Abstract
Measles cases in the European Region have been increasing in the last decade; this illustrates the challenge of what we are now encountering in the form of pediatric preventable diseases. In Catalonia, autochthonous measles was declared eliminated in the year 2000 as the result of high measles-mumps-rubella vaccine (MMR) coverage for first and second dose (15 mo and 4 y) since the mid-1990s. From then on, sporadic imported cases and small outbreaks appeared, until in 2006–2007 a large measles outbreak affecting mostly unvaccinated toddlers hit the Barcelona Health Region. Consequently, in January 2008, first dose administration of MMR was lowered from 15 to 12 mo of age. A new honeymoon period went by until the end of 2010, when several importations of cases triggered new sustained transmission of different wild measles virus genotypes, but this time striking young adults. The aim of this study is to show the effect of a change in MMR vaccination schedule policy, and the difference in age incidence and hospitalization rates of affected individuals between both outbreaks.
Epidemiologic data were obtained by case interviews and review of medical records. Samples for virological confirmation and genotyping of cases were collected as established in the Measles Elimination plan guidelines. Incidence rate (IR), rate ratio (RR) and their 95% CI and hospitalization rate (HR) by age group were determined. Statistic z was used for comparing proportions. Total number of confirmed cases was 305 in the 2010 outbreak and 381 in the 2006–2007 outbreak; mean age 20 y (SD 14.8 y; 3 mo to 51 y) vs. 15 mo (SD 13.1 y; 1 mo to 50 y). Highest proportion of cases was set in ≥ 25 y (47%) vs. 24.2% in 2006 (p < 0.001). Differences in IR for ≤ 15 mo (49/100,000 vs. 278.2/100,000; RR: 3,9; 95%CI 2,9–5.4) and in overall HR 29.8% vs. 15.7% were all statistically significant (p < 0.001).
The change of the month of age for the administration of the first MMR dose proved successful to protect infants. Yet, given the current epidemiological situation, continued awareness and efforts to reach young adult population, especially those at high risk of infection and transmission such as healthcare workers and travelers, are needed to stop the spread of the virus when importations occur.
Introduction
Measles is a highly contagious, vaccine-preventable disease caused by a single-strand RNA virus of the genus Morbillivirus in the family Paramyxoviridae characterized in 1954 by Enders and Peebles with 23 known genotypes. It is spread by droplets or by direct contact with nasal or throat secretions of infected persons. It is also, less commonly, airborne or spread through articles freshly soiled with secretions of nose and throat. Measles is one of the most readily transmitted communicable diseases and probably the best known and most deadly of all childhood rash/fever illnesses. Measles is characterized by rash, fever and cough, coryza or conjunctivitis, and is transmitted by pharyngeal or nasal secretions, normally from four days before to four days after the onset of rash. The incubation period is normally 10–14 d and the possible complications include otitis media, laryngotracheobronchitis, pneumonia, diarrhea, encephalitis and secondary bacterial infections. Children aged < 5 y who are living in poor conditions or are malnourished, and adults or patients with immune deficiencies have a greater risk of severe complications.Citation1 Subacute sclerosing panencephalitis (SSPE), a degenerative neurological disease that occurs several years after infection, is the most severe condition related to measles infection especially in the very young. The increased risk of developing SSPE after measles virus infection in young children underscores the importance of childhood immunization programs that decrease measles virus transmission and, therefore, reduce the risk of exposure to measles among infants and prevent the devastating disease SSPE.Citation2 Measles can be effectively prevented by vaccination, which provides lifelong immunity to most recipients against all 23 recognized genotypes.
