and Methods
Study design and subjects
The study period was January 2002 to December 2011. The study was performed in Catalonia, a region in the northeast of Spain with more than 7.5 million inhabitants, of whom around 15% are immigrants.
Case definitions
A suspected case of postnatal rubella was defined as acute onset of generalized maculopapular rash, temperature > 37°C, arthralgia, arthritis, lymphadenopathy or conjunctivitis. A confirmed case of postnatal rubella was defined as a suspected case with or without symptoms with laboratory evidence of rubella infection confirmed by one or more of the following tests: isolation of rubella virus, detection of rubella-virus specific nucleic acid by amplification techniques (RT-PCR), positive serologic test for rubella immunoglobulin M (IgM) antibody, or significant rise between acute-and convalescent-phase titers in serum rubella immunoglobulin G (IgG) antibody level by any standard serologic assay. Alternatively, a suspected case that was epidemiologically linked to a laboratory-confirmed case was also considered a confirmed case.
A suspected case of CRS was defined as an infant with one or more of the following clinical findings: cataracts, congenital glaucoma, congenital heart disease, hearing impairment, pigmentary retinopathy, purpura, hepatosplenomegaly, jaundice, microcephaly, developmental delay, meningoencephalitis or radiolucent bone disease. A confirmed case of CRS was defined as a suspected case with laboratory evidence of CRS infection demonstrated by one or more of the following tests: isolation of rubella virus; detection of rubella-virus specific nucleic acid by amplification techniques, detection of rubella-specific (IgM) antibody, or infant rubella antibody level that persisted at a higher level and for a longer period than expected from passive transfer of maternal antibody or recent immunization.
An imported case was defined as a case in which rubella resulted from exposure to rubella virus outside Catalonia during 14–23 d before rash onset, or rash began within 23 d of entering Catalonia, or was not related to the transmission chain of an indigenous case.
Laboratory analyses
Serological analyses were performed at the Microbiology Laboratory, Hospital Clinic of Barcelona and RT-PCR analysis in CRS suspected cases was performed at the National Center of Microbiology, Majadahonda (Madrid).
Data sources
The data sources used were: Urgent Notification to the Statutory Disease Reporting System, the Individualized Disease Reporting System, screening for other viruses included in the Measles Elimination Program, the Microbiological Reporting System and the Minimum Hospital Discharge Data. The Urgent Notification to the Statutory Disease Reporting System is a component of the Statutory Disease Reporting System that was introduced in the Rubella Elimination Program launched by the Autonomous Government of Catalonia: all suspected cases of postnatal rubella and CRS must be reported telephonically by physicians (whether public or private) within 24 h to the Epidemiological Surveillance Units of the Department of Health. The Individualized Disease Reporting System obliges all public and private physicians in Catalonia to send a report of all suspected cases of postnatal rubella and CRS (according to the standard case definition), including patient demographic and epidemiological data to the Department of Health. Virus screening in the Measles Elimination Program allows IgM specific measles antibody determination in any cases of rash with fever. Cases in which measles was ruled out were tested for rubella virus, herpesvirus 6, cytomegalovirus and parvovirus B19. The Microbiological Reporting System is a voluntary system participated in by 50 centers representing 84% of public hospital beds. Each week, participant laboratories report the number and characteristics of specific microbiological agents of special interest to public health services. The Minimum Hospital Discharge Data provides information on the discharge records for cases of rubella or rubella-related disease and CRS (International Classification of Diseases, 9th revision codes 056.0 [rubella with neurological complications], and 771.0 [congenital rubella]). This source was reviewed twice yearly.
Statistical analysis
The sensitivity of the different sources of data was calculated as the proportion of all laboratory confirmed cases detected by the system. The PPV was calculated as the proportion of suspected cases detected by the system that were laboratory confirmed cases . The 95% confidence intervals were calculated by the exact binomial method. The analysis was performed using the SPSS v18.0 statistical package for windows (SPSS; Chicago, USA) and the R v2.14.1 statistical software.
| Abbreviations: | ||
| CI | = | confidence interval |
| CRS | = | congenital rubella syndrome |
| d | = | days |
| h | = | hours |
| IgG | = | immunoglobulin G |
| IgM | = | immunoglobulin M |
| MMR | = | measles mumps and rubella vaccine |
| mo | = | months |
| PPV | = | positive predictive value |
| RT-PCR | = | reverse-transcription polymerase chain reaction |
| WHO | = | World Health Organization |
| y | = | years |
Acknowledgments
We thank Dr Fernando de Ory, Centro Nacional de Microbiología (Instituto de Salud Carlos III) for support in CRS confirmation, the physicians and microbiologists that have reported cases, the staff of the regional units of epidemiological surveillance of Public Health Agency of Catalonia and the Public Health Agency of Barcelona for their support in the epidemiological investigation of cases, and the staff of the Catalan Health Service for providing the information of the Minimum Hospital Discharge Data.
