Abstract
Current cancer immunotherapies predominantly rely on CD8+ T cells to fight against tumors. However accumulative evidence showed that proinflammatory CD4+ helper T cells are critical determinants of effective antitumor immunity. The utilization of universal tumor-reactive helper peptides from telomerase represents a powerful approach to the fully use of CD4+ T cell-based immunotherapy.
Keywords: :
Critical Roles of CD4+ Helper T Cells in Antitumor Immunity
The cancer immunoediting hypothesis indicates that tumor cells could be immunogenic and that the adaptive immune system is involved in the active elimination and selection of tumor cells.Citation1 Among adaptive immune cells involved in antitumor responses, CD8+ T cells (CTL) have been considered as the main protagonists because they exhibit a direct cytotoxic activity toward cancer cells. However, recent advances on the fields indicate that different subpopulations of CD4+ T helper (TH) lymphocytes regulate the antitumor response.Citation2 Among them, tumor-reactive CD4+ T helper 1 cells (TH1), which produce IFN-γ, TNF-α and IL-2, play a critical role in the orchestration of cell-mediated immunity against tumors.Citation3 The concept of CD4+ T-cell help initially emerged from studies showing that successful generation of antitumor CTL depends on the presence of CD4+ T cells. Hence, adoptive cell transfer with CD4+ TH cells induces tumor protection or regression, whereas depletion of CD4+ T cells inhibits vaccine-induced protective immunity.Citation4,Citation5
One generally accepted model implies that CD4+ T cells are necessary to license dendritic cells (DC) for efficient CD8+ T-cell priming through the interaction of costimulatory receptors such as CD40-CD40L.Citation6,Citation7 TH1 cells also promote NK cells and macrophages (M1) activation in vivo.Citation2,Citation8 They have also been shown to contribute to the inhibition of tumor angiogenesis via an IFN-γ and TSP-1 dependent pathway.Citation9,Citation10 Accordingly, in human, high density of tumor-infiltrating TH1 cells has been shown as a good prognostic marker in several cancers.Citation11 The expression of the TH1 specific transcription factor Tbet in tumor infiltrating lymphocytes predicts survival of breast cancer patients treated with traztuzumab and chemotherapy.Citation12 All above emphasize the growing interest to specifically target tumor-reactive CD4 TH1 cells for cancer immunotherapy.
Use of Universal CD4 Helper Peptides from Telomerase as a Relevant Tool to Target CD4 T Cells in Vivo
Because CTLs have been shown as the most powerful effector cells, most previous cancer vaccines targeted MHC class I-restricted peptides derived from tumor antigens to stimulate anticancer CTL responses.Citation13 In the meanwhile; CD4+ TH cells have gained interest in antitumor immunity and immunotherapy. As a result, increasing attention has focused on identifying MHC class II epitopes from tumor antigens to actively target antitumor CD4+ T cells in vivo.Citation14,Citation15 However, the use of tumor-reactive MHC class II helper peptide should require particular caution to prevent the induction of detrimental immune response as different subpopulations of CD4+ T cells are known to regulate host immune responses.Citation16 For instance, TH2 and regulatory T cell are frequently associated with an inhibitory environment within the tumorCitation17 and the role of TH17 cells in the antitumor response is still controversial and seems to depend on the type of cancer.Citation11,Citation18
In a recent study, we used an optimized reverse immunology approach to identify four novel MHC class II-restricted peptides derived from human telomerase reverse transcriptase (TERT).Citation19 TERT maintains telomere length in dividing cells and its expression is the predominant mechanism developed by malignant cells to escape telomere-dependent cell death.Citation20 Telomerase activity has been observed in the vast majority of cancers and emerges as a clinically relevant tumor antigen for immunotherapy.Citation21 These novel TERT-derived peptides referred as “Universal Cancer Peptides” (UCP), effectively bind to most commonly found HLA-DR molecules which increase their applicability in a large number of cancer patients.Citation19 This promiscuous binding capacity of UCP circumvents one major limit of the clinical use of tumor-derived helper peptides that only bind few MHC class II alleles.Citation14 UCP-specific CD4+ T-cell repertoire is present in human and naturally occurring CD4+ T-cells responses against UCP were detected in patients with various types of cancers and these cells mainly produce IFN-γ and TNF-α revealing their TH1 polarization.
