Abstract
We investigated the incidence and distribution of cases of invasive pneumococcal disease (IPD), invasive meningococcal disease (IMD) and invasive Hemophilus influenzae disease (IHiD) notified by hospital laboratories to the Microbiological Reporting System of Catalonia between 2005 and 2009. Incidence rates were compared using the rate ratio (RR) and 95% CI were calculated. A value of p < 0.05 was considered statistically significant. Of the 6,661 cases, 6,012 were IPD, 436 IMD and 213 IHiD. The global annual incidence per 105 inhabitants was 16.62 (95% CI 16.20–17.04) for IPD, 1.21 (95% CI 1.09–1.32) for IMD and 0.59 (95% CI 0.51–0.67) for IHiD. IPD increased in 2009 compared with 2005 (RR:1.55, 95%CI: 1.43–1.70) and IMD and IHiD remained stable. Pneumonia was the most-frequent clinical manifestation of IPD (75.6%) and IHiD (44.1%) and meningoencephalitis with or without sepsis for IMD (70.6%). The male:female ratio was 1.37 for IPD, 1.0 for IMD and 1.15 for IHiD. The age groups with the highest incidence were the ≤ 2 y and 2–4 y groups for IPD (66.40 and 50.66/100,000 persons-year) and IMD (14.88 and 7.26/100,000 persons-year) and the ≤ 2 y and ≥ 65 y groups for IHiD (1.88 and 1.89/100,000 persons-year). The most-frequent serotypes were serotype 1 (19.0%) in IPD and untypeable serotypes (60.8%) in IHiD. Serogroup B (78.3%) was the most frequent in IMD. S. pneumoniae is the most-frequent agent causing invasive disease in Catalonia. The main clinical manifestations were pneumonia in IPD and IHiD and meningitis in IMD. The main causative agent of meningitis was N. meningitidis in people aged < 20 y and S. pneumoniae in people aged ≥ 20 y. Vaccination with conjugate vaccines may reduce the risk of infectious disease in our setting.
Introduction
Invasive bacteria such as Neisseria meningitidis (Nm), Streptococcus pneumoniae (Sp) and Hemophilus influenzae (Hi) result in high morbidity and mortality in spite of the preventive antibiotic use and highly-effective vaccines.Citation1 Globally, these three bacteria are responsible for > 80% of cases of bacterial meningitis in children.Citation2 Sp and Hi are also a major cause of bacteremia, pneumonia and acute otitis media in children aged > 5 y.Citation3
After the introduction of conjugate H. influenzae type b (Hib) and N. meningitidis serogroup C (NmC) vaccines, a reduction in the number of cases produced by these bacteria has been observed and this has changed the epidemiology of these diseases. Countries that have introduced vaccination with the 7-valent conjugate pneumococcal vaccine (PCV7) have detected a substantial reduction in the number of cases caused by vaccine serotypes followed by a rise in cases due to non-vaccine serotypes.Citation4-Citation6
In Catalonia, a region in the northeast of Spain, the conjugate Hib vaccine was the first of these vaccines to be included in the vaccination schedule, in 1999. Invasive H. influenzae disease occurred mainly in children and, in 95% of cases, was due to serotype b.Citation7 The estimated vaccination coverage was 91.5%.Citation8 In 2000, the meningococcal C conjugated vaccine (MCCV) was included in the vaccination schedule, due mainly to a substantial increase in the number of cases of meningococcal disease caused by this serotype in Catalonia and other Spanish regions and European countries during the 1990s, although the increases were not simultaneous.Citation9 It is estimated that serogroup C vaccine coverage in Catalonia is 88.5%.Citation8 Although meningococcal serogoup B disease now causes the majority of cases in Catalonia, no licensed vaccine is yet available.
PCV7 (only conjugated vaccine available during the study period) was licensed in Catalonia in 2001 but is not included in the routine vaccination schedule, and is only indicated in children aged < 5 y with risk factors.Citation10 The Spanish Society of Paediatrics recommends vaccination,Citation11 and the vaccine is administered in private pediatric clinics. The estimated vaccine coverage with PCV7 is around 50%.Citation12
In addition to immunization and the use of antibiotics, other intrinsic and extrinsic host factors such as age, sex, risk disease and geographical region influence the epidemiology of invasive disease.
The aim of this study was to investigate the epidemiological characteristics of vaccine-preventable invasive bacterial disease (VPIBD) in Catalonia between 2005 and 2009. The diseases studied were invasive pneumococcal disease (IPD), invasive meningococcal disease (IMD) and invasive Hemophilus influenzae disease (IHiD).
Results
Of the 6,661 confirmed cases, 6,012 were IPD, 436 were IMD and 213 were IHiD. The global annual incidence per 100,000 inhabitants was 16.62 (95% CI 16.20–17.04) () for IPD, 1.21 (95% CI 1.09–1.32) () for IMD and 0.59 (95% CI 0.51–0.67) for IHiD ().
Table 1. Distribution of invasive pneumococcal disease by clinical manifestations and age group. Catalonia, 2005–2009.
Table 3. Distribution of invasive meningococcal disease by clinical manifestations and age group. Catalonia, 2005–2009.
Table 5. Distribution of invasive Hemophilus influenzae disease by clinical manifestations and age group. Catalonia, 2005–2009.
The incidence of IPD increased from 12.07 in 2005 to 18.75 /100,000 in 2009 (RR: 1.55, 95% CI: 1.43–1.70) (), but remained stable from 2007 onwards. The male:female ratio was 1.37. The highest incidence was in the < 2 y and 2–4 y age groups (66.40 and 50.66/100,000 persons-year, respectively) (). Pneumonia was the most-frequent clinical manifestation of IPD, with 4,546 cases (75.6%), of which pneumoniae with empyema represented 11.1% (506 cases). Information on the serotyped was obtained in 4,235 of the 6,012 cases (70.4%). More than 70 serotypes were identified. The most-frequent serotypes were 1 (19.0%), 19A (9.9%), 7F (7.8%), 3 (7.7%), 14 (6.8%) and 5 (4.4%) ().