The other members of the Rubella Surveillance Working Group of Catalonia are: Miquel Alsedà, Josep Álvarez, César Arias, Antoni Artigues, Pilar Jorgina Balañà, Neus Camps, Mónica Carol, Maria Company, Nuria Follia, Pere Godoy, Conchita Izquierdo, Sofia Minguell, Ignasi Parrón, Elsa Plasencia, Ana Rodés, Ariadna Rovira, Laura Ruiz, M Rosa Sala, Roser Torra, Luis Urbiztondo (Agency of Public Health of Catalonia), Joan Caylà, Sara Lafuente, Cristina Rius, Cecilia Tortajada (Agency of Public Health of Barcelona) and Teresa Salas (Catalan Service of Health).
Funding
This study was partially supported by the Catalan Agency for the Management of Grants for University Research (AGAUR Grant number 2009/ SGR 42) and CIBERESP (expedient number CB06/02/0076, Instituto de Salud Carlos III).
Submitted
10/19/12
Accepted
10/29/12
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hobman T, Chanter J. Rubella virus. In: Knipe DM, Howley PM. Fields Virology. 5th ed. Philadelphia: Wolters Kluwer 2007:1069-100.
- Cooper LZ. The burden of congenital rubella syndrome. In: de Quadros CA, ed. Vaccines. Preventing Disease & Protecting Health. Washington: Pan American Health Organization, 2004:53-64.
- Gershon AA. Rubella virus (German measles). In: Mandell Gl, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Churchill Livingstone,2010: 2127-32.
- American Academy of Pediatrics. Rubella. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2012: 629-34.
- Goodson JL, Chu SY, Rota PA, Moss WJ, Featherstone DA, Vijayaraghavan M, et al. Research priorities for global measles and rubella control and eradication. Vaccine 2012; 30:4709 - 16; http://dx.doi.org/10.1016/j.vaccine.2012.04.058; PMID: 22549089
- Banatvala JE, Brown DW. Rubella. Lancet 2004; 363:1127 - 37; http://dx.doi.org/10.1016/S0140-6736(04)15897-2; PMID: 15064032
- World Health Organization. Eliminating measles and rubella and preventing congenital rubella. WHO European Region Strategic Plan 2005-2010. Geneva, 2005.
- Red Nacional de Vigilancia Epidemiológica. Guidelines for the surveillance of rubella and congenital rubella syndrome [Spanish]. Available at http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/fd-enfermedades-prevenibles vacunacion/Protocoloeliminacionrubeola.pdf. Accessed on September 16, 2012.
- World Health Organization. Controlling rubella and preventing congenital rubella syndrome – global progress, 2009. Wkly Epidemiol Rec 2010; 85:413 - 8; PMID: 20949700
- World Health Organization. Global measles and rubella. Strategic Plan 2012-2020. Geneva: World Health Organization, 2012.