Measles is a highly communicable disease, for which these conditions for eradication are favorable: humans are the only reservoir for the measles virus (MV), the vaccine is safe, inexpensive and produces life-long immunity, diagnostic tests are both specific and sensitive, all infected people develop symptoms, and there are no chronic carriers.Citation3,Citation4 Eradicating measles would represent a major public health achievement, well worth the investment it requires. For the EU, the first step toward eradication of measles is effective control within its own borders. Finally, eradication will be the result of elimination of transmission on all continents. Elimination of measles by 2015 is part of the WHO strategic plan for measles in the World Health Organization (WHO) European Region. High immunization coverage has dramatically reduced the incidence of measles in Catalonia since measles vaccine was included in vaccination schedule in 1981. Despite overall high vaccination coverage, measles continues to cause frequent outbreaks. However, given the current epidemiological situation,Citation5-Citation9 continued awareness and efforts are needed. Especial efforts should be set concerning mass-gathering events and high traveling frequency among their population as well as from other parts of the world which offer favorable conditions for the spread of the virus between countries.
In Catalonia, a region in the Northeast of Spain with a population of more than 7.5 million inhabitants, autochthonous measles was declared eliminated in the year 2000Citation10 as the result of high Measles Mumps Rubella vaccine (MMR) coverage for first and second dose (15 mo and 4 y) since mid-1990s, from then on then sporadic imported cases and small outbreaks appeared until August 2006, when a large measles outbreak appeared affecting 381 people, 50% of which were below 15 mo of age.Citation11 From January 2008 first dose administration of MMR was in consequence lowered to 12 mo of age. A new honeymoon period went by until at the end of 2010, again, several new importations of different genotypes of wild MV, from neighboring countries triggered another outbreak on November 2010 with a different age distribution sparing small children from infection and striking young adults, mainly adults > 25 y.
The aim of this study is to compare age distribution and incidence rates (IR) of cases resulting from first dose MMR vaccine administration changed from 15 to 12 mo of age and to underscore the importance of enhanced surveillance and implementation of actions to prevent disease and hospitalization for all ages and especially in hard to reach susceptible population.
Results
During the study period 489 suspected measles cases were notified to the corresponding regional epidemiological surveillance units vs. 549 in the 2006–2007 outbreak. Total number of confirmed cases was 305 vs. 381 in 2006; showing slight statistical difference in confirmation rates (62.4% vs. 69.1%) [OR:0.73; 95%CI: 0.56–0.95 (p = 0.02)]. Global IR showed statistical significance (4.05/100,000 vs. 6.6/100,000; RR: 1.3; 95% CI 1.08–1.46). Mean age of cases was 20 y (SD 14.8 y; range 3 mo–51 y) vs. 15 mo (SD 13.1 y; range 1 mo–50 y) in 2006. Highest proportion of cases was set in ≥ 25 y (47.4%) vs. 24.2% in 2006 (p < 0.001). Statistically significant differences were also observed in IR for ≤ 15 mo (49/100,000 vs. 278.2/100,000; RR: 3,9; 95%CI 2.9–5.4) () and in HR 29.8% vs. 15.7% [OR:2.3;95%CI: 1.54–3.45 (p < 0.001)]. The highest percentage of hospitalized patients occurred in those older than 25 y was 37.4% vs. 25.0% in 2006 [OR:1.79;95%CI: 1.01–3.18 (p = 0.05)] (). Eighty percent of hospitalized cases presented complications in contrast to 58.3% in the 2006 outbreak, being gastrointestinal symptoms such as diarrhea and vomiting (33%) the most frequent. A higher, although not significant, proportion of pneumonia was observed (23 cases: 26%) when compared with 2006 (8 cases: 13.3%) [OR: 1.90; 95%CI: 0.74–4.96 (p = 0.21)].
Figure 1. Differences in incidence rates of confirmed measles cases of two outbreaks according to age group. Catalonia 2006–2007 and 2010–2011 outbreaks.
