Abstract
Although progress has been made in the treatment of herpes zoster (HZ) and postherpetic neuralgia (PHN), available therapeutic options are only partially effective. Given evidence that a live-attenuated varicella-zoster-virus vaccine is effective at reducing the incidence of HZ, PHN and the burden of illness, policymakers and clinicians are being asked to make recommendations regarding the use of the zoster vaccine. In this report, we summarize the evidence regarding the: (1) burden of illness; (2) vaccine efficacy and safety; and (3) cost-effectiveness of vaccination, to assist evidence-based policy making and guide clinicians in their recommendations. First, there is general agreement that the overall burden of illness associated with HZ and PHN is substantial. Second, the safety and efficacy of the zoster vaccine at reducing the burden of illness due to HZ and the incidence of PHN have been clearly demonstrated in large placebo-controlled trials. However, uncertainty remains about the vaccine’s duration of protection. Third, vaccination against HZ is likely to be cost-effective when the vaccine is given at approximately 65 y of age, if vaccine duration is longer than 10 y.
Herpes zoster (HZ), or shingles, results from reactivation of the varicella-zoster virus (VZV) latent in sensory ganglia since the time of primary infection.Citation1,Citation2 It is characterized by dermatomal pain and vesicular rash.Citation3,Citation4 The most common complication of HZ is postherpetic neuralgia (PHN),Citation5,Citation6 often defined as clinically significant pain persisting more than 90 d after rash onset.Citation7 HZ can also lead to other complications, particularly in immunosuppressed individuals. These include disseminated zoster, ophthalmic zoster, motor paresis (including facial paralysis) and inflammation of the spinal cord and brain.Citation8
Although progress has been made in the treatment of HZ and PHN, available therapeutic options are only partially effective. Aggressive treatment with antivirals (famciclovir, valacyclovir) reduces acute pain and hastens rash resolution, but their ability to prevent the development of PHN remains controversial.Citation9 In addition, antiviral therapy is most effective when initiated within 72 h of HZ rash onset, but the diagnosis of HZ is often delayed.Citation10 PHN is debilitating and its management is often unsuccessful, despite the combined use of tricyclic antidepressants, gabapentinoids, strong analgesics such as opioids and topical agents such as lidocaine medicated plasters or high-concentration capsaicin patches.Citation8,Citation11-Citation13
Given evidence that a live-attenuated VZV vaccine (zoster vaccine) is effective at reducing the incidence of HZ, PHN and the burden of illness due to HZ,Citation14 vaccination against HZ is promising. In many countries, policymakers and clinicians are being asked to make recommendations regarding the use of the zoster vaccine, in the light of competing demands on limited public health resources. The main criteria considered in recommendations of immunization programs include the: (1) burden of illness; (2) vaccine efficacy and safety; and (3) cost-effectiveness of vaccination. In this report, we summarize the information available for each of the above criteria to assist evidence-based policymaking and guide clinicians in their recommendations in developed countries.
Burden of Illness
Epidemiology of herpes zoster and post-herpetic neuralgia
The age-specific incidence of HZ is highly consistent between developed countries.Citation8,Citation15,Citation16 About 20–35% of individuals living in developed countries will develop HZ at some point in their lifeCitation8,Citation15,Citation16 and complications of HZ occur in 13–40% of cases.Citation6,Citation17,Citation18 PHN is the most common complication, with 8 to 27% of individuals with HZ developing PHN.Citation14,Citation19-Citation21 Moreover, the risk of developing PHN as a complication of HZ increases markedly with age; it is about four times higher among individuals older than 70 y of age compared with those younger than 60.Citation15,Citation21
The overall incidence of HZ has been increasing for a number of years.Citation16,Citation22,Citation23 For example, the incidence of HZ consultations in the US increased from 1.7 in 1993 to 4.4 per 1000 person-year in 2006, and is rising in most age groups.Citation22 Although the cause of the rise in HZ remains unclear, it has been suggested that it may be due to: (1) the aging of the population, (2) increasing numbers of immunocompromised individuals in the population and, (3) decreasing exposure to childhood varicella. First, since the incidence rate of HZ increases with age, increasing age of the population in most developed countries will likely lead to a greater number of cases. Second, changes in the management of malignant or auto-immune diseases, as well as the increasing use of corticosteroids and immunosuppressant medication (particularly for organ transplants) are all likely to increase the number of immunocompromised subjects, at greater risk of HZ.Citation23,Citation24 Finally, although still controversial, several epidemiological studies support the hypothesis that exposure to childhood varicella reduces the risk of developing HZ by boosting immunity against VZV.Citation25,Citation26 Exposure to childhood varicella has been decreasing due to important demographic and societal changes in industrialized counties (e.g., decreasing proportions of women with at least one child, decreasing numbers of children per woman, increasing numbers of single-parent families and decreasing contact between grandparents and grandchildren) and the introduction of routine childhood varicella vaccination. However, a clear association between childhood varicella vaccination and the increase in age-specific incidence of HZ has yet to be established.Citation22,Citation27-Citation29 Close monitoring of HZ in countries that have introduced childhood varicella vaccination will help improve our understanding of the impact of these different factors on HZ incidence.