- Domínguez A, Vidal J, Plans P, Carmona G, Godoy P, Batalla J, et al. Measles immunity and vaccination policy in Catalonia. Vaccine 1999; 17:530 - 4; http://dx.doi.org/10.1016/S0264-410X(98)00230-8; PMID: 10075158
- Domínguez A, Torner N, Barrabeig I, Rovira A, Rius C, Caylà J, et al, Working Group for the Study of the Measles Outbreak in Catalonia. Large outbreak of measles in a community with high vaccination coverage: implications for the vaccination schedule. Clin Infect Dis 2008; 47:1143 - 9; http://dx.doi.org/10.1086/592258; PMID: 18823269
- Borràs E, Urbiztondo L, Costa J, Batalla J, Torner N, Plasencia A, et al, Working Group for the Study of Measles Immunity in Children. Measles antibodies and response to vaccination in children aged less than 14 months: implications for age of vaccination. Epidemiol Infect 2012; 140:1599 - 606; http://dx.doi.org/10.1017/S0950268811002184; PMID: 22074684
- Borràs E, Domínguez A, Batalla J, Torner N, Cardeñosa N, Nebot M, et al. Vaccination coverage in indigenous and immigrant children under 3 years of age in Catalonia (Spain). Vaccine 2007; 25:3240 - 3; http://dx.doi.org/10.1016/j.vaccine.2007.01.026; PMID: 17320249
- Salleras L, Domínguez A, Torner N. Confirmed interruption of indigenous measles transmission in Catalonia, Spain. Euro Surveill 2001; 6:113 - 7; PMID: 12631955
- Department of Health. The elimination of rubella in Catalonia by the year 2005. [Catalan]. Barcelona: Generalitat of Catalonia 2002.
- World Health Organization. Surveillance Guidelines for measles, rubella and congenital rubella in the WHO European Region. Copenhagen 2009.
- Zimmerman LA, Muscat M, Jankovic D, Goel A, Bang H, Khetsuriani N, et al. Status of rubella and congenital rubella syndrome surveillance, 2005-2009, the World Health Organization European Region. J Infect Dis 2011; 204:Suppl 1 S381 - 8; http://dx.doi.org/10.1093/infdis/jir104; PMID: 21666188
- Reef SE, Redd SB, Abernathy E, Kutty P, Icenogle JP. Evidence used to support the achievement and maintenance of elimination of rubella and congenital rubella syndrome in the United States. J Infect Dis 2011; 204:Suppl 2 S593 - 7; http://dx.doi.org/10.1093/infdis/jir420; PMID: 21954252
- Muscat M, Zimmerman L, Bacci S, Bang H, Glismann S, Mølbak K, et al, EUVAC.NET group. Toward rubella elimination in Europe: an epidemiological assessment. Vaccine 2012; 30:1999 - 2007; http://dx.doi.org/10.1016/j.vaccine.2011.12.016; PMID: 22178098
- Domínguez A, Plans P, Costa J, Torner N, Cardeñosa N, Batalla J, et al. Seroprevalence of measles, rubella, and mumps antibodies in Catalonia, Spain: results of a cross-sectional study. Eur J Clin Microbiol Infect Dis 2006; 25:310 - 7; http://dx.doi.org/10.1007/s10096-006-0133-z; PMID: 16786377
- Bechini A, Boccalini S, Tiscione E, Pesavento G, Mannelli F, Peruzzi M, et al. Progress towards measles and rubella elimination in Tuscany, Italy: the role of population seroepidemiological profile. Eur J Public Health 2012; 22:133 - 9; http://dx.doi.org/10.1093/eurpub/ckq134; PMID: 20880991
- Andrus JK, de Quadros CA, Solórzano CC, Periago MR, Henderson DA. Measles and rubella eradication in the Americas. Vaccine 2011; 29:Suppl 4 D91 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.04.059; PMID: 22185837
- Icenogle JP, Frey TK, Abernathy E, Reef SE, Schnurr D, Stewart JA. Genetic analysis of rubella viruses found in the United States between 1966 and 2004: evidence that indigenous rubella viruses have been eliminated. Clin Infect Dis 2006; 43:Suppl 3 S133 - 40; http://dx.doi.org/10.1086/505945; PMID: 16998772
- Castillo-Solórzano C, Reef SE, Morice A, Andrus JK, Ruiz Matus C, Tambini G, et al. Guidelines for the documentation and verification of measles, rubella, and congenital rubella syndrome elimination in the region of the Americas. J Infect Dis 2011; 204:Suppl 2 S683 - 9; http://dx.doi.org/10.1093/infdis/jir471; PMID: 21954267