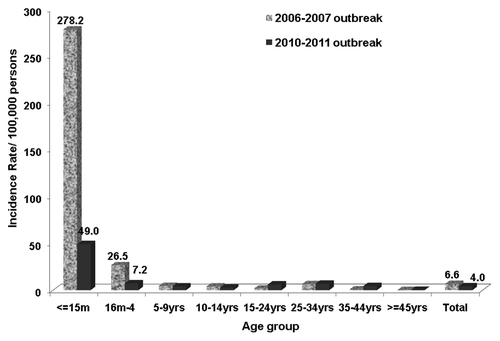
Table 1. Differences in hospitalization rates of confirmed measles cases of two outbreaks according to age group
Laboratory testing was performed in 452 out of 489 suspected cases (92.4%) and of these, 262 (58%) were confirmed cases and 190 were classified as non-measles cases. Of the 262 laboratory confirmed cases, 238 (90.8%) were positive for MV by real-time RT-PCR, 81 (31%) were positive for IgM measles specific antibodies and 54 (20.6%) cases were both positive for real-time RT-PCR and IgM. Seventy percent of cases were confirmed on basis of positive urine and/or pharyngeal swab positive RT-PCR MV test whereas in the 2006 outbreak this percentage accounted for only 19.7% of laboratory confirmed cases.
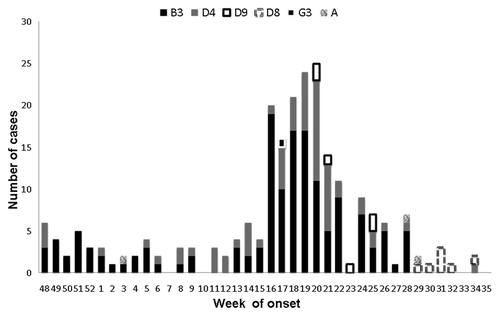
Phylogenetic analysis of the minimum recommended 450 nucleotides of N gene of 227/238 (91%) out of all RT-PCR positive samples revealed that the strains belonged to six different genotypes: A (3; 1.6%), B3 (147; 59.5%), D4 (66; 33.2%), D8 (7; 2.8%), D9 (6; 2.4%) and G3 (1; 0.4%) (). Genotype A was related to vaccine-induced virus infection. Two hundred and 70 one cases /305 (89%) were unvaccinated people of these 36/271 (13.3%) cases were below vaccination age (12 mo) and 32 (11.8%) refused vaccination on philosophical beliefs. Twenty-six cases (8%) had one dose and 8 (3%) had 2 doses of MMR vaccine. One of these cases vaccinated with 2 doses of MMR occurred in a physician working at a hospital emergency department. Seventy-eight cases were of foreign origin (25.3%) vs. 39 (10.2%) in the 2006 outbreak [OR: 2.90; 95%CI: 1.87–4.53 (p < 0.001)] and 11 cases (3.6%) occurred in healthcare settings vs. 11(2.9%)in the 2006 outbreak [OR: 1.25; 95%CI: 0.50–3.17 (p = 0.75)]
Discussion
The increase in measles cases in 2010 occurred despite a steady rise in regional and global MMR coverage. Measles surveillance data and outbreak investigations provide critical information to identify gaps in population immunity and lead to corrective actions and refinements of vaccination strategies.
Adapting vaccination strategies to the epidemiological scenario is important to control of the disease, thus with the evidence gathered from one large outbreak,Citation11infants have been spared from measles infection in this second large outbreak 4 y later when other European countries have had high incidence in infants below vaccination scheduled ageCitation5,Citation12-Citation15 Surveillance data analyses and outbreak investigations should continue to be used to complement vaccination coverage monitoring to identify gaps in vaccination programs.Citation16 Yet measles transmission has been firmly re-established in some European Union (EU) Member States to the extent of even exporting measles to the rest of the world, threatening to undermine years of efforts to eliminate endemic transmission of the measles virus.Citation6,Citation17
The difference in global hospitalization rate (29.8% vs. 15.7%) and higher proportion of complications (80% vs. 53.7%) could be explained by the higher proportion of adult cases affected in this second outbreak in which the mean age of cases was 20 y (SD 14.8 y; range 3 mo–51 y) vs. 15 mo in the 2006 outbreak.Citation18 Yet hospitalization rate in infants below vaccination age was still high (27.3% vs. 13.2%) compared with the previous and other outbreaks.Citation19 This could reflect a higher sensitivity and therefore higher degree of hospitalization not solely on severity of disease. Although further studies should explore whether the fact that cases were infected by different genotypes that might also have different severity.