Burden of illness from the patient perspective
Prodromal pain
Prodromal pain, which reflects the reactivation of latent VZV and its subsequent replication in neural tissue, is reported by most patients with HZ.Citation24,Citation30-Citation32 In MASTER (Monitoring and Assessing Shingles Through Education and Research), a pan-Canadian prospective observational study, 74% of individuals experienced prodromal pain, most often described as a burning, itching or shooting sensationCitation33 (). This pain generally begins 4–6 d before rash onset, with a mean severity of approximately 6 out of 1033. Older individuals, the unemployed and/or those with impaired immune status were more likely to report prodromal painCitation33 (). Because the identification of prodromal pain as a manifestation of HZ is only possible when the HZ rash appears, prodromal HZ is often misdiagnosed and mistaken for other diseases associated with unilateral pain, such as angina, renal stones, cholecystitis, or lumbar radiculopathy.Citation34 However, it is frequent and contributes to the overall burden of illness of HZ.Citation33 Finally, prodromal pain and greater severity of this pain are generally recognized as markers of more severe HZ and PHN.Citation33-Citation35
Table 1. Evolution of pain over the course of the diseaseCitation19,Citation33,Citation36
Table 2. Predictors of higher burden of illness for the different phases of herpes zosterCitation19,Citation33,Citation36
Acute and subacute phases of HZ
Acute phase of HZ is characterized by a unilateral, dermatomal and vesicular rash, most often accompanied by pain or discomfort.Citation3,Citation4 Acute HZ pain probably results from a combination of nociceptive pain due to the inflammation of the skin and neuropathic pain caused by neuronal damage.Citation30 The pain can be intermittent or constant and is often described as a burning, itching, shooting or throbbing sensation. Allodynia, which is defined as pain in response to a normally non-painful stimulus, is also commonly reported in the area of the HZ rash.Citation34 Virtually all subjects in MASTER reported pain at recruitment, with 45%, 39% and 11% reporting, severe, moderate and mild pain.Citation36
The rash and pain associated with HZ generally disappear within one month, although pain may persists for several weeks, months, or even years after the rash has healed in some patients.Citation34,Citation36 In MASTER, the severity of pain decreased significantly during the first 30 d after rash onset, from an average of 6.3/10.0 to 2.4/10.0. Between 30 and 90 d, the mean pain severity continued to decrease significantly, but at a slower rate. Median duration of pain was 33 d (), which is consistent with other studies using similar methods.Citation37,Citation38 Individuals of lower socio-economic status, who were immunocompromised, and those with a greater number of lesions were more likely to report severe acute pain (). Interestingly, age was not associated with the severity of acute pain, with younger patients as likely as older ones to experience a considerable burden of illness during the acute phase of HZ.Citation36,Citation39
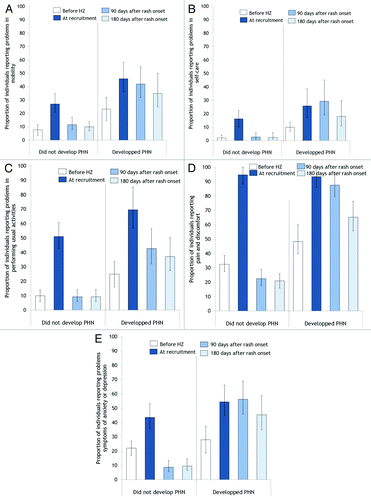
Acute HZ has a major impact on quality of life and functional status.Citation40-Citation43 For example, among individuals who did not develop PHN in MASTER, 95%, 51% and 44% reported, at recruitment (i.e., within 14 d of rash onset), pain and discomfort, problems in performing their usual activities and symptoms of anxiety and depression, respectively, compared with 32%, 10% and 22% prior to HZCitation42 (). These proportions returned to pre-HZ values after pain cessation. Significant impairments across different age groups also indicated that younger patients were as likely as older ones to be adversely affected during the acute phase of HZ.Citation42 In accordance with data from Schmader et al.,Citation40 the majority of participants in MASTER reported interference with sleep (64%), enjoyment of life (58%) and general activities (53%)Citation42 during the acute phase of HZ. A few studies also indicated that HZ had a negative impact on productive work life.Citation44-Citation47 In these studies, the great majority of employed individuals with HZ reported missing time off work for a mean absenteeism of 26 to 32 h per employee.Citation44-Citation47 Moreover, presenteeism (i.e., time with decreased effectiveness at work because of illnessCitation48,Citation49) has been reported to affect 72% of employed individuals during the acute phase of HZ, with a mean of 22 h of presenteeism per employee.Citation47
Postherpetic neuralgia