- WHO. Standardization of the nomenclature for genetic characteristics of wild-type rubella viruses. Wkly Epidemiol Rec 2005; 80:126 - 32; PMID: 15850226
- Domínguez A, Plans P, Espuñes J, Costa J, Torner N, Cardeñosa N, et al. Rubella immune status of indigenous and immigrant pregnant women in Catalonia, Spain. Eur J Public Health 2007; 17:560 - 4; http://dx.doi.org/10.1093/eurpub/ckm034; PMID: 17442703
- Vargas-Leguás H, Campins Martí M, Juste Sánchez C, Martínez Gómez X, Hermosilla Pérez E, Cabero Roura L. [Rubella susceptibility of immigrant pregnant women in Catalonia]. Med Clin (Barc) 2009; 132:344 - 7; PMID: 19268322
- Kasper S, Allerberger F, Aberle S, Holzmann H, Redlberger M, Daghofer E, et al. Rubella in Austria 2008-2009: no longer a typical childhood disease. Pediatr Infect Dis J 2010; 29:448 - 52; http://dx.doi.org/10.1097/INF.0b013e3181cc3db6; PMID: 20032808
- Canepa P, Valle L, Cristina E, De Florentiis D, Parodi V, Banfi F, et al. Role of congenital rubella reference laboratory: 21-months-surveillance in Liguria, Italy. J Prev Med Hyg 2009; 50:221 - 6; PMID: 20812517
- Berger BE, Omer SB. Could the United States experience rubella outbreaks as a result of vaccine refusal and disease importation?. Hum Vaccin 2010; 6:1016 - 20; http://dx.doi.org/10.4161/hv.6.12.13398; PMID: 21150305
- Bellini WJ, Icenogle JP. Measles and rubella virus. In: Versalov J, Jorgensen JH, Landry ML, Warnock DW, eds. Manual of Clinical Microbiology. 10th ed. Washington: American Society of Microbiology, 2011: 1372-87.
- Rota PA, Brown KE, Hübschen JM, Muller CP, Icenogle J, Chen MH, et al. Improving global virologic surveillance for measles and rubella. J Infect Dis 2011; 204:Suppl 1 S506 - 13; http://dx.doi.org/10.1093/infdis/jir117; PMID: 21666207
- Bispo de Filippis AM, Icenogle J, Matus CR, Andrus JK. Enhanced laboratory surveillance for the elimination of rubella and congenital rubella syndrome in the Americas. J Infect Dis 2011; 204:Suppl 2 S652 - 8; http://dx.doi.org/10.1093/infdis/jir405; PMID: 21954262
- Tipples GA. Rubella diagnostic issues in Canada. J Infect Dis 2011; 204:Suppl 2 S659 - 63; http://dx.doi.org/10.1093/infdis/jir430; PMID: 21954263
- Davidkin I, Valle M, Peltola H, Hovi T, Paunio M, Roivainen M, et al. Etiology of measles- and rubella-like illnesses in measles, mumps, and rubella-vaccinated children. J Infect Dis 1998; 178:1567 - 70; http://dx.doi.org/10.1086/314513; PMID: 9815205
- Macey JF, Tam T, Lipskie T, Tipples G, Eisbrenner T. Rubella elimination, the Canadian experience. J Infect Dis 2011; 204:Suppl 2 S585 - 92; http://dx.doi.org/10.1093/infdis/jir406; PMID: 21954251
- Song N, Gao Z, Wood JG, Hueston L, Gilbert GL, MacIntyre CR, et al. Current epidemiology of rubella and congenital rubella syndrome in Australia: progress towards elimination. Vaccine 2012; 30:4073 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.04.025; PMID: 22554644
- Carnicer-Pont D, Peña-Rey I, Martínez V, de Ory F, Domínguez A, Torner JA, et al. Eliminating congenital rubella syndrome in Spain: does massive immigration have any influence?. Eur J Public Health 2008; 18:688 - 90; http://dx.doi.org/10.1093/eurpub/ckn098
- Salleras L, Campins M, Castrodezaq J, Domínguez A Fernández-Crehuet J, Fernández S, et al. Vaccination calendar in adolescents and adults recommended by the Spanish Society of Preventive Medicine, Public Health and Hygiene (consensus 2009). Vacunas 2010; 11:Supl 2 204 - 15
- Torner N, Anton A, Barrabeig I, Lafuente S, Parrón I, Arias C, et al. Epidemiology of two large measles virus outbreaks in Catalonia: what a difference the month of administration of the first dose of vaccine makes. Hum Vaccin In press PMID: 21285535
- Fine PEM. Mulholland K. Community immunity. In: Plotkin SA, Orenstein W, Offit P. eds. Vaccines 5th ed. Philadelphia: Saunders Elsevier2008: 1573-92.