The implementation of molecular diagnostic and genotyping techniques allowed to gather epidemiological information on measles virus circulating types. Genotypes B3 and D4 were the predominant genotypes in the second measles outbreaks in Catalonia while the first was entirely identified as genotype D4.Citation20
Genotypes B3 and D4 showed genetic differences between sequences with a maximum genetic distance of 2 nucleotides in the genomic region studied, revealing different genetic viral variants within the same genetic group. The remaining genotypes D8 and D9 appeared in sporadic cases or related to small limited outbreaks during the study period. Measles genotype G3 is generally associated with measles infections in south-east Asia, or in sporadic cases with links to south-east Asia.Citation21 There had been no reported cases of measles G3 in Europe since 2006 until by the end of 2010 it reappeared in several different countries in Europe.Citation22 Unlike other outbreaks,Citation11,Citation12,Citation23 six different genotypes have been isolated in Catalonia during the study period, showing several importations as a result of the high incidence in other neighboring territories.
The high proportion of cases in immigrant population (24.9%) reflects the fact that, although immigrants are offered the same health care services as the indigenous population, the rate of MMR vaccination coverage is lower in this population.Citation24 In the 2006 outbreak, this proportion was significantly lower (10.2%) probably because, immigrant parents do adhere to pediatric vaccination schedules in a greater proportion than adults. This fact stresses the need to offer complete adult vaccination schedule to this population when consulting primary care services.
Nosocomial infection has been described as an important source for measles infection,Citation15,Citation25 in this, as in the previous outbreak, 11 cases (3.6% and 2.9% respectively) were related to healthcare workers with few secondary cases arising from them, this fact underscores the importance of maintaining high MMR immunization coverage and of the efforts addressed to improve this coverage in order to reach zero cases in healthcare workers in future outbreaks.
First cases identified in this 2010–2011 outbreak occurred within a setting of unvaccinated children due to philosophical reasons (11.8%) giving place to transmission in an area where anti-vaccine movement is active. This was not so in the previous outbreak where rejection of vaccination for philosophical reasons (1.5%) would not have greatly influenced maintained transmission of chains.Citation11 Parents who refuse to vaccinate their children is an important issue because of the influence it can have on sustaining transmission after an importation of MV within a community. Several authors have studied this phenomenon to find out which are the keys to this belief.Citation26,Citation27 The anti-vaccine movement represents ongoing groups who share concerns based on misconceptions, unfortunately, they not only put their own children at higher risk for disease but they also contribute to the failure of communities to achieve protective vaccination rates and to herd immunity failure even among highly vaccinated populations.Citation28
The fact that a physician correctly vaccinated with 2 doses of MMR became ill has also been observed by other authors.Citation29 It might indicate that in an outbreak setting with persistent close contact with MV, waning of immunity over time is another issue to be followed up closely, especially in regions where circulation of wild MV is low and could pose the possibility of recommending a booster dose for healthcare workers in an outbreak setting.Citation30,Citation31
Since the interruption of endemic measles transmission in December of 2000 and in spite of the high-immunization coverage, measles outbreaks and sporadic infections have occurred in Catalonia due to importations of measles, yet no sustained transmission had occurred and outbreaks, to the exception of those described in this study, were quickly set under control. Surveillance data and results of molecular epidemiology indicate that there is a continuous exposure to MV from other regions of Europe and of the world. The cocirculation of different genotypes and several viral variants for genotypes B3 and D4 revealed that 2010–2011 outbreak was caused by multiple imports from abroad or other Spanish regions (Andalusia, Madrid) and confirms the absence of endemic infection. The change of the month of administration of the first dose proved successful in preventing disease and hospitalization in unvaccinated infants, but young adult population are far harder to reach than children. In this pouch of susceptible, achieving high coverage is difficult and furthermore they are the most mobile population, greatly prone to travel and be a source for importation themselves.