PHN is the most common debilitating complication of HZ, one of the most challenging to treat and the cause of the greatest HZ-related burden of illness. However, controversy exists regarding the exact definition of PHN. Furthermore, the instruments used to measure PHN greatly differ between studies and the overall risk varies according to the age distribution of the studied group. For these reasons, important variations in the estimated risk of developing PHN (from 8 to 27%Citation14,Citation19,Citation20,Citation32) are found in the literature. This has led to conflicting messages to health professionals and difficulty in quantifying the burden of PHN. In MASTER, the risk of developing PHN was 22% among immunocompetent participants, when defined as a worst pain score ≥ 3/10 during the last 24 h (as measured by the Zoster Brief Pain Inventory (ZBPI)Citation7) persisting more than 90 d after rash onset.Citation19 However, this risk decreased to 14% when defined by the average pain during the last 24 h or by current pain (also measured by the ZBPI). These observations illustrate that methodological choices can largely influence the estimates of the risk of PHN, and therefore should be taken into account when comparing studies.
Very few clinical studies have estimated the complete duration and burden of PHN, due to the prohibitive cost of following-up PHN patients for years.Citation18,Citation50 Helgason et al. estimated that the average duration of PHN is about 1.5 y.Citation50 At the beginning of the PHN period, 69% of subjects in MASTER reported moderate pain and 15% reported severe pain. In addition, half of subjects with PHN still reported clinically significant pain at the end of study follow-up (i.e., 180 d after rash onset).Citation19 Older age, greater rash severity and greater acute pain have been consistently identified as predictors of PHN.Citation19,Citation32,Citation50-Citation54 Other risk factors, such as prodromal pain,Citation32 female genderCitation32 and ophthalmic localization of HZ rashCitation52 were identified only in some studies. In addition, MASTER results indicated that reduced premorbid functional status, possibly a marker of poor health or immune function, also increased the risk of developing PHN.Citation19
Clinical observations suggest that PHN has a severe impact on several aspects of quality of life and on functional status.Citation55 For example, individuals with PHN often report a variety of symptoms such as sleeping problems, chronic fatigue, anorexia, weight loss, anxiety and depression.Citation55 Furthermore, the social life of individuals with PHN is often seriously affected by the disease.Citation55 However, very few studies have quantified the magnitude of these clinical observations.Citation42,Citation43,Citation56 In MASTER, all five health domains assessed by the EuroqolCitation57,Citation58 were significantly affected during PHN.Citation42 For example, at the onset of PHN (i.e., 90 d after rash onset), significantly higher proportions of individuals reported anxiety and depression (56%) and problems with self-care (29%), compared with 28% and 10% prior to HZ (). Interestingly, individuals who developed PHN were more likely to report problems in different health domains prior to HZ compared with those who did not develop PHN (). These data once again support the hypothesis that limitations in premorbid functional status may increase the risk of developing PHN. Consistent with data from Bouhassira et al. and Johnson et al., PHN also interfered with enjoyment of life (31%), mood (30%), sleep (29%) and general activity (25%).Citation42,Citation43,Citation56 Finally, PHN has a negative impact on the productive life of individuals.Citation47 In MASTER, employed subjects who developed PHN reported absenteeism and presenteeism for a mean of 36 and 122 h per employee.Citation47
Impact of herpes zoster and postherpetic neuralgia on the healthcare system
In addition to the burden of HZ and PHN from the patient’s perspective, the management of these conditions results in important costs for the health care system. The main cost drivers of HZ and its complications include outpatient physician visits, hospitalizations and drug prescriptions. In the US, the healthcare cost per acute episode was estimated to be $431 ($US 2007).Citation75 In UK, the average costs for treating HZ and PHN were estimated at £75 and £340 per case (£ 2009).Citation59 In Canada, the diagnosis and treatment of HZ and its complications was estimated to cost $521 per episode for an annual cost of $68 million ($CAN 2009).Citation60 Most of health care use is among adults over the age of 60 y.Citation15,Citation60,Citation61 In Canada, about 40% of HZ consultations, 75% of HZ hospitalization and 71% of PHN episodes occur in adults over 60 y of age.Citation60