Figure 1. Annual incidence of vaccine-preventable invasive bacterial disease. Catalonia 2005–2009. Note: IPD: invasive pneumococcal disease; IMD: invasive meningococcal disease; IHiD: invasive H. influenzae disease; IR: incidence rate.
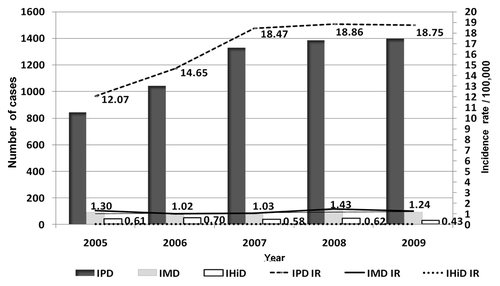
Figure 2. Distribution of invasive pneumococcal disease serotypes. Catalonia 2005–2009. *Percentage of cases caused by serotypes included in each vaccine. Note: Serotype 6A is not included in PPV23
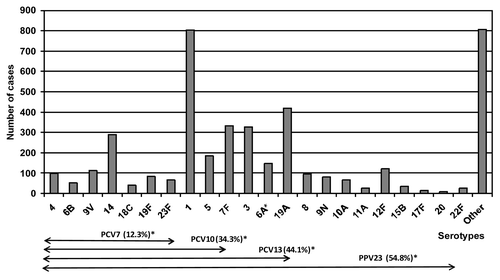
Comparison of the evolution of serotypes during the study period showed a reduction in PCV7 serotypes [20.9% and 13.2% in 2005 and 2009, respectively; (RR: 0.63, 95% CI: 0.49–0.80)] and in serotypes not included in any of the available vaccines [(23.6% and 18.0% in 2005 and 2009, respectively; (RR: 0.76, 95%CI: 0.61–0.95)]. No global changes were observed in PCV13 serotypes [(69.4% and 67.5% in 2005 and 2009, respectively; (RR: 0.97, 95%CI: 0.86–1.10)], in PVC10 [(49.7% and 45.0% in 2005 and 2009, respectively; (RR: 0.91, 95%CI: 0.78–1.05)], although analysis of the most-frequent serotypes showed an increase in serotype 7F [(0.8% and 9.8% in 2005 and 2009, respectively; (RR: 11.65, 95%CI: 4.86–36.52)] and a reduction in serotype 5 [13.5% and 4.0% in 2005 and 2009, respectively; (RR: 0.29, 95%CI: 0.20–0.43)].
shows the incidence rates of the most-frequent serotypes according to age groups. The highest incidence rates were for serotype 1 in children aged 2 -4 y (14.96/100,000 persons-year) and serotype 19A in children aged < 2 y (12.38/100,000 persons-year).
Table 2. Incidence rate of most-frequent serotypes of S. pneumoniae by age group. Catalonia, 2005–2009.
Serotype 1 was the most-frequent serotype in children aged 2–4 y (171 cases; 41.3%) and 5–19 y (163 cases; 53.1%) and adults aged 20–64 y (315 cases; 19.4%). In adults aged ≥ 65 y, serotype 3 was the most frequent (165 cases; 11.5%). Serotype 1 was associated with the 2–4 y (OR:3.56; 95% CI:2.87–4.41) and 5–19 y (OR:5.83; 95% CI:4.59–7.41) age groups. Serotypes 19A and 14 were associated with the < 2 y age group (OR:3.44; 95% CI:2.67–4.43; OR:1.94; 95% CI:1.40–2.70, respectively). Serotype 7F was associated with the 20–64 y age group (OR:1.62; 95% CI:1.30–2.03) and serotype 3 and serotype 14 with the ≥ 65 y age group (OR:2.14; 95% CI:1.71–2.69; OR:1.31; 95% CI:1.03–1.68, respectively).
The distribution of the 4,235 cases serotyped according with the clinical presentation was: 3,402 pneumonia (366 pneumonia with empiema), 402 bacteriemia without focus, 306 meningoencefalitis and 105 other clinical forms.
Comparing the clinical presentation with the most frequent serotypes vs. the rest of serotypes, associations were found between serotype 1 and pneumonia (738 cases; OR:3.27; 95% CI:2.51–4.27), serotype 1 and empiema (136 cases; OR:2.84; 95% CI:2.26–3.56), serotype 19A and bacteriemia without focus (53 cases; OR:1.40; 95% CI:1.03–1.91), serotype 3 and empiema (51 presentations; OR:2.11; 95% CI:1.53–2.90), serotype 7F and pneumonia (285 cases; OR: 1.56; 95% CI: 1.15–2.15) and serotype 5 and pneumonia (159 cases; OR: 1.52; 95% CI: 1.01–2.31). There was no association between serotype 14 and any clinical presentations.
Pneumonia was the most-frequent clinical presentation in all age groups (), ranging between 59.1% in children aged < 2 y and 83.6% in children aged 2–4 y.
The incidence rates of IMD remained stable [1.30 and 1.24/100,000 in 2005 and 2009, respectively (RR: 0.96, 95%CI: 0.71–1.29)] (). The male:female ratio was 1.0. The highest incidence was in the < 2 y and 2–4 y age groups (14.88 and 7.26/100,000 persons-year, respectively) (). Meningoencephalitis, with or without sepsis, was the most-frequent clinical manifestation, with 308 cases (70.6%).
In 422 (96.8%) of 436 cases, information was obtained on the serogroup (). Five serogroups were detected, with serogroup B being the most-frequent (336 cases; 79.6%). The other serogroups were: C (45 cases; 10.7%), A (9 cases; 2.1%), W135 (6 cases; 1.4%), Y (5 cases; 1.2%) and W135/Y (4 cases; 9.5%). In 17 cases (4.0%) the strain was non-groupable/autoagluttinable.
