Abstract
We previously demonstrated that our second-generation DNA-based Alzheimer disease (AD) epitope vaccine comprising three copies of a short amyloid-β (Aβ) B cell epitope, Aβ11 fused with the foreign promiscuous Th epitope, PADRE (p3Aβ11-PADRE) was immunogenic in mice. However, since DNA vaccines exhibit poor immunogenicity in large animals and humans, in this study, we sought to improve the immunogenicity of p3Aβ11-PADRE by modifying this vaccine to express protein 3Aβ11-PADRE with a free N-terminal aspartic acid fused with eight additional promiscuous Th epitopes. Generated pN-3Aβ11-PADRE-Thep vaccine has been designated as AV-1955. We also delivered this vaccine using the TriGrid electroporation system to improve the efficiency of DNA transfection. This third-generation DNA epitope vaccine was evaluated for immunogenicity in rabbits in comparison to the parent construct p3Aβ11-PADRE. AV-1955 vaccination induced significantly stronger humoral immune responses in rabbits compared with p3Aβ11-PADRE vaccine. Anti-Aβ11 antibodies recognized all forms of human β-amyloid peptide (monomers, oligomers and fibrils), bound to amyloid plaques in brain sections from an AD case and reduced oligomer- and fibril-mediated cytotoxicity ex vivo. These findings suggest that AV-1955 could represent an effective DNA epitope vaccine for AD therapy, pending safety and efficacy studies that are currently being conducted in Rhesus monkeys.
Introduction
Vaccination approaches against AD must be designed to induce strong antibody responses and avoid pro-inflammatory autoreactive T cell responses that are likely responsible for meningoencephalitis in subset of AD patients enrolled in AN1792 trials.Citation1-Citation8 Therefore, it is crucial to develop a vaccine that is safe enough to be used as an “early therapeutic” or preventative measure. Previously we reported on immunogenicity, safety and therapeutic efficacy of an AD DNA epitope vaccine in wild-type and 3xTg-AD mice.Citation9 This vaccine was specifically designed to reduce the risk of T cell-mediated autoimmunity by encoding a non-self T helper cell epitope (PADRE) and a short self B cell epitope from the N-terminus of Aβ. Although this vaccine induced strong humoral B cell responses in mice, the fact that DNA vaccines generally exhibit weak immune responses in large animals and humans, particularly due to low transfection efficacy of naked DNA, is another major consideration for the design of novel vaccine strategies. To improve transfection efficiency of DNA vaccines for humans, various DNA delivery systems such as jet injectors, gene gun and electroporation (EP) have been developed. EP enhances DNA uptake into cells through the delivery of brief electrical pulses, which transiently destabilize the cell membrane to allow DNA uptake into the cell, possibly by electrophoretic movement of the negatively charged DNA within the electrical field.Citation10 EP can increase gene expression in vivo by 100- to 1000-fold compared with needle injection of naked plasmid DNA.Citation11,Citation12 Several electroporation devices from VGXi, Inc., Ichor Medical Systems Inc., BTX Harvard Apparatus are now being tested in more than in 30 Phase I-III clinical trials worldwide (http://clinicaltrials.gov/ct2/results?term=electroporation+device). Specifically, a clinical grade EP device (Intramuscular TriGridTM Delivery System, TDS-IM) developed by Ichor Medical Systems is currently being evaluated for DNA vaccine delivery in several clinical trialsCitation13 and has been shown to markedly enhance responses to an HIV vaccine,Citation14 therefore, we aimed to test this delivery system for a novel DNA-based epitope vaccine against AD. In this translational study, we tested TDS-IM and the efficacy of a modified version of the p3Aβ11-PADRE vaccine engineered to express 3Aβ11-PADRE protein with free N-terminal aspartic acid fused with eight additional promiscuous Th epitopes (pN-3Aβ11-PADRE-Thep) in rabbits.
Results
Immunogenicity of second- and third-generation DNA epitope vaccines delivered in rabbits by EP
To evaluate whether anti-Aβ responses to our second-generation DNA epitope vaccine could be scaled up from mice to a larger species, rabbits were immunized intramuscularly with p3Aβ11-PADRE vaccine (). All 14 animals responded to immunization with concentrations of anti-Aβ antibodies in ranging from 3.1–19.4µg/ml () and these antibodies were mostly of IgG isotype (). Next, we used two different approaches to refine the p3Aβ11-PADRE vaccine to enhance its immunogenicity ( and ). First, to enhance the immunogenicity of a vaccine for potential clinical use in humans with highly polymorphic “classical” MHC class II genes, we incorporated eight promiscuous foreign Th cell epitopes from conventional vaccines into this construct (). Fine epitope mapping of sera from patients enrolled in the AN1792 trial suggested that the free N-terminal aspartic acid of Aβ42 may be essential for induction of antibodies in humans,Citation15 which was also supported by studies in monkeysCitation16 and rabbits.Citation17 Therefore, we next modified p3Aβ11-PADRE-Thep vaccine to generate a construct that would encode an immunogen possessing a free N-terminal aspartic acid following signal sequence cleavage ().