Herpes Zoster Vaccine Efficacy and Safety
The Shingles Prevention Study (SPS), a randomized, double-blind, placebo-controlled trial, conducted among 38,546 adults ≥ 60 y of age, showed that a live-attenuated zoster vaccine was effective at reducing the incidence of HZ and PHN by 51% and 66%, respectively.Citation14 In addition, the vaccine significantly reduced the burden of illness, a composite measure of incidence, duration and intensity of pain by 61%Citation14 (). A second randomized, placebo-controlled trial, conducted among 22,439 individuals aged 50–59 y old also showed that the zoster vaccine was effective at reducing the incidence of HZ and the burden of illness by 70% and 73%, respectively among these younger subjects.Citation62 As illustrated in , the vaccine efficacy at preventing HZ significantly decreased with older age at vaccination, but was similar for the burden of illness and prevention of PHN across the different age groups.Citation63 These results suggest that, for younger individuals, most of the benefit of HZ vaccination resulted from the reduction of HZ incidence, whereas for older individuals, much of the benefit resulted from the reduction in disease severity and the incidence of PHN incidence. Furthermore, the zoster vaccine significantly reduced the burden of HZ-related interference with activities of daily living in the overall population of vaccinees, as well as in individuals who developed HZ.Citation64 The Short-Term Persistence Substudy (STPS), a continuation of follow-up of 14,000 SPS subjects, confirmed that vaccine efficacy was maintained through year 5 after vaccination. However, a decline in vaccine efficacy was observed over the 5-y follow-up, particularly for vaccine efficacy against HZ. Because of the small number of cases observed during the STPS, the study power was insufficient to draw conclusion about the persistence of vaccine efficacy beyond 5 y post-vaccination.Citation65 These results seem to confirm a modeling study, which estimated that the zoster vaccine protection waned over time.Citation66
Table 3. Herpes zoster vaccine efficacy according to age at vaccinationCitation14,Citation62
The vaccine was well tolerated in the SPS with similar proportions of participants who received the zoster vaccine (n = 19,270) or the placebo (n = 19,276) reporting serious adverse events (1.4% for both, zoster vaccine and placebo groups).Citation14 Varicella-like rash at the site of injection was the only adverse event statistically more frequent in the vaccinated arm (0.1%) compared with the placebo group (0.04%). The safety of the vaccine was confirmed in the clinical trial among younger subjects with similar proportions of serious adverse events occurring after vaccination (zoster vaccine:0.6%; placebo:0.5%).Citation62
Cost-Effectiveness of Vaccination Against Herpes Zoster
Many studies have examined the cost-effectiveness of vaccination against HZ.Citation15,Citation59-Citation61,Citation67-Citation74 These studies report that the incidence, morbidity and costs associated with the management of HZ and PHN are substantial. Furthermore, they suggest that HZ vaccination is cost-effective if given at 60–70 y of age, assuming vaccine duration of protection is longer than 10 y and vaccine efficacy against PHN is around 67% across all ages.Citation15,Citation59,Citation60,Citation67,Citation69
For a given country, the cost-effectiveness of HZ vaccination is highly sensitive to three key elements: age at vaccination, vaccine duration of protection and vaccine efficacy against PHN. Given that the incidence of HZ and risk of PHN increase markedly after 60 y of age, the optimal age at vaccination is the one that strikes the optimal balance between higher vaccine efficacy among younger individuals and possible waning of vaccine protection. Thus, most studies suggest that the optimal age at HZ vaccination is at around 65 y.Citation15,Citation59,Citation60 Vaccination beyond 65 y of age is less cost-effective as a result of decreasing vaccine efficacy and shorter life expectancy. On the other hand, vaccination before the age of 65 y is less cost-effective because of the uncertainties related to duration of vaccine protection. Duration of the vaccine efficacy, particularly against PHN, has a great impact on cost-effectiveness estimates and contributes most to the uncertainty regarding the cost-effectiveness of HZ vaccination. The recent results from the STPS, indicating possible waning of protection against HZ over time,Citation65 can thus have an important impact on whether the vaccine is cost-effective. However, the key to zoster vaccine cost-effectiveness is the efficacy against PHN across older age groups and the duration of this efficacy.