Table 4. Incidence rate of serogroups of N. meningitidis by age group. Catalonia, 2005–2009.
Serogroups B and C remained stable during the study period compared with the other serogroups [(RR: 1.21, 95%CI: 0.85–1.73) and (RR: 0.48, 95%CI: 0.15–1.36), respectively].
Serogroup B was the most-frequent serogroup in all age groups. The highest incidence rates were in children aged < 2 y and 2–4 y (13.38 and 6.21/100,000 persons-year, respectively) ().
For all clinical presentations studied, serogroup B was the most-frequent serogroup, causing 239 (80.5%) of the 297 cases of meningitis with or without sepsis and 41 (64.1%) of the 64 cases of bacteremia without focus. Serogroup C caused 32 cases (10.8%) of meningitis with or without sepsis and 11 cases (17.2%) of bacteremia without focus. shows that, in all age groups, the most-frequent clinical presentation was meningitis with or without sepsis, ranging between 60.9% in people aged ≥ 65 y and 80.8% in people aged 20–64 y.
The incidence of IHiD remained stable during the study period [0.61 and 0.43 /100,000 in 2005 and 2009, respectively (RR: 0.70, 95%CI: 0.43–1.13)] (). The male:female ratio was 1.15. The highest incidence was in the < 2 y and ≥ 65 y age groups (1.88 and 1.89/100,000 persons-year, respectively) (). Pneumonia was the most-frequent clinical manifestation, with 96 cases (45.1%).
Serotyping was performed in 166 (77.9%) of 213 cases. Non-typeable Hi was the most-frequent result (101 cases; 60.8%), followed by serotype b (32 cases; 19.3%). Serotypes a, d, f and c represented 7.2% (12 cases), 1.2% (2 cases), 1.2% (2 cases) and 0.6% (1 case), respectively. In 9.6% (16 cases) were identified as serotype non-b ().
Table 6. Incidence rate of serotypes of Hemophilus influenzae by age group. Catalonia, 2005–2009.
Cases of non-typeable Hi, Hib and Hi non-b remained stable compared with other serotypes [(RR: 1.0, 95%CI: 0.47–2.09), (RR: 0.90, 95%CI: 0.23–3.11) and (RR: 1.03, 95%CI: 0.53–1.97), respectively].
The highest incidence rates were for non-typeable Hi in the < 2 y and ≥ 65 y age groups (1.25 and 0.88/100,000 persons-year, respectively), followed by serotype b in children aged < 2 y (0.38/100,000 persons-year) and adults aged ≥ 65 y (0.27/100,000 persons-year) (). No Hi serotype were associated with groups of age.
Non-typeable H.influenzae was the most frequent serotyped in cases of pneumonia (68.1%), followed by serotype b (10.1%) and non-b (10.1%). In cases with sepsis, serotype b was the most-frequent serotype (83.3%; OR: 30.0, 95%CI: 6.16–146.21), followed by serotype non-b (16.7%).
Discussion
The incidence of invasive bacterial disease varies according to the epidemiological characteristics of the disease, the geographical areaCitation13 and the use of preventive measures such as vaccination, and the sensitivity of the disease surveillance system.Citation14
Our results show that the incidence of IPD was much higher than IMD and IHiD, as occurs in other countries,Citation15 due mainly to the systematic introduction of conjugated vaccines in the vaccination schedule (NmC and Hib vaccines). The current vaccination schedule is three doses at 2, 4 and 6 mo and a booster dose in the second year of life. In Catalonia, although the PCV7 has not been incorporated in the routine vaccination schedule, many pediatricians recommend vaccination, and it is paid for by parents. However, children aged < 5 y with risk factors receive the vaccine free of charge, following the same schedule as the NmC and Hib conjugated vaccines. The current estimated coverage of the PCV7 is around 50%,Citation12 compared with 88.5% for the NmC and 91.5% for the Hib vaccines.Citation8
The global incidence of IPD observed was 16.62/100,000 persons-year, within the range observed in comparable developed countries of 8–34/100,000,Citation16 and in European countries (0.4–20/100,000).Citation17 The incidence in Catalonia was slightly higher than observed in the Community of Madrid (Spain) between 2007 and 2009 (10.74/100,000 persons-year), which has incorporated the PCV7 in the routine vaccination schedule since 2006.Citation18
Children aged < 4 y, and especially aged < 2 y, had the highest rates of the disease (66.40/100,000 persons- year and 50.66/100,000 persons-year in < 2 y and in 2–4 y, respectively). In these age groups, the incidence observed was also somewhat higher than observed in other Spanish regions, such as the Community of Madrid, which had an incidence of 58.10/100,000 in infants aged < 1 y and 32.68/100,000 in children aged 1–4 y, one year after the incorporation of the PVC7 in the routine schedule.Citation18
The main clinical presentation was pneumonia, which was much more frequent than cases of bacteremia without focus and meningitis with or without sepsis as is described in other regions.Citation19,Citation20
Globally, the most-frequent cause of meningitis was Sp, although the behavior was not the same in different age groups. Nm was the most-frequent cause of meningitis in people aged < 20 y and Sp in people aged ≥ 20 y.
In countries which have incorporated the PCV7 in the routine schedule,Citation5,Citation6,Citation21 considerable reductions in the number of cases of IPD caused by PCV7 serotypes, due to the effectiveness of the vaccine and the herd immunity it provides. PCV7 effectiveness against the seven vaccine serotypes is estimated as > 90%.Citation5,Citation22 However, a rise has been observed in cases due to non-vaccine serotypes, especially serotype 19A.Citation5,Citation23,Citation24
Our results show an increase in the global number of cases, with a reduction in cases due to serotypes not included in any currently available pneumococcal vaccine and an increase in serotypes such as 7F.