Figure 1. (A) Schematic representation of construct encoding epitope vaccine p3Aβ11-PADRE. (B) p3Aβ11-PADRE induces anti-Aβ antibody responses in all immunized rabbits. Antibody responses were analyzed in individual sera after 2nd, 3rd and 4th immunizations by ELISA. Lines indicate the mean (n = 14). (C) All animals immunized two times with p3Aβ11-PADRE produced anti-Aβ antibodies of IgG isotype. IgG and IgM isotypes of antibodies were analyzed in individual sera of immunized animals at dilution 1:200. Error bars indicate SD (n = 14).
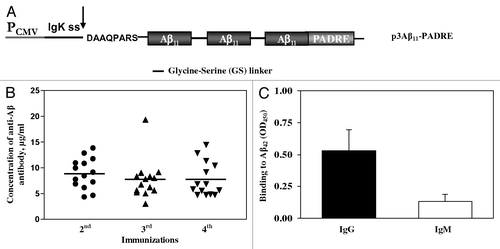
Figure 2. (A) Schematic representation of third generation epitope vaccines. Parental construct (p3Aβ11-PADRE) was modified to express protein composed of three Aβ11 B cell epitopes and nine different foreign Th cell epitopes each separated by a small glycine-serine spacer. In addition, extra amino acids between signal sequence and the Aβ11 was removed to generate protein with free N-terminal aspartic acid after cleavage of signal sequence. (B and C) Correct cleavage of signal sequence and generation of free N-terminus aspartic acid in a first copy of Aβ11 in N-3Aβ11-PADRE-Thep was analyzed in conditioned media (CM) of CHO cells transfected with p3Aβ11-PADRE-Thep (Lane 1) and pN-3Aβ11-PADRE-Thep (Lane 2) by IP/WB. Both proteins were immunoprecipitated with 6E10 MoAb. Blots were stained with 6E10 (B) or rabbit antibody specific to the N-terminus of Aβ peptide (C).
Table 1. CD4+ T cell epitopes forming the Th epitope string
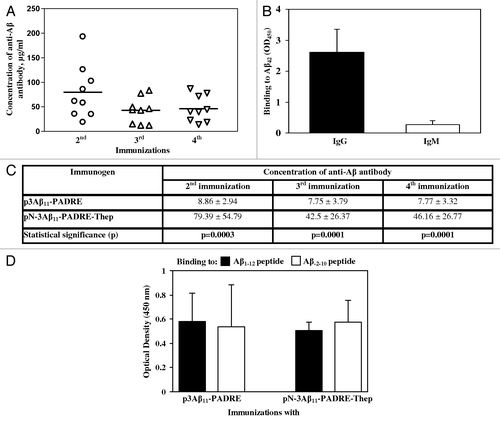
We first verified that the protein encoded by pN-3Aβ11-PADRE-Thep, designated as AV-1955 is expressed and the signal sequence is cleaved appropriately. CHO cells were transfected with this plasmid and the expression was evaluated by IP/WB. The control construct was p3Aβ11-PADRE-Thep that upon secretion contains eight extra amino acids at the N-terminus (). The primary antibodies in WB were commercial 6E10 anti-Aβ monoclonal antibody that recognizes amino acid residues 3–8, or rabbit anti-Aβ free N-terminus specific polyclonal antibodies (sera was prepared in Dr. Cribbs’ laboratory, UCI). As shown in , 6E10 antibody bound to both peptides: 3Aβ11-PADRE-Thep and N-3Aβ11-PADRE-Thep, whereas rabbit anti-Aβ N-terminus specific antibody recognized only N-3Aβ11-PADRE-Thep (), demonstrating that signal sequence cleavage produced a protein with a free aspartic acid at the one position of Aβ. As anticipated, anti-mouse () but not anti-rabbit secondary antibody () recognized heavy and light chains of mouse 6E10 Abs used for IP.Animals immunized twice with AV-1955 induced high concentrations of anti-Aβ antibodies in all 9 rabbits. The third and fourth immunizations with AV-1955 caused a modest reduction of the anti-Aβ antibody concentrations although the results were not significantly different in comparison to two immunizations (). Of note, AV-1955 immunizations induced production of anti-Aβ antibodies of IgG isotype indicating that humoral response is T helper cell dependent (). The immunogenicity of AV-1955 was significantly higher (p ≤ 0.001) than that of parental p3Aβ11-PADRE vaccine after 2nd, 3rd, 4th immunizations ().
Figure 3. (A) The DNA construct possessing free aspartic acid at the N-terminus and additional Th epitopes, AV-1955, induced high level of antibody after two, three and four immunizations. Lines indicate the mean (n = 9). (B) All animals immunized two times with AV-1955 produced anti-Aβ antibodies of IgG isotype. IgG and IgM isotypes of antibodies were analyzed in individual sera of immunized animals at dilution 1:200. Error bars indicate SD (n = 9). (C) Average data (mean value ± SD) of the concentration of antibodies generated in all rabbits in each group, i.e., n = 14 rabbits vaccinated with p3Aβ11-PADRE and n = 9 rabbits vaccinated with AV-1955 are presented. (D) Sera from rabbits vaccinated with either p3Aβ11-PADRE or AV-1955 bound equally to peptides possessing free or hidden N-terminal aspartic acid.