Conclusion
In light of the current evidence, vaccination against HZ is promising. First, there is general agreement that the overall burden of illness associated with HZ is substantial, not only for individuals who will develop PHN, but also during the prodromal and acute phases of HZ. Available therapeutic options are only partially effective, and the management of PHN is complex and often unsuccessful. Severe detriments to the quality of life have been reported throughout the course of HZ, and the economic burden is substantial. Second, the safety profile of the vaccine and its efficacy at reducing the burden of illness due to HZ and the incidence of PHN have been clearly demonstrated in large placebo-controlled trials. However, uncertainty remains about the vaccine’s duration of protection against HZ and, more importantly, PHN. More research is needed to estimate the duration of protection against PHN, through long-term follow-up and through modeling studies of decay of vaccine protection. Third, vaccination against HZ is likely to be cost-effective when the vaccine is given at approximately 65 y of age, assuming average vaccine protection against moderate/severe pain and PHN is longer than 10 y.
Acknowledgments
This work was funded in part by the Canada Research Chairs (support for Dr. Brisson). MASTER was funded by Merck Frosst Canada Ltd. and was conducted under a collaborative research agreement between Merck Frosst Canada Ltd. and the study’s scientific steering committee, which comprised Marc Brisson, Mélanie Drolet, Myron Levin, Kenneth Schmader, Michael Oxman, Robert Johnson, David Patrick and James Mansi. The scientific steering committee was responsible for the development of the study protocol and for the direction of MASTER. Merck Frosst Canada Ltd. does not have any ownership rights to the findings and results of MASTER. The scientific steering committee has absolute discretion and the final decision over the contents and authorship of all publications or presentations.
Disclosure of Potential Conflicts of Interest
Drs Drolet and Patrick have no conflicts of interest to declare. Dr Oxman has consulted for Merck Frosst and is national chairman of the Shingles Prevention Study (VA Cooperative Study no. 403) and its substudies, which have been supported in part by grants from Merck to the VA Cooperative Studies Program, to the VA San Diego Medical Research Foundation and to the VA Connecticut Research and Education Foundation. Dr Levin has consulted for Merck Frosst; he has received research funds from Merck and is on their speakers bureau. Dr Schmader has received grant support from Merck and Wyeth and has consulted for Merck and GlaxoSmithKline. Dr Johnson has consulted and has received honoraria for public speaking from Merck Frosst, Merck (US), Sanofi Pasteur Merck, Astellas, Novartis and GlaxoSmithKline. Dr Mansi was an employee of Merck Frosst Canada Ltd. Dr. Brisson has consulted and received reimbursement for travel expenses from Merck Frosst and GlaxoSmithKline.
References
- Hope-Simpson RE. The Nature of Herpes Zoster: a Long-Term Study and a New Hypothesis. Proc R Soc Med 1965; 58:9 - 20; PMID: 14267505
- Weller TH. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med 1983; 309:1434 - 40; http://dx.doi.org/10.1056/NEJM198312083092306; PMID: 6195526
- Gnann JW Jr., Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med 2002; 347:340 - 6; http://dx.doi.org/10.1056/NEJMcp013211; PMID: 12151472
- Head H, Campbell AW, Kennedy PG. The pathology of Herpes Zoster and its bearing on sensory localisation. Rev Med Virol 1997; 7:131 - 43; http://dx.doi.org/10.1002/(SICI)1099-1654(199709)7:3<131::AID-RMV198>3.0.CO;2-7; PMID: 10398478
- Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain 1996; 67:241 - 51; http://dx.doi.org/10.1016/0304-3959(96)03122-3; PMID: 8951917
- Oxman MN. Clinical manifestation of herpes zoster. In Arvin AM, Gerson AA, eds. Varicella zoster virus: virology and clinical management. Cambridge, England: Cambridge University press. 246-275., 2000.
- Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain 2004; 5:344 - 56; http://dx.doi.org/10.1016/j.jpain.2004.06.001; PMID: 15336639
- Johnson RW, Wasner G, Saddier P, Baron R. Herpes zoster and postherpetic neuralgia: optimizing management in the elderly patient. Drugs Aging 2008; 25:991 - 1006; http://dx.doi.org/10.2165/0002512-200825120-00002; PMID: 19021299
- Li Q, Chen N, Yang J, Zhou M, Zhou D, Zhang Q, et al. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2009; CD006866; PMID: 19370655
- Bruxelle J, Pinchinat S. Effectiveness of antiviral treatment on acute phase of herpes zoster and development of post herpetic neuralgia: review of international publications. Med Mal Infect 2012; 42:53 - 8; http://dx.doi.org/10.1016/j.medmal.2011.11.001; PMID: 22169279
- Mick G, Correa-Illanes G. Topical pain management with the 5% lidocaine medicated plaster--a review. Curr Med Res Opin 2012; 28:937 - 51; http://dx.doi.org/10.1185/03007995.2012.690339; PMID: 22551228
- Backonja M, Wallace MS, Blonsky ER, Cutler BJ, Malan P Jr., Rauck R, et al, NGX-4010 C116 Study Group. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol 2008; 7:1106 - 12; http://dx.doi.org/10.1016/S1474-4422(08)70228-X; PMID: 18977178
- Irving G, Backonja M, Rauck R, Webster LR, Tobias JK, Vanhove GF. NGX-4010, a capsaicin 8% dermal patch, administered alone or in combination with systemic neuropathic pain medications, reduces pain in patients with postherpetic neuralgia. Clin J Pain 2012; 28:101 - 7; http://dx.doi.org/10.1097/AJP.0b013e318227403d; PMID: 21753727
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al, Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271 - 84; http://dx.doi.org/10.1056/NEJMoa051016; PMID: 15930418
- Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine 2001; 19:3076 - 90; http://dx.doi.org/10.1016/S0264-410X(01)00044-5; PMID: 11312002
- Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect 2001; 127:305 - 14; http://dx.doi.org/10.1017/S0950268801005921; PMID: 11693508
- De Moragas JM, Kierland RR. The outcome of patients with herpes zoster. AMA Arch Derm 1957; 75:193 - 6; http://dx.doi.org/10.1001/archderm.1957.01550140037006; PMID: 13393787
- Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract 1975; 25:571 - 5; PMID: 1195231
- Drolet M, Brisson M, Schmader K, Levin M, Johnson R, Oxman M, et al. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain 2010; 11:1211 - 21; http://dx.doi.org/10.1016/j.jpain.2010.02.020; PMID: 20434957
- Opstelten W, Zuithoff NP, van Essen GA, van Loon AM, van Wijck AJ, Kalkman CJ, et al. Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: prospective prognostic study. Pain 2007; 132:Suppl 1 S52 - 9; http://dx.doi.org/10.1016/j.pain.2007.02.004; PMID: 17379412
- Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341 - 9; http://dx.doi.org/10.4065/82.11.1341; PMID: 17976353
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52:332 - 40; http://dx.doi.org/10.1093/cid/ciq077; PMID: 21217180
- Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997-2002. Epidemiol Infect 2005; 133:245 - 53; http://dx.doi.org/10.1017/S095026880400281X; PMID: 15816149
- Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44:Suppl 1 S1 - 26; http://dx.doi.org/10.1086/510206; PMID: 17143845
- Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine 2002; 20:2500 - 7; http://dx.doi.org/10.1016/S0264-410X(02)00180-9; PMID: 12057605
- Thomas SL, Wheeler JG, Hall AJ. Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study. Lancet 2002; 360:678 - 82; http://dx.doi.org/10.1016/S0140-6736(02)09837-9; PMID: 12241874
- Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis 2005; 191:2002 - 7; http://dx.doi.org/10.1086/430325; PMID: 15897984
- Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, et al. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998-2003. BMC Public Health 2005; 5:68; http://dx.doi.org/10.1186/1471-2458-5-68; PMID: 15960856
- Tanuseputro P, Zagorski B, Chan KJ, Kwong JC. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine 2011; 29:8580 - 4; http://dx.doi.org/10.1016/j.vaccine.2011.09.024; PMID: 21939721
- Haanpää M, Laippala P, Nurmikko T. Pain and somatosensory dysfunction in acute herpes zoster. Clin J Pain 1999; 15:78 - 84; http://dx.doi.org/10.1097/00002508-199906000-00003; PMID: 10382920
- Dworkin RH, Nagasako EM, Johnson RW, Griffin DR. Acute pain in herpes zoster: the famciclovir database project. Pain 2001; 94:113 - 9; http://dx.doi.org/10.1016/S0304-3959(01)00347-5; PMID: 11576750
- Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology 2004; 62:1545 - 51; http://dx.doi.org/10.1212/01.WNL.0000123261.00004.29; PMID: 15136679
- Benbernou A, Drolet M, Levin MJ, Schmader KE, Oxman MN, Johnson R, et al. Association between prodromal pain and the severity of acute herpes zoster and utilization of health care resources. Eur J Pain 2011; 15:1100 - 6; http://dx.doi.org/10.1016/j.ejpain.2011.04.014; PMID: 21600819
- Dworkin RH, Gnann JW Jr., Oaklander AL, Raja SN, Schmader KE, Whitley RJ. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain 2008; 9:Suppl 1 S37 - 44; http://dx.doi.org/10.1016/j.jpain.2007.10.008; PMID: 18166464
- Johnson R, McElhaney J, Pedalino B, Levin M. Prevention of herpes zoster and its painful and debilitating complications. Int J Infect Dis 2007; 11:Suppl 2 S43 - 8; http://dx.doi.org/10.1016/S1201-9712(07)60021-6; PMID: 18162246
- Drolet M, Brisson M, Levin MJ, Schmader KE, Oxman MN, Johnson RW, et al. A prospective study of the herpes zoster severity of illness. Clin J Pain 2010; 26:656 - 66; PMID: 20842005
- Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother 1995; 39:1546 - 53; http://dx.doi.org/10.1128/AAC.39.7.1546; PMID: 7492102
- Tyring SK, Beutner KR, Tucker BA, Anderson WC, Crooks RJ. Antiviral therapy for herpes zoster: randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med 2000; 9:863 - 9; http://dx.doi.org/10.1001/archfami.9.9.863; PMID: 11031393
- Opstelten W. Herpes zoster and postherpetic neuralgia in general practice. The Netherland: Utrecht University, 2005:186.
- Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain 2007; 23:490 - 6; http://dx.doi.org/10.1097/AJP.0b013e318065b6c9; PMID: 17575488
- Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis 2004; 39:342 - 8; http://dx.doi.org/10.1086/421942; PMID: 15307000
- Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ 2010; 182:1731 - 6; http://dx.doi.org/10.1503/cmaj.091711; PMID: 20921251
- Johnson RW, Bouhassira D, Kassianos G, Leplège A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med 2010; 8:37; http://dx.doi.org/10.1186/1741-7015-8-37; PMID: 20565946
- Scott FT, Johnson RW, Leedham-Green M, Davies E, Edmunds WJ, Breuer J. The burden of Herpes Zoster: a prospective population based study. Vaccine 2006; 24:1308 - 14; http://dx.doi.org/10.1016/j.vaccine.2005.09.026; PMID: 16352376
- Singhal PK, Makin C, Pellissier J, Sy L, White R, Saddier P. Work and productivity loss related to herpes zoster. J Med Econ 2011; 14:639 - 45; http://dx.doi.org/10.3111/13696998.2011.607482; PMID: 21838599
- White RR, Lenhart G, Singhal PK, Insinga RP, Itzler RF, Pellissier JM, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics 2009; 27:781 - 92; http://dx.doi.org/10.2165/11317560-000000000-00000; PMID: 19757871
- Drolet M, Levin MJ, Schmader KE, Johnson R, Oxman MN, Patrick D, et al. Employment related productivity loss associated with herpes zoster and postherpetic neuralgia: a 6-month prospective study. Vaccine 2012; 30:2047 - 50; http://dx.doi.org/10.1016/j.vaccine.2012.01.045; PMID: 22285632
- Aronsson G, Gustafsson K, Dallner M. Sick but yet at work. An empirical study of sickness presenteeism. J Epidemiol Community Health 2000; 54:502 - 9; http://dx.doi.org/10.1136/jech.54.7.502; PMID: 10846192
- Prochaska JO, Evers KE, Johnson JL, Castle PH, Prochaska JM, Sears LE, et al. The well-being assessment for productivity: a well-being approach to presenteeism. J Occup Environ Med 2011; 53:735 - 42; http://dx.doi.org/10.1097/JOM.0b013e318222af48; PMID: 21691220
- Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ 2000; 321:794 - 6; http://dx.doi.org/10.1136/bmj.321.7264.794; PMID: 11009518
- Whitley RJ, Weiss HL, Soong SJ, Gnann JW. Herpes zoster: risk categories for persistent pain. J Infect Dis 1999; 179:9 - 15; http://dx.doi.org/10.1086/314562; PMID: 9841816
- Opstelten W, Mauritz JW, de Wit NJ, van Wijck AJ, Stalman WA, van Essen GA. Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Pract 2002; 19:471 - 5; http://dx.doi.org/10.1093/fampra/19.5.471; PMID: 12356697
- Coen PG, Scott F, Leedham-Green M, Nia T, Jamil A, Johnson RW, et al. Predicting and preventing post-herpetic neuralgia: are current risk factors useful in clinical practice?. Eur J Pain 2006; 10:695 - 700; http://dx.doi.org/10.1016/j.ejpain.2005.11.002; PMID: 16427792
- Volpi A, Gatti A, Pica F, Bellino S, Marsella LT, Sabato AF. Clinical and psychosocial correlates of post-herpetic neuralgia. J Med Virol 2008; 80:1646 - 52; http://dx.doi.org/10.1002/jmv.21254; PMID: 18649332
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain 2002; 18:350 - 4; http://dx.doi.org/10.1097/00002508-200211000-00002; PMID: 12441828
- Bouhassira D, Chassany O, Gaillat J, Hanslik T, Launay O, Mann C, et al. Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. Pain 2012; 153:342 - 9; http://dx.doi.org/10.1016/j.pain.2011.10.026; PMID: 22138256
- Szende A, Oppe M, Devlin N. EQ-5D Value Sets: Inventory, comparative review and user Guide. 2007.