More than 90 serotypes of Sp have been reported, but only 20 of these cause more than 80% of cases of invasive disease.Citation25,Citation26 However, there are temporal changes in the distribution of pathogenic serotypes.Citation27,Citation28 Our study detected more than 70 serotypes, and the most-frequent serotypes were those most-often detected in other Spanish and European regions.Citation19,Citation20 The most-frequent serotypes were, in order, 1, 19A, 7F and 3. These PCV13 serotypes caused 44.4% of cases and have emerged after the introduction of the PCV7.Citation20,Citation23
As suggested by other authors,Citation18,Citation19,Citation29 different IPD serotypes were associated with specific clinical presentations and with different age groups. There was an association between pneumonia and serotype 1, between empyema and serotypes 1 and 3 and between non-focal bacteremia and serotype 19A, as reported in other regions.Citation18,Citation20 Serotype 19A was associated with children aged < 2 y, serotype 1 with people aged 2–19 y, serotype 7 with the 20–64 y age group and serotype 3 with the ≥ 65 y age group.
The incidence rate of IMD remained stable during the study period, with a higher incidence in children aged < 4 y. The global incidence reported by other authors is 0.5–5/100,000 persons-year.Citation30 Our results were inside of this range. The most-frequent clinical presentation was meningitis with or without sepsis. Nm was the main cause of bacterial meningitis in children and adolescents aged < 20 y, as reported in other studies.Citation4
Serogroup B was the most-frequent serotype, causing the majority of cases of invasive disease. Serogroup B causes the majority of meningococcal disease in Europe and is a major health problem.Citation31,Citation32 Serogroup C, the second in frequency, caused 10.7% of the cases. In the 1990s, various European countries, including Spain, saw an increase in the incidence of cases due to serogroup C. In the United Kingdom, in 1996, 32% of notified cases of IMD were caused by serogroup C.Citation33 After the inclusion of the vaccine in the routine schedule,Citation34 the number of cases decreased by 80% to levels seen before the introduction of the vaccine.Citation35 In Catalonia, as in other Spanish regions, there was also an increase in the incidence, with cases produced by this serogroup representing 32% of all cases in 1996 and 46% in 1997, a substantial rise compared with the preceding years.Citation36 The polysaccharide vaccine campaign resulted in a substantial reduction in the incidence in 1998.Citation37,Citation38 The introduction of the conjugated vaccine in 2000Citation39 resulted in a substantial decrease in the number of cases caused by this serogroup.
Hib was the leading cause of meningitis in children in the US and in most European countries before the introduction of the conjugate Hib vaccine.Citation4,Citation40 Hib has been virtually erradicated as a cause of meningitis in children in countries that have incorporated the vaccine in the routine schedule.Citation4 The conjugated Hib vaccine was the first of the three conjugated vaccines, including the meningococcal C and PCV7, to become available. Vaccine effectiveness is estimated at around 98%Citation26,Citation41,Citation42 and its introduction was followed by a marked reduction in all clinical presentations of IHiD, including meningitis.Citation25 Surveillance data from European countries with routine Hib vaccination show an incidence rate of 0.28/100,000 persons-year of Hi in the post-vaccine era, with only 28% of Hib compared with 80% in the pre-vaccination era.Citation43 There has been a non-significant increase in invasive disease caused by non-b serotypes in these countries since the introduction of the vaccine.Citation43
In Catalonia, before Hib vaccination was introduced, the incidence of invasive disease due to Hib was not as high as in other European countries, ranging between 4.9 and 9.6/100,000.Citation44,Citation45 After the introduction of the vaccine, disease incidence has fallen to minimum levels, ranging between 0.61 and 0.43 /100,000 persons-year during the study period. The highest incidence was in the < 2 y and ≥ 65 y age groups. The mean incidence in Europe in the < 5 y age group in 2007 remained stable at 0.58/100,000 persons-years.Citation46 In contrast, the worldwide incidence rate in the < 5 y age group is 20–60 cases/100,000 persons-year.Citation47
Hib mainly causes meningitis and pneumonia.Citation47 After the introduction of the vaccine, the most severe forms of the disease almost disappeared in children, although invasive disease due to other serotypes and non-typeable strains has increased.Citation48 Non-typeable strains of Hi are an important cause of cases of pneumonia worldwide.Citation48 Non-typeable strains of Hi were the most-frequent cause of disease in our study and the Hib does not protect against other serotypes or non-typeable strains.
Strain replacement is an important but unanswered question in the epidemiology of IHiD. It is not clear whether the elimination of Hib has increased the incidence of invasive disease caused by other serotypes.Citation49 Some data suggests this may be occurring in some countries such as Canada.Citation50
Between 1996 and 2001, 60% of cases of invasive disease in children were caused by Hi non-b, mainly non-typeable strains.Citation51 Studies in some countries suggest a possible change in the epidemiology of Hi towards non-b serotypes,Citation52,Citation53 while in other countries, no substantial increase in non-b serotypes has been observed.Citation53
However, our knowledge of the immunology of the conjugated vaccines is far from complete. Increases in the incidence of invasive disease due to Hi, NmC and recently detected emerging pneumococcal serotypes confirm the importante of continuous and enhanced surveillance.Citation25
The potential for the replacement of strains after the introduction of effective conjugated vaccines is an important factor in both Hib and in other capsulated bacteria such as Sp and Nm.Citation54,Citation55
The main strength of this study is that the data come from the MRSC and were confirmed and completed by the use of other sources such as the Obligatory Disease Reporting System of Catalonia and the microbiological records of the national and regional reference centers of Spain and Catalonia, respectively. The sample size is another advantage, as it allows conclusions to be drawn on the epidemiology of all the invasive diseases studied.
One limitation of the study is that the serotype was not identified in a large number of strains of Hi, which could make it difficult to obtain conclusive results with respect to IHiD.
Conclusions
Sp is the most-frequent agent causing invasive disease in Catalonia. The incidence of IHiD and IMD has remained stable during the study period, while an increase in the incidence of IPD has been observed due to increase in non-PCV7 serotypes.