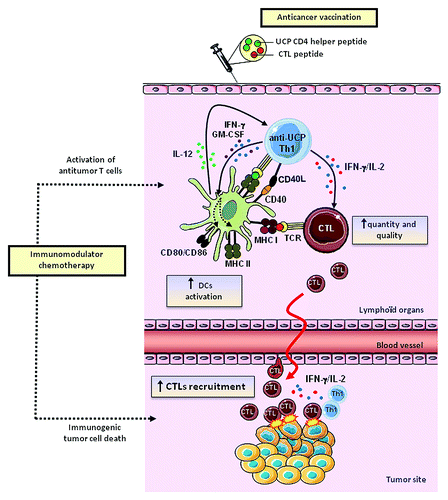
In a second study, UCP were use to actively target CD4+ T cells in a preclinical tumor model and the helper properties of UCP-specific CD4+ T cells were systematically analyzed.Citation22 Using the HLA-A2/HLA-DR1 transgenic mouse model, we showed that UCP vaccinations induce high avidity and tumor-specific CD4+ TH1polarized responses. The UCP-specific CD4+ T cells produced high amount of IFN-γ and IL-2 and but no IL-4, IL-5, IL-10 and IL-17. Co-immunization of MHC class I restricted tumor-derived peptides with or without UCP showed that UCP-specific CD4+ T cells fulfill helper features necessary to generate potent cellular antitumor responses. Indeed, the addition of UCP as helper peptide drastically increased tumor-specific CD8+ T cell responses. We showed that UCP-based vaccine was associated with high antitumor CTL avidity and memory, two critical functions for tumor eradication. Furthermore, the magnitude and quality of the CD8+ T cell responses were closely correlated with the number of IFN-γ and IL-2-secreting UCP-specific CD4+ T cells in vivo. The induction of DC activation represents one major helper mechanism used by CD4+ TH1 cells to sustain antigen presentation and to provide costimulatory signals to CTL. This is referred as the “ménage à trois” model.Citation23 Fully DC activation was also found increased in vivo following UCP vaccination and in vitro after coculture of immature DC with UCP-specific CD4+ T cells. The upregulation of activation markers such as CD86 and MHC class II on DC depends on both IFN-γ and GM-CSF secretion and CD40L expression by UCP-specific CD4+ T cells (). Finally, by using a model of transplantable mouse melanoma (B16-HLA-A2),Citation24 we showed that the addition of UCP as helper peptide was required for effective protection against tumor growth in a therapeutic peptide vaccine using the HLA-A2+ self/TERT CTL peptides (pY988 or pY572).Citation25,Citation26 Collectively our results provide a robust method to comprehensively analyze tumor-derived helper peptides and support that the stimulation of tumor-reactive CD4 TH1 cells is a powerful method to improve the efficacy of cancer vaccines.
Figure 1. Antitumor immune effects of novel universal CD4 helper peptides derived from telomerase (UCP). UCP-based antitumor vaccines favor DC activation which provides costimulatory signals to antitumor CTL, enhancing their quantity, quality and recruitment at the tumor site. Concomittantly chemotherapy can act by promoting immunogenic cell death and antitumor T cell activation.