In conclusion, given the current epidemiological situation, continued awareness and efforts to reach young adult population (especially healthcare workers and travelers) are needed to stop the spread of the virus. Enhanced measles surveillance is critical to disease control by early identification of measles cases and thus allowing for early detection and control of outbreaks, assessing on-going transmission patterns in order to mount more effective vaccination measures.
Material and Methods
Subjects of the study
Urgent reported suspected cases of measles to the Public Health Surveillance units were registered and data on age, vaccination status, clinical course and epidemiological information were obtained by case interview and review of medical records.
Laboratory analysis
Samples for virological confirmation and genotyping of cases were collected as established in the Measles Elimination plan guidelines and delivered to the Microbiology Department of the Hospital Clinic of Barcelona. Serum samples were collected after 3rd day of onset and measles specific antibodies IgG and IgM were determined by an ELISA Assay (Vircell ®). Nasopharyngeal and urine samples were collected and tested by real-time RT-PCR. In accordance with WHO recommendation for molecular epidemiology of measles, phylogenetic analysis of the 450 nucleotides that code for the C-terminal 150 amino acids of the measles nucleoprotein (N) gene was used for genotype determination. Sequences obtained during this study were submitted to Health Protection Agency (HPA) measles database.
Statistical analysis
Statistical assessment of incidence rates (IR) and risk ratios (RR) and their 95%CI, hospitalization rate (HR) by age group were determined. Statistic Chi,Citation2 Fisher’s test and statistic z were used for comparing variables and proportions. Statistical analysis was performed by means of the SPSS® 18.0 statistical package for windows (SPSS; Chicago, USA). Statistical significance set at α = 0.05
| Abbreviations: | ||
| CI | = | confidence interval |
| d | = | day |
| ELISA | = | enzyme linked immuno sorbent assay |
| HR | = | hospitalization rate |
| HPA | = | Health Protection Agency |
| IR | = | incidence rate |
| MMR | = | measles-mumps-rubella |
| MV | = | measles virus |
| mo | = | months |
| N | = | neuraminidase |
| OR | = | odds ratio |
| RR | = | rate ratio |
| RT-PCR | = | reverse-transcription polymerase chain reaction |
| SD | = | standard deviation |
| SSPE | = | subacute sclerosing panencephalitis |
| vs. | = | versus |
| WHO | = | World Health Organization |
| y | = | years |
Acknowledgments
We thank all reporting physicians and the other members of the Measles Elimination Program Surveillance Network of Catalonia: Alseda M, Alvarez J, Artigues A, Balaña PJ, Borràs E, Carmona G, Carol M, Ciruela P, Company M, Follia N, Hernandez S, Izquierdo C, Plasencia E, Rovira A, Ruiz L, Sala MR, Serrano J, Torres J, Batalla J, Urbitzondo L (Public Health Agency of Catalonia) and Caylà J, Rius C, Tortajada C, Santomà J, Clos R, Masdeu E, Simon P, Gorrindo P (Public Health Agency of Barcelona) and Ricard Isanta (Service of Microbiology, H Clinic of Barcelona).
Financial disclosure
This work was partially supported by the Agency for the Management of Grants for University Research (AGAUR Grant number 2009 SGR 42) and Spanish Network for the Research in Infectious Diseases (REIPI 06/0008) and European Regional Development Fund (ERDF).