The < 4 y and ≥ 65 y age groups were those most affected by the three invasive diseases studied. Pneumonia was the main clinical presentations in cases due to IPD and IHiD and meningitis the principal clinical presentation in IMD. The main causal agent of meningitis was Nm in people aged < 20 y and Sp in adults aged ≥ 20 y.
The conjugate vaccines have been a determining factor in reducing the incidence of invasive disease. Correct surveillance of these diseases is necessary to determine the best public health activities at any given time and situation.
Materials and Methods
We analyzed all notifications by participating microbiologists to the Microbiological Reporting System of Catalonia (MRSC) between 2005 and 2009.
The MRSC consists of 50 centers (hospital and primary health care laboratories) representing 84% of hospital beds in public hospitals in Catalonia. This passive surveillance system covers bacterial, viral and parasitic infections including Sp, Nm and Hi.Citation56
Notifications of vaccine-preventable invasive disease to the MRSC were compared with other sources of information, in order to capture non-reported cases and complete demographic and microbiological data to do enhanced surveillance. All cases were compared with sources from national (Spanish National Microbiology Center, Madrid) and regional (Hospital Sant Joan de Déu, Barcelona) reference centers. For IMD and IHiD, we also consulted notifications by physicians to the Obligatory Disease Reporting System.
Inclusion criteria
IPD was defined as the isolation of Sp, the detection of DNA by real-time PCR or the detection of antigen in any normally sterile site with compatible clinical manifestations. IMD and HiID were defined as the isolation of Nm or Hi, respectively, in any normal sterile site with compatible clinical manifestations. Strains isolated by culture were identified and serotyped by standard microbiological methods.Citation57 Serogrouping and serotyping were performed in the regional and national reference centers.
Variables
Sociodemographic, clinical and microbiological data collected included: age, sex, clinical presentation, serogroup and serotype. The clinical presentations evaluated were: pneumonia, meningitis (with or without sepsis), bacteremia without focus, sepsis and others (arthritis, peritonitis, etc.).
Data analysis
Descriptive statistics of cases of VPIBD and the distribution according to age, sex, clinical presentations, serogroup and serotype were analyzed. The incidence was calculated per 100,000 persons-year. The reference population was collected from the Statistical Institute of Catalonia.Citation58 Incidence rates were compared using the rate ratio (RR) and the 95% confidence intervals (CI) were calculated. The associations between serotypes and age groups, and between serogroups and clinical presentations were calculated using the odds ratios and their 95% CI. A value of p < 0.05 was considered statistically significant.
| Abbreviations: | ||
| Nm | = | Neisseria meningitidis |
| Sp | = | Streptococcus pneumoniae |
| Hi | = | Hemophilus influenzae |
| Hib | = | H. influenzae type b |
| NmC | = | N. meningitidis serogroup C |
| IPD | = | invasive pneumococcal disease |
| IMD | = | invasive meningococcal disease |
| IHiD | = | invasive Haemophilus influenzae disease |
| IR | = | incidence rate |
| CI | = | confidence interval |
| MRSC | = | Microbiological Reporting System of Catalonia |
| OR | = | odds ratio |
| PCR | = | polymerase chain reaction |
| PCV7 | = | 7-valent pneumococcal conjugate vaccine |
| PCV10 | = | 10-valent pneumococcal conjugate vaccine |
| PCV13 | = | 13-valent pneumococcal conjugate vaccine |
| RR | = | rate ratio |
| VPIBD | = | vaccine-preventable invasive bacterial disease |
| y | = | year |
| mo | = | month |
Acknowledgments
We thank Dr Fenoll of the National Center of Microbiology, Majadahonda, Madrid, Spain.
The Microbiological Reporting System of Catalonia Study Group: Hospital Universitari General de la Vall d’Hebron (Barcelona); Cap Drassanes – Unitat de Medicina Tropical i Internacional (Raval Sud, Barcelona); Hospital de la Santa Creu i Sant Pau (Barcelona); Hospital Casa de Maternitat (Barcelona); Hospital Clínic i Provincial de Barcelona; Hospital de l’Esperança – Laboratori de Referència de Catalunya (Barcelona); Hospital del Mar – Laboratori de Referència de Catalunya (Barcelona); Hospital Dos de Maig – Consorci Laboratori Intercomarcal (Barcelona); Consorci del Laboratori Intercomarcal de l’Alt Penedès, l’Anoia i Garraf (Vilafranca del Penedès); Hospital Residència Sant Camil – Consorci Laboratori Intercomarcal (Sant Pere de Ribes); Hospital Sant Joan de Déu (Esplugues de Llobregat); Hospital Sant Joan de Déu (Martorell); Hospital Comarcal de l’Alt Penedès – Consorci Laboratori Intercomarcal (Vilafranca del Penedès); Hospital General de l’Hospitalet – Consorci Laboratori Intercomarcal (L’Hospitalet de Llobregat); Hospital de Sant Joan Despí Moisès Broggi – Consorci Laboratori Intercomarcal (Sant Joan Despí); Hospital Universitari de Bellvitge (L’Hospitalet de Llobregat); Hospital de Sant Jaume de Calella; Hospital Municipal de Badalona; Hospital Universitari Germans Trias i Pujol (Badalona); Hospital de Mataró; Catlab – Centre Analítiques Terrassa, AIE (Viladecavalls); Hospital de Sabadell; Hospital de Sant Celoni – Laboratori de Referència de Catalunya; Hospital de Terrassa – Catlab; Hospital General de Catalunya (Sant Cugat del Vallès); Hospital General de Granollers; Hospital Universitari Mútua Terrassa – Catlab (Terrassa); Centre Hospitalari (Manresa); Hospital Comarcal Sant Bernabè (Berga); Hospital de Sant Joan de Déu (Manresa); Hospital d’Igualada – Consorci Laboratori Intercomarcal; Hospital General de Vic; Hospital de Sant Pau i Santa Tecla (Tarragona); Hospital Universitari Joan XXIII de Tarragona; Hospital Universitari de Sant Joan de Reus; Hospital del Vendrell; Clínica Terres de l’Ebre (Tortosa); Hospital Comarcal d’Amposta; Hospital Comarcal de Móra d’Ebre; Hospital de Tortosa Verge de la Cinta; Hospital Comarcal de Blanes; Hospital de Figueres; Hospital Sant Jaume d’Olot; Hospital Universitari de Girona Dr. Josep Trueta (Girona); Hospital Santa Maria (Lleida); Hospital Universitari Arnau de Vilanova de Lleida.