Tumor-Reactive Helper Peptides are More Effective for Intratumoral Recruitment of Effector CD8+ T Cells
A critical consideration for vaccination is the nature of the helper peptide used. One approach to induction of CD4+ T cell help is to use of xenogenic or non-tumor antigens that stimulate recall responses or nonspecific help. The synthetic helper peptide PADRE derived from keyhole limpet hemocyanin (KLH), and the tetanus toxoid-derived helper peptide are commonly used in anticancer vaccines.Citation27-Citation30 Although, tumor-specific CTL responses appear to be increased by co-administration of non-tumor helper peptide, the clinical benefit of this strategy has not been clearly established, as exemplified in a recent study in melanoma. In this report, patients were vaccinated with MHC class I tumor-derived peptides in conjunction with either helper peptides derived from melanoma antigens or the tetanus-derived helper peptide. Although higher CD8+ T cell responses were induced in the arm with tetanus helper peptide than in melanoma helper peptides one, the clinical impact was quite similar in the two groups.Citation31 One explanation could be related to the inefficiency of non-tumor specific CD4+ T cells to guide effector CD8+ T cells within the tumor as recently demonstrated by Sherman L and colleagues.Citation32,Citation33 Indeed, CD8+ effector T-cell recruitment within the tumor was enhanced by tumor-specific CD4+ T cells and this effect was promoted by IFN-γ-dependent production of chemokines such as CXCL9 and CXCL10. In addition, the production of IL-2 by tumor resident CD4+ T cells enhanced CD8+ T-cell proliferation and function.Citation34
In our study, the use of UCP as helper peptide during therapeutic vaccine promoted tumor infiltration by tumor-specific CD8+ T cells which explain the best tumor control observed in mice ().Citation22 In a previous related study, Gross and colleagues reported that vaccination with the same self/TERT CTL peptides (pY572 and pY988) induced tumor protection only when coupled with a CD4+ helper peptide derived from the hepatitis B virus.Citation35 However around 25% of mice vaccinated prophylactically achieved tumor protection compared with 60% in our therapeutic vaccine study using TERT-derived UCP. Thus we speculate that the difference observed in the two studies could be related to the specificity of the helper signal delivered by CD4+ T cells. This new role of CD4+ helper T cells on CD8 T attraction to the site of attack emerges as a new general mechanism and has also been reported by Nakanishi et al. in the case of infected mucosa.Citation36 Thus only tumor-reactive CD4+ T cells are effectively able to induce a better homing of killer cells at the tumor site.
Interplay Between TERT-Specific CD4+ T Immune Responses and Cytotoxic Chemotherapy: An Emerging Synergistic Antitumor Effects
In a report by Godet and colleagues, we used the universal characteristic of UCP to monitor the UCP-specific CD4+ T-cell responses in metastatic non-small cell lung carcinoma (NSCLC) patients treated by platinum-based doublet chemotherapy. Naturally occurring UCP-specific TH1 CD4 responses were found in 38% of these patients prior chemotherapy. We observed that the presence of this response prior treatment significantly increases the survival of chemotherapy responding patients (median overall survival: 13.2 vs 10 mo, p = 0.034).Citation19 Of note, antiviral T-cell responses measured at the same time in the two groups of patients were similar and had no effect on survival. On other hand, patients with progressive disease after first line chemotherapy do not benefit from UCP-specific immune responses. These results strongly suggested the interplay between UCP-specific CD4 TH1-cell immunity and chemotherapy efficacy.
Original work from Laurence Zitvogel and Guido Kroemer highlighted the capacity of several anticancer agents including classical chemotherapeutics, and targeted compounds stimulate tumor-specific immune responses either by inducing the immunogenic death of tumor cells or by engaging immune effector mechanisms.Citation37 Such an immunogenic cell death relies on the coordinated emission of specific signals from dying cancer cells and their perception by the host immune system. The molecular mechanisms imply the early exposition of calreticulin, ERP57 and the MRP on the cell surface together with the secretion of ATP.Citation38 In line with these observations, our data suggest that the tumor cell lysis induced by chemotherapy promotes an immunological milieu and the release of TERT, which is taken up by antigen presenting cells that subsequently amplify preexisting tumor-specific CD4+ T cells.Citation39 An additional postchemotherapy monitoring of UCP-specific CD4+ T cell responses will be performed for a complete study of CD4+ T cell modulation.
In contrast to CD8 T cell responses, few data are available on the mechanisms by which anticancer drugs modulate antitumor CD4+ T cell immunity.Citation38 Although cyclophosphamide (CTX) has been thought as the most potent CD4-activating anticancer drug in several experimental models; the mechanisms underlying its effect are not well understood. In addition to its well-known effect of depleting suppressor T cells, recent data suggest a link between CD4+ T-cell responses and an immunogenic milieu such as proinflammatory cytokines induced by CTX.Citation40 We and others have shown that blocking VEGF/VEGFR pathway with antiangiogenic drugs modulates CD4+ Foxp3+ regulatory T cells number and functions in tumor-bearing mice and in metastatic cancer patients.Citation41,Citation42 Thus, understanding how the efficiency of conventional chemotherapy influenced CD4+ helper T cell response is a challenging study for chemoimmunotherapy.