Submitted
10/19/12
Accepted
10/30/12
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189:Suppl 1 S4 - 16; http://dx.doi.org/10.1086/377712; PMID: 15106083
- Bellini WJ, Rota JS, Lowe LE, Katz RS, Dyken PR, Zaki SR, et al. Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J Infect Dis 2005; 192:1686 - 93; http://dx.doi.org/10.1086/497169; PMID: 16235165
- World Health Organization. Proceedings of the Global Technical Consultation to assess the feasibility of measles eradication, 28-30 July 2010. J Infect Dis 2011; 204:Suppl 1 S4 - 13; http://dx.doi.org/10.1093/infdis/jir100; PMID: 21666191
- Moss WJ, Strebel P. Biological feasibility of measles eradication. J Infect Dis 2011; 204:Suppl 1 S47 - 53; http://dx.doi.org/10.1093/infdis/jir065; PMID: 21666201
- Filia A, Tavilla A, Bella A, Magurano F, Ansaldi F, Chironna M, et al. Measles in Italy, July 2009 to September 2010. Euro Surveill 2011; 16:16; PMID: 21801692
- Muscat M, Bang H, Wohlfahrt J, Glismann S, Mølbak K, EUVAC.NET Group. Measles in Europe: an epidemiological assessment. Lancet 2009; 373:383 - 9; http://dx.doi.org/10.1016/S0140-6736(08)61849-8; PMID: 19131097
- Delaporte E, Richard JL, Wyler Lazarevic CA, Lacour O, Girard M, Ginet C, et al. Ongoing measles outbreak, Geneva, Switzerland, January to March 2011. Euro Surveill 2011; 16:16; PMID: 21435325
- Mankertz A, Mihneva Z, Gold H, Baumgarte S, Baillot A, Helble R, et al. Spread of measles virus D4-Hamburg, Europe, 2008-2011. Emerg Infect Dis 2011; 17:1396 - 401; PMID: 21801615
- Benkimoun P. Outbreak of measles in France shows no signs of abating. BMJ 2011; 342:d3161; http://dx.doi.org/10.1136/bmj.d3161; PMID: 21602242
- Salleras L, Dominguez A, Torner N. Confirmed interruption of indigenous measles transmission in Catalonia. Euro Surveill 2001; 6:113 - 7; PMID: 12631955
- Domínguez A, Torner N, Barrabeig I, Rovira A, Rius C, Cayla J, et al, Working Group for the Study of the Measles Outbreak in Catalonia. Large outbreak of measles in a community with high vaccination coverage: implications for the vaccination schedule. Clin Infect Dis 2008; 47:1143 - 9; http://dx.doi.org/10.1086/592258; PMID: 18823269
- Hegasy G, Kätzner K, Helle M, Mankertz A, Baumgarte S, Wille A, et al. Description of measles D4-Hamburg outbreak in Hamburg, Germany, December 2008 to June 2009, which disproportionally affected a local Roma community. Euro Surveill 2012; 17:17; PMID: 22720769
- Stanescu A, Janta D, Lupulescu E, Necula G, Lazar M, Molnar G, et al. Ongoing measles outbreak in Romania, 2011. Euro Surveill 2011; 16:16; PMID: 21871218
- Vivancos R, Keenan A, Farmer S, Atkinson J, Coffey E, Dardamissis E, et al. An ongoing large outbreak of measles in Merseyside, England, January to June 2012. Euro Surveill 2012; 17:17; PMID: 22835470
- Komitova R, Kunchev A, Mihneva Z, Marinova L. Nosocomial transmission of measles among healthcare workers, Bulgaria, 2010. Euro Surveill 2011; 16:16; PMID: 21507322
- Centers for Disease Control and Prevention (CDC). Progress in global measles control, 2000-2010. MMWR Morb Mortal Wkly Rep 2012; 61:73 - 8; PMID: 22298303
- Castillo-Solorzano C, Marsigli C, Danovaro-Holliday MC, Ruiz-Matus C, Tambini G, Andrus JK. Measles and rubella elimination initiatives in the Americas: lessons learned and best practices. J Infect Dis 2011; 204:Suppl 1 S279 - 83; http://dx.doi.org/10.1093/infdis/jir216; PMID: 21666173