Submitted
10/19/12
Accepted
11/02/12
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- American Academy of Pediatrics. Red Book: 2012 Report of the Committee on Infectious Diseases. Pickering LK, ed. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012.
- WHO. Control of epidemic meningococcal disease, 2nd ed. WHO practical guidelines; 1998. Available at: http://www.who.int/csr/resources/publications/meningitis/WHO_EMC_BAC_98_3_EN/en
- Hausdorff WP, Hajjeh R, Al-Mazrou A, Shibl A, Soriano-Gabarro M, Middle East & North Africa Vaccine-Preventable Diseases Regional Advisory Group. The epidemiology of pneumococcal, meningococcal, and Haemophilus disease in the Middle East and North Africa (MENA) region--current status and needs. Vaccine 2007; 25:1935 - 44; http://dx.doi.org/10.1016/j.vaccine.2006.11.018; PMID: 17241707
- Agrawal S, Nadel S. Acute bacterial meningitis in infants and children: epidemiology and management. Paediatr Drugs 2011; 13:385 - 400; http://dx.doi.org/10.2165/11593340-000000000-00000; PMID: 21999651
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al, Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737 - 46; http://dx.doi.org/10.1056/NEJMoa022823; PMID: 12724479
- Centers for Disease Control and Prevention (CDC). Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease--United States, 1998-2003. MMWR Morb Mortal Wkly Rep 2005; 54:893 - 7; PMID: 16163262
- Domínguez A, Prats G. Vacuna anti-Haemophilus influenzae tipo b. In: Salleras L, ed. Vacunaciones Preventivas, Principios y aplicaciones. 2ª Ed. Barcelona: Masson, 2003: 163-90.
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Coberturas de vacunación, datos estadísticos. Available at: http://www.msc.es/profesionales/saludPublica/prevPromocion/vacunaciones/coberturas.htm#segundo (accessed 14 September 2012)
- Martínez AI, Domínguez A, Oviedo M, Minguell S, Jansà JM, Codina G, et al, Grupo de Trabajo sobre Enfermedad Meningocócica de Cataluña. [Epidemiology of the meningococcal disease in Catalonia before and after vaccination against serogroup C]. Rev Esp Salud Publica 2009; 83:725 - 35; PMID: 20111820
- Generalitat de Catalunya. Departament de Salut. Manual de vacunacions. Col·leció: Quaderns de salut pública, 14. 4th ed. Direcció General de Salut Pública. Barcelona 2006 Available at: http://www20.gencat.cat/docs/canalsalut/Home%20Canal%20Salut/Professionals/Temes_de_salut/Vacunacions/documents/manualvacunes06.pdf (accessed 14 September 2012).
- Blanco A, Gimenez F, Asensi F, Bernaola E, de Juan F, García J, et al, Comité Asesor de Vacunas de la Asociación Española de Pediatría. Vaccination schedule of the spanish association of pediatrics: recommendations 2004. An Pediatr (Barc) 2004; 60:468 - 72; PMID: 15105003
- Ciruela P, Soldevila N, Hernández S, Selva L. F. de Sevilla M, García-García JJ, et al. Risk factors for invasive pneumococcal disease in a community with a high proportion of non vaccine serotypes. Vaccine 2013; In Press
- Hausdorff WP. Invasive pneumococcal disease in children: geographic and temporal variations in incidence and serotype distribution. Eur J Pediatr 2002; 161:Suppl 2 S135 - 9; http://dx.doi.org/10.1007/s00431-002-1066-x; PMID: 12494260
- Hanquet G, Perrocheau A, Kissling E, Bruhl DL, Tarragó D, Stuart J, et al, ECDC Country Experts for Pneumococcal Disease. Surveillance of invasive pneumococcal disease in 30 EU countries: Towards a European system?. Vaccine 2010; 28:3920 - 8; http://dx.doi.org/10.1016/j.vaccine.2010.03.069; PMID: 20394721
- Roberts J, Chandra M, Pebody R, Stuart J. Variation in incidence of pneumococcal and meningococcal disease across Europe. Euro Surveill. 2007;12(46):pii=3310. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3310.
- WHO. Pneumococcal vaccines. Wordl Health Organization. Immunization, Vaccines and Biologicals. Available at: www.who.int/vaccines/in/pneumococcus.shtml.