In conclusion, there is great interest for cancer vaccines to stimulate tumor-reactive TH1 responses by using tumor-reactive CD4 helper peptides such as TERT-derived UCP that would extend the potential application to various types of cancers. These results also provide a new tool for comprehensive monitoring of antitumor CD4+ T cell responses and support the concept of the immunomodulation of chemotherapy efficacy in cancer patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to acknowledge funding received from Ligue Contre le Cancer, and Association pour la Recherche contre le Cancer, France and grant from the province of Franche Comté, France.
References
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235 - 71; http://dx.doi.org/10.1146/annurev-immunol-031210-101324; PMID: 21219185
- Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev 2008; 222:129 - 44; http://dx.doi.org/10.1111/j.1600-065X.2008.00616.x; PMID: 18363998
- Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol 1998; 10:588 - 94; http://dx.doi.org/10.1016/S0952-7915(98)80228-8; PMID: 9794842
- Fayolle C, Deriaud E, Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol 1991; 147:4069 - 73; PMID: 1684372
- Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 2008; 358:2698 - 703; http://dx.doi.org/10.1056/NEJMoa0800251; PMID: 18565862
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 1998; 393:478 - 80; http://dx.doi.org/10.1038/30996; PMID: 9624004
- Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol 2004; 5:1143 - 8; http://dx.doi.org/10.1038/ni1129; PMID: 15475958
- Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 2003; 101:3052 - 7; http://dx.doi.org/10.1182/blood-2002-09-2876; PMID: 12480696
- Qin Z, Blankenstein T. CD4+ T cell--mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity 2000; 12:677 - 86; http://dx.doi.org/10.1016/S1074-7613(00)80218-6; PMID: 10894167
- Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 2010; 18:485 - 98; http://dx.doi.org/10.1016/j.ccr.2010.10.002; PMID: 21035406
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298 - 306; http://dx.doi.org/10.1038/nrc3245; PMID: 22419253
- Ladoire S, Arnould L, Mignot G, Apetoh L, Rébé C, Martin F, et al. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival. Br J Cancer 2011; 105:366 - 71; http://dx.doi.org/10.1038/bjc.2011.261; PMID: 21750556
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10:909 - 15; http://dx.doi.org/10.1038/nm1100; PMID: 15340416
- Kobayashi H, Celis E. Peptide epitope identification for tumor-reactive CD4 T cells. Curr Opin Immunol 2008; 20:221 - 7; http://dx.doi.org/10.1016/j.coi.2008.04.011; PMID: 18499419
- Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 2005; 54:721 - 8; http://dx.doi.org/10.1007/s00262-004-0653-2; PMID: 16010587
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 2008; 112:1557 - 69; http://dx.doi.org/10.1182/blood-2008-05-078154; PMID: 18725574
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 2011; 208:469 - 78; http://dx.doi.org/10.1084/jem.20101876; PMID: 21339327
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263 - 71; http://dx.doi.org/10.1158/0008-5472.CAN-10-2907; PMID: 21303976
- Godet Y, Fabre E, Dosset M, Lamuraglia M, Levionnois E, Ravel P, et al. Analysis of spontaneous tumor-specific CD4 T-cell immunity in lung cancer using promiscuous HLA-DR telomerase-derived epitopes: potential synergistic effect with chemotherapy response. Clin Cancer Res 2012; 18:2943 - 53; http://dx.doi.org/10.1158/1078-0432.CCR-11-3185; PMID: 22407833
- Martínez P, Blasco MA. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat Rev Cancer 2011; 11:161 - 76; http://dx.doi.org/10.1038/nrc3025; PMID: 21346783
- Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer 2008; 8:167 - 79; http://dx.doi.org/10.1038/nrc2275; PMID: 18256617
- Dosset M, Godet Y, Vauchy C, Beziaud L, Lone YC, Sedlik C, et al. Universal cancer peptide-based therapeutic vaccine breaks tolerance against telomerase and eradicates established tumor. Clin Cancer Res 2012; 18:6284 - 95; http://dx.doi.org/10.1158/1078-0432.CCR-12-0896; PMID: 23032748