- Bassetti M, Schenone E, Calzi A, Camera M, Valle L, Ansaldi F, et al. Measles outbreak in adults in Italy. Infez Med 2011; 19:16 - 9; PMID: 21471742
- Ciofi Degli Atti ML, Filia A, Massari M, Pizzuti R, Nicoletti L, D’Argenzio A, et al, SPES Study Group. Assessment of measles incidence, measles-related complications and hospitalisations during an outbreak in a southern Italian region. Vaccine 2006; 24:1332 - 8; http://dx.doi.org/10.1016/j.vaccine.2005.09.031; PMID: 16219394
- Barrabeig I, Rovira A, Muñoz P, Batalla J, Rius C, Sánchez JA, et al. MMR vaccine effectiveness in an outbreak that involved day-care and primary schools. Vaccine 2011; 29:8024 - 31; http://dx.doi.org/10.1016/j.vaccine.2011.08.056; PMID: 21893145
- Rota PA, Brown K, Mankertz A, Santibanez S, Shulga S, Muller CP, et al. Global distribution of measles genotypes and measles molecular epidemiology. J Infect Dis 2011; 204:Suppl 1 S514 - 23; http://dx.doi.org/10.1093/infdis/jir118; PMID: 21666208
- Cilla G, Montes M, Artieda J, Pineiro L, Arriola L, Perez-Trallero E. Measles genotypes D4 and G3 reintroduced by multiple foci after 15 years without measles virus circulation, Gipuzkoa, the Basque Country, Spain, March to June 2011. Euro Surveill 2011; 16:16; PMID: 22085599
- Melidou A, Gioula G, Pogka V, Exindari M, Moutoussi A, Sgouras D, et al. Molecular and phylogenetic analysis of Greek measles 2010 strains. Epidemiol Infect 2012; 140:432 - 8; http://dx.doi.org/10.1017/S095026881100094X; PMID: 21676352
- Borràs E, Domínguez A, Batalla J, Torner N, Cardeñosa N, Nebot M, et al. Vaccination coverage in indigenous and immigrant children under 3 years of age in Catalonia (Spain). Vaccine 2007; 25:3240 - 3; http://dx.doi.org/10.1016/j.vaccine.2007.01.026; PMID: 17320249
- Botelho-Nevers E, Cassir N, Minodier P, Laporte R, Gautret P, Badiaga S, et al. Measles among healthcare workers: a potential for nosocomial outbreaks. Euro Surveill 2011; 16:16; PMID: 21251488
- Paulussen TG, Hoekstra F, Lanting CI, Buijs GB, Hirasing RA. Determinants of Dutch parents’ decisions to vaccinate their child. Vaccine 2006; 24:644 - 51; http://dx.doi.org/10.1016/j.vaccine.2005.08.053; PMID: 16157423
- Jacobson RM, Targonski PV, Poland GA. A taxonomy of reasoning flaws in the anti-vaccine movement. Vaccine 2007; 25:3146 - 52; http://dx.doi.org/10.1016/j.vaccine.2007.01.046; PMID: 17292515
- May T, Silverman RD. ‘Clustering of exemptions’ as a collective action threat to herd immunity. Vaccine 2003; 21:1048 - 51; http://dx.doi.org/10.1016/S0264-410X(02)00627-8; PMID: 12559778
- Rota JS, Hickman CJ, Sowers SB, Rota PA, Mercader S, Bellini WJ. Two case studies of modified measles in vaccinated physicians exposed to primary measles cases: high risk of infection but low risk of transmission. J Infect Dis 2011; 204:Suppl 1 S559 - 63; http://dx.doi.org/10.1093/infdis/jir098; PMID: 21666213
- Viana PO, Ono E, Miyamoto M, Salomao R, Costa-Carvalho BT, Weckx LY, et al. Humoral and cellular immune responses to measles and tetanus: the importance of elapsed time since last exposure and the nature of the antigen. J Clin Immunol 2010; 30:574 - 82; http://dx.doi.org/10.1007/s10875-010-9420-7; PMID: 20405177
- Hickman CJ, Hyde TB, Sowers SB, Mercader S, McGrew M, Williams NJ, et al. Laboratory characterization of measles virus infection in previously vaccinated and unvaccinated individuals. J Infect Dis 2011; 204:Suppl 1 S549 - 58; http://dx.doi.org/10.1093/infdis/jir106; PMID: 21666212