- Szende A, Williams A. Measuring self-reported population health: An international perspective based on EQ-5D. 2007.
- van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine 2009; 27:1454 - 67; http://dx.doi.org/10.1016/j.vaccine.2008.12.024; PMID: 19135492
- Brisson M, Pellissier JM, Camden S, Quach C, De Wals P. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Hum Vaccin 2008; 4:238 - 45; http://dx.doi.org/10.4161/hv.4.3.5686; PMID: 18382137
- Bilcke J, Marais C, Ogunjimi B, Willem L, Hens N, Beutels P. Cost-effectiveness of vaccination against herpes zoster in adults aged over 60 years in Belgium. Vaccine 2012; 30:675 - 84; http://dx.doi.org/10.1016/j.vaccine.2011.10.036; PMID: 22120193
- Schmader KE, Levin MJ, Gnann JW Jr., McNeil SA, Vesikari T, Betts RF, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922 - 8; http://dx.doi.org/10.1093/cid/cir970; PMID: 22291101
- Brisson M, Pellissier JM, Levin MJ. Cost-effectiveness of herpes zoster vaccine: flawed assumptions regarding efficacy against postherpetic neuralgia. Clin Infect Dis 2007; 45:1527 - 9; http://dx.doi.org/10.1086/523011; PMID: 17990240
- Schmader KE, Johnson GR, Saddier P, Ciarleglio M, Wang WW, Zhang JH, et al, Shingles Prevention Study Group. Effect of a zoster vaccine on herpes zoster-related interference with functional status and health-related quality-of-life measures in older adults. J Am Geriatr Soc 2010; 58:1634 - 41; http://dx.doi.org/10.1111/j.1532-5415.2010.03021.x; PMID: 20863322
- Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, et al, Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55:1320 - 8; http://dx.doi.org/10.1093/cid/cis638; PMID: 22828595
- Bilcke J, Ogunjimi B, Hulstaert F, Van Damme P, Hens N, Beutels P. Estimating the age-specific duration of herpes zoster vaccine protection: a matter of model choice?. Vaccine 2012; 30:2795 - 800; http://dx.doi.org/10.1016/j.vaccine.2011.09.079; PMID: 21964056
- Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med 2006; 145:317 - 25; PMID: 16954357
- Rothberg MB, Virapongse A, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis 2007; 44:1280 - 8; http://dx.doi.org/10.1086/514342; PMID: 17443464
- Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine 2007; 25:8326 - 37; http://dx.doi.org/10.1016/j.vaccine.2007.09.066; PMID: 17980938
- Najafzadeh M, Marra CA, Galanis E, Patrick DM. Cost effectiveness of herpes zoster vaccine in Canada. Pharmacoeconomics 2009; 27:991 - 1004; http://dx.doi.org/10.2165/11314010-000000000-00000; PMID: 19908924
- Moore L, Remy V, Martin M, Beillat M, McGuire A. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc 2010; 8:7; http://dx.doi.org/10.1186/1478-7547-8-7; PMID: 20433704
- Annemans L, Bresse X, Gobbo C, Papageorgiou M. Health economic evaluation of a vaccine for the prevention of herpes zoster (shingles) and post-herpetic neuralgia in adults in Belgium. J Med Econ 2010; 13:537 - 51; http://dx.doi.org/10.3111/13696998.2010.502854; PMID: 20707768
- van Hoek AJ, Melegaro A, Gay N, Bilcke J, Edmunds WJ. The cost-effectiveness of varicella and combined varicella and herpes zoster vaccination programmes in the United Kingdom. Vaccine 2012; 30:1225 - 34; http://dx.doi.org/10.1016/j.vaccine.2011.11.026; PMID: 22119592
- van Lier A, van Hoek AJ, Opstelten W, Boot HJ, de Melker HE. Assessing the potential effects and cost-effectiveness of programmatic herpes zoster vaccination of elderly in the Netherlands. BMC Health Serv Res 2010; 10:237; http://dx.doi.org/10.1186/1472-6963-10-237; PMID: 20707884
- Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics 2007; 25:155 - 69; http://dx.doi.org/10.2165/00019053-200725020-00007; PMID: 17249857