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 1998; 393:474 - 8; http://dx.doi.org/10.1038/30989; PMID: 9624003
- Adotévi O, Mollier K, Neuveut C, Dosset M, Ravel P, Fridman WH, et al. Targeting human telomerase reverse transcriptase with recombinant lentivector is highly effective to stimulate antitumor CD8 T-cell immunity in vivo. Blood 2010; 115:3025 - 32; http://dx.doi.org/10.1182/blood-2009-11-253641; PMID: 20130242
- Hernandez J, Garcia-Pons F, Lone YC, Firat H, Schmidt JD, Langlade-Demoyen P, et al. Identification of a human telomerase reverse transcriptase peptide of low affinity for HLA A2.1 that induces cytotoxic T lymphocytes and mediates lysis of tumor cells. Proc Natl Acad Sci U S A 2002; 99:12275 - 80; http://dx.doi.org/10.1073/pnas.182418399; PMID: 12218171
- Scardino A, Gross DA, Alves P, Schultze JL, Graff-Dubois S, Faure O, et al. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J Immunol 2002; 168:5900 - 6; PMID: 12023395
- del Guercio MF, Alexander J, Kubo RT, Arrhenius T, Maewal A, Appella E, et al. Potent immunogenic short linear peptide constructs composed of B cell epitopes and Pan DR T helper epitopes (PADRE) for antibody responses in vivo. Vaccine 1997; 15:441 - 8; http://dx.doi.org/10.1016/S0264-410X(97)00186-2; PMID: 9141216
- Helling F, Zhang S, Shang A, Adluri S, Calves M, Koganty R, et al. GM2-KLH conjugate vaccine: increased immunogenicity in melanoma patients after administration with immunological adjuvant QS-21. Cancer Res 1995; 55:2783 - 8; PMID: 7796403
- Scheibenbogen C, Schadendorf D, Bechrakis NE, Nagorsen D, Hofmann U, Servetopoulou F, et al. Effects of granulocyte-macrophage colony-stimulating factor and foreign helper protein as immunologic adjuvants on the T-cell response to vaccination with tyrosinase peptides. Int J Cancer 2003; 104:188 - 94; http://dx.doi.org/10.1002/ijc.10961; PMID: 12569574
- Valmori D, Pessi A, Bianchi E, Corradin G. Use of human universally antigenic tetanus toxin T cell epitopes as carriers for human vaccination. J Immunol 1992; 149:717 - 21; PMID: 1378079
- Slingluff CL Jr., Petroni GR, Chianese-Bullock KA, Smolkin ME, Ross MI, Haas NB, et al. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol 2011; 29:2924 - 32; http://dx.doi.org/10.1200/JCO.2010.33.8053; PMID: 21690475
- Wong SB, Bos R, Sherman LA. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol 2008; 180:3122 - 31; PMID: 18292535
- Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, et al. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol 2000; 165:6047 - 55; PMID: 11086036
- Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res 2010; 70:8368 - 77; http://dx.doi.org/10.1158/0008-5472.CAN-10-1322; PMID: 20940398
- Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, et al. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest 2004; 113:425 - 33; PMID: 14755339
- Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature 2009; 462:510 - 3; http://dx.doi.org/10.1038/nature08511; PMID: 19898495
- Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011; 8:151 - 60; http://dx.doi.org/10.1038/nrclinonc.2010.223; PMID: 21364688
- Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11:215 - 33; http://dx.doi.org/10.1038/nrd3626; PMID: 22301798
- Godet Y, Dosset M, Borg C, Adotevi O. Is preexisting antitumor CD4 T cell response indispensable for the chemotherapy induced immune regression of cancer?. Oncoimmunology 2012; 1:1617 - 9; http://dx.doi.org/10.4161/onci.21513; PMID: 23264913
- Ding ZC, Zhou G. Cytotoxic chemotherapy and CD4+ effector T cells: an emerging alliance for durable antitumor effects. Clin Dev Immunol 2012; 2012:890178; http://dx.doi.org/10.1155/2012/890178; PMID: 22400040
- Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGF Receptor pathway blockade inhibits tumor-induced regulatory T cell proliferation in colorectal cancer. Cancer Res 2013; 73:539 - 49; http://dx.doi.org/10.1158/0008-5472.CAN-12-2325; PMID: 23108136
- Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother 2010; 33:991 - 8; http://dx.doi.org/10.1097/CJI.0b013e3181f4c208; PMID: 20948437