- Pebody RG, Hellenbrand W, D’Ancona F, Ruutu P, European Union funded Pnc-EURO contributing group. Pneumococcal disease surveillance in Europe. Euro Surveill 2006; 11:171 - 8; PMID: 17075159
- Rodríguez MA, González AV, Gavín MA, Martínez FM, Marín NG, Blázquez BR, et al. Invasive pneumococcal disease: association between serotype, clinical presentation and lethality. Vaccine 2011; 29:5740 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.05.099; PMID: 21683112
- Picazo J, Ruiz-Contreras J, Casado-Flores J, Giangaspro E, Del Castillo F, Hernández-Sampelayo T, et al, Heracles Study Group. Relationship between serotypes, age, and clinical presentation of invasive pneumococcal disease in Madrid, Spain, after introduction of the 7-valent pneumococcal conjugate vaccine into the vaccination calendar. Clin Vaccine Immunol 2011; 18:89 - 94; http://dx.doi.org/10.1128/CVI.00317-10; PMID: 21047996
- Aguiar SI, Brito MJ, Gonçalo-Marques J, Melo-Cristino J, Ramirez M. Serotypes 1, 7F and 19A became the leading causes of pediatric invasive pneumococcal infections in Portugal after 7 years of heptavalent conjugate vaccine use. Vaccine 2010; 28:5167 - 73; http://dx.doi.org/10.1016/j.vaccine.2010.06.008; PMID: 20558247
- Kaplan SL, Mason EO Jr., Wald ER, Schutze GE, Bradley JS, Tan TQ, et al. Decrease of invasive infections in children among 8 children’s hospitals in the United States after the introduction of the 7-valent pneumococcal vaccine. Pediatrics 2004; 113:443 - 9; http://dx.doi.org/10.1542/peds.113.3.443; PMID: 14993532
- Domínguez A, Ciruela P, García-García JJ, Moraga F, de Sevilla MF, Selva L, et al. Effectiveness of 7-valent pneumococcal conjugate vaccine in the prevention of invasive pneumococcal disease in children aged 7-59 months. A matched case-control study. Vaccine 2011; 29:9020 - 5; http://dx.doi.org/10.1016/j.vaccine.2011.09.034; PMID: 21939724
- Muñoz-Almagro C, Jordan I, Gene A, Latorre C, Garcia-Garcia JJ, Pallares R. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis 2008; 46:174 - 82; http://dx.doi.org/10.1086/524660; PMID: 18171247
- Moore MR. Rethinking replacement and resistance. J Infect Dis 2009; 199:771 - 3; http://dx.doi.org/10.1086/597045; PMID: 19434926
- Makwana N, Riordan FAI. Bacterial meningitis: the impact of vaccination. CNS Drugs 2007; 21:355 - 66; http://dx.doi.org/10.2165/00023210-200721050-00001; PMID: 17447825
- WHO. Meningococcal meningitis [online]. Available from URL: http://www.who.int/mediacentre/factsheets/fs141/in/index.html [Accessed 2011 Feb 18]
- Hsieh YC, Lin PY, Chiu CH, Huang YC, Chang KY, Liao CH, et al. National survey of invasive pneumococcal diseases in Taiwan under partial PCV7 vaccination in 2007: emergence of serotype 19A with high invasive potential. Vaccine 2009; 27:5513 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.06.091; PMID: 19615960
- Jansen AG, Rodenburg GD, van der Ende A, van Alphen L, Veenhoven RH, Spanjaard L, et al. Invasive pneumococcal disease among adults: associations among serotypes, disease characteristics, and outcome. Clin Infect Dis 2009; 49:e23 - 9; http://dx.doi.org/10.1086/600045; PMID: 19522653
- Mothibeli KM, du Plessis M, von Gottberg A, de Gouveia L, Adrian P, Madhi SA, et al, Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa (GERMS-SA). An unusual pneumococcal sequence type is the predominant cause of serotype 3 invasive disease in South Africa. J Clin Microbiol 2010; 48:184 - 91; http://dx.doi.org/10.1128/JCM.01011-09; PMID: 19889905
- Centers for Diseases Control and Prevention. Meningococcal disease: technical and clinical information [online]. Available from URL: http://www.cdc.gov/meningitis/clinica-info.html [Accessed 2001 Aug 17].
- Cartwright K, Noah N, Peltola H, Meningococcal Disease Advisory Board. Meningococcal disease in Europe: epidemiology, mortality, and prevention with conjugate vaccines. Report of a European advisory board meeting Vienna, Austria, 6-8 October, 2000. Vaccine 2001; 19:4347 - 56; http://dx.doi.org/10.1016/S0264-410X(01)00205-5; PMID: 11534497
- Connolly M, Noah N, European Meningitis Surveillance Group. Is group C meningococcal disease increasing in Europe? A report of surveillance of meningococcal infection in Europe 1993-6. Epidemiol Infect 1999; 122:41 - 9; http://dx.doi.org/10.1017/S0950268898001848; PMID: 10098784
- Goldblatt D. Recent developments in bacterial conjugate vaccines. J Med Microbiol 1998; 47:563 - 7; http://dx.doi.org/10.1099/00222615-47-7-563; PMID: 9839559
- Ramsay ME, Andrews N, Kaczmarski EB, Miller E. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 2001; 357:195 - 6; http://dx.doi.org/10.1016/S0140-6736(00)03594-7; PMID: 11213098
- Trotter CL, Ramsay ME, Kaczmarski EB. Meningococcal serogroup C conjugate vaccination in England and Wales: coverage and initial impact of the campaign. Commun Dis Public Health 2002; 5:220 - 5; PMID: 12434692
- Domínguez A, Muñoz P, Cardeñosa N, Martínez A, Caylà JA, Meningococcal Disease Study Group. Time-series analyses of meningococcal disease in Catalonia. Ann Epidemiol 2007; 17:654 - 62; http://dx.doi.org/10.1016/j.annepidem.2007.03.006; PMID: 17555986
- Cardeñosa N, Domínguez A, Martínez A, Alvarez J, Pañella H, Godoy P, et al, Working Group on Meningococcal Disease. Meningococcal disease in Catalonia 1 year after mass vaccination campaign with meningococcal group C polysaccharide vaccine. Infection 2003; 31:392 - 7; PMID: 14735381
- Salleras Ll, Domínguez A, Prats G, Parrón I, Muñoz P. Dramatic decline of serogroup C meningococcal disease incidence in Catalonia (Spain) 24 months after a mass vaccination programme of children and young people. J Epidemiol Community Health 2001; 55:283 - 7; http://dx.doi.org/10.1136/jech.55.4.283; PMID: 11238585
- Generalitat de Catalunya. Departament de Sanitat i Seguretat Social. Decret 318/2000 de 27 de setembre, pel qual es modifica l’annex del Decret 60/1999 de 9 de març, pel qual s’estableix calendari de vacunacions sistemàtiques. DOGC 3.242 de 10 d’octubre de 2000.
- Morant A, Díez J, Gimeno C, de la Muela N, Pereiró I, Brines J. [Epidemiology of Haemophilus influenzae type b, Neisseria meningitidis, and Streptococcus pneumoniae in children in the Valencia Community, Spain. Acute diseases study group]. Rev Neurol 1998; 26:34 - 7; PMID: 9533202
- Centers for Diseases Control and Prevention. The impact of Haemophilus influenzae immunisation on invasive infection in children. Commun Dis Rep CDR Wkly 1993; 3:231; PMID: 7508794
- Booy R, Heath PT, Slack MP, Begg N, Moxon ER. Vaccine failures after primary immunisation with Haemophilus influenzae type-b conjugate vaccine without booster. Lancet 1997; 349:1197 - 202; http://dx.doi.org/10.1016/S0140-6736(96)06392-1; PMID: 9130940
- Ladhani S, Slack MP, Heath PT, von Gottberg A, Chandra M, Ramsay ME, European Union Invasive Bacterial Infection Surveillance participants. Invasive Haemophilus influenzae disease, Europe, 1996-2006. Emerg Infect Dis 2010; 16:455 - 63; http://dx.doi.org/10.3201/eid1603.090290; PMID: 20202421
- Departament de Sanitat i Seguretat Social. Generalitat de Catalunya. Resum de les Malalties de Declaració Obligatòria durant l’any 1993. Butlletí Epidemiològic de Catalunya (BEC) 1994, núm 15 (extraordinari): 104-105.
- Departament de Sanitat i Seguretat Social. Generalitat de Catalunya. Resum de les Malalties de Declaració Individualitzada. Distribució quadrisetmanal. Butlletí Epidemiològic de Catalunya (BEC) 1995, núm 16 (extraordinari): 31-33.
- ECDC. (2009). Annual Epidemiological Report on Communicable Diseases in Europe 2009, pp.162. Stockholm: European Centre for Disease Prevention and Control.
- WHO. Haemophilus influenzae type b vaccine. World Health Organization. Immunization, Vaccines and Biologicals. Available at: http://www.who.int/immunization/topics/hib/en/index.html
- Campos J, Román F, Pérez-Vázquez M, Oteo J, Aracil B, Cercenado E, Spanish Study Group for Haemophilus influenzae Type E. Infections due to Haemophilus influenzae serotype E: microbiological, clinical, and epidemiological features. Clin Infect Dis 2003; 37:841 - 5; http://dx.doi.org/10.1086/377232; PMID: 12955648
- Lipsitch M. Vaccination against colonizing bacteria with multiple serotypes. Proc Natl Acad Sci U S A 1997; 94:6571 - 6; http://dx.doi.org/10.1073/pnas.94.12.6571; PMID: 9177259
- Adam HJ, Richardson SE, Jamieson FB, Rawte P, Low DE, Fisman DN. Changing epidemiology of invasive Haemophilus influenzae in Ontario, Canada: evidence for herd effects and strain replacement due to Hib vaccination. Vaccine 2010; 28:4073 - 8; http://dx.doi.org/10.1016/j.vaccine.2010.03.075; PMID: 20398617
- McConnell A, Tan B, Scheifele D, Halperin S, Vaudry W, Law B, et al, of The Canadian Immunization Monitoring Program, ACTive (IMPACT). Invasive infections caused by haemophilus influenzae serotypes in twelve Canadian IMPACT centers, 1996-2001. Pediatr Infect Dis J 2007; 26:1025 - 31; http://dx.doi.org/10.1097/INF.0b013e31812f4f5b; PMID: 17984810
- Tsang RS, Mubareka S, Sill ML, Wylie J, Skinner S, Law DK. Invasive Haemophilus influenzae in Manitoba, Canada, in the postvaccination era. J Clin Microbiol 2006; 44:1530 - 5; http://dx.doi.org/10.1128/JCM.44.4.1530-1535.2006; PMID: 16597886
- Singleton R, Hammitt L, Hennessy T, Bulkow L, DeByle C, Parkinson A, et al. The Alaska Haemophilus influenzae type b experience: lessons in controlling a vaccine-preventable disease. Pediatrics 2006; 118:e421 - 9; http://dx.doi.org/10.1542/peds.2006-0287; PMID: 16882783
- Centers for Disease Control and Prevention (CDC). Emergence of antimicrobial-resistant serotype 19A Streptococcus pneumoniae--Massachusetts, 2001-2006. MMWR Morb Mortal Wkly Rep 2007; 56:1077 - 80; PMID: 17947966
- Kinlin LM, Jamieson F, Brown EM, Brown S, Rawte P, Dolman S, et al. Rapid identification of herd effects with the introduction of serogroup C meningococcal conjugate vaccine in Ontario, Canada, 2000-2006. Vaccine 2009; 27:1735 - 40; http://dx.doi.org/10.1016/j.vaccine.2009.01.026; PMID: 19186206
- Izquierdo C, Ciruela P, Hernandez S, Domínguez A. Vigilància epidemiològica de la infecció respiratòria aguda a Catalunya. Sistema de notificació microbiològica de Catalunya, 2005-2008. Butlleti Epidemiologic de Catalunya 2011;32 (4):38-42. Available at: http://www20.gencat.cat/docs/canalsalut/Home%20Canal%20Salut/Professionals/Recursos/Butlletins_de_salut/PROMOCIO_I_PROTECCIO_DE_LA_SALUT/BEC_Butlleti_epidemiologic_de_Catalunya/2011/Arxius/bec042011.pdf
- Fenoll A, Jado I, Vicioso D, Casal J. Dot blot assay for the serotyping of pneumococci. J Clin Microbiol 1997; 35:764 - 6; PMID: 9041430
- Statistical institut of Catalonia. Available at: http://www.idescat.cat/cat/poblacio/poblestructura.html.