Characterization of anti-Aβ11 antibody binding to Aβ42 monomeric and aggregated species
We believe that the AV-1955 vaccine could be more beneficial than p3Aβ11-PADRE because it should activate not only naïve T cells that are reduced in the elderly but also memory Th cells, to thus generate strong cellular responses in virtually all vaccinated individuals. Accordingly, we further characterized the antibodies generated in rabbits by this more promicing AV-1955 vaccine.
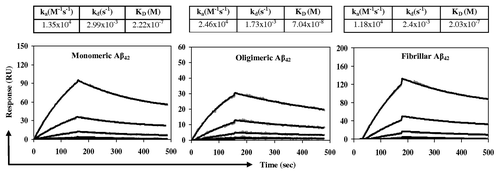
One of the most important characteristics of therapeutically potent anti-Aβ antibodies is their ability to recognize the aggregated pathological forms of Aβ42 peptide.Citation18 We used SPR based assay for determination the binding capability of purified anti-Aβ antibodies generated after immunizations with AV-1955 to different Aβ species. Monomeric, oligomeric and fibrillar states of Aβ42 peptide were detected by EM and by dot blot using mouse monoclonal 6E10 antibody specific to all forms of Aβ42, oligomer specific rabbit A11 antibodies, monomer and fibril specific rabbit OC antibodies (data not shown).Citation18-Citation20 We demonstrated that anti-Aβ antibodies bound to monomeric and fibrillar forms of amyloid similarly, while binding to oligomeric Aβ42 was much stronger (). Dissociation constants (KD) of anti-Aβ11/peptide complexes for oligomeric, monomeric and fibrillar Aβ42 were 7.04 × 10-8 M, 2.22 × 10-7 M and 2.03 × 10-7 M, respectively. Of note, irrelevant rabbit IgG interacted with Aβ42 peptide nonspecifically (data not shown).
Figure 4. Rabbit anti-Aβ11 antibodies bind to Aβ42 monomeric, oligomeric, or fibrillar forms as measured using the Biacore. Different species of Aβ42 peptides were immobilized on the surface of biosensor chip CM5 and purified rabbit anti-Aβ11 antibody were run over each immobilized form of peptide. The kinetics of binding/dissociation was measured as change of the SPR signal using BIAevaluation 4.1.1 software. The gray dots represent individual data points, while the black lines represent fitted curves.
Ex vivo functional characteristics of anti-Aβ antibodies generated by AV-1955
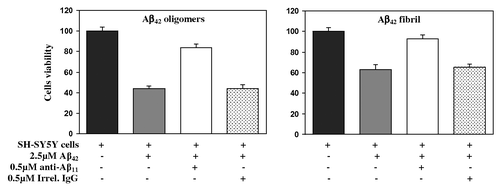
A critical feature of functional anti-Aβ antibodies is inhibition of the toxicity of Aβ42 oligomers and fibrils. To test the protective effect of the rabbit anti-Aβ antibodies generated in response to AV-1955 on Aβ-induced neurotoxicity, we performed an in vitro assessment using human neuroblastoma SH-SY5Y cells as targets. The data showed that both Aβ42 fibrils and oligomers are cytotoxic, reducing cell viability to about 63% and 44%, respectively (). Pre-incubation of Aβ42 fibrils with rabbit anti-Aβ42 antibodies isolated from sera of rabbits vaccinated with AV-1955 rescued the cell viability to approximately 93%. Similarly, pre-incubation of Aβ42 oligomers with rabbit anti-Aβ11 antibody increased cell viability to approximately 83.7% whereas an irrelevant rabbit antibody (control) did not affect cell survival. Of note, purified antibodies had no effect on the viability of SH-SY5Y cells (data not shown).
Figure 5. Rabbit anti-Aβ11 antibodies inhibit Aβ42 fibrils- and oligomer-mediated neurotoxicity. Human neuroblastoma SH-SY5Y cells were incubated with Aβ42 oligomers and fibrils, in the presence or absence of anti-Aβ11 antibody or irrelevant rabbit IgG. Control cells were treated with the vehicle, and cell viability was assayed in all cultures using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data were collected (four replicates) and were expressed as percentages of control ± s.d.
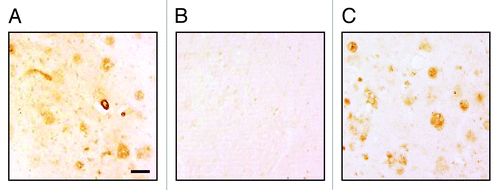
To investigate the functional potential of antibodies generated after immunizations of rabbits with AV-1955, we analyzed binding of immune sera to Aβ plaques in the brain tissue from an AD case. As shown in , the serum from vaccinated rabbits bound to amyloid-β plaques and this binding was specific to Aβ since it was blocked by pre-absorption of antisera with Aβ42 peptide (). Anti-Aβ monoclonal antibody, 6E10 was used as a positive control (). Sera collected from the same rabbits prior to immunization did not bind to the AD brain tissue (data not shown). Collectively, these results suggest that AV-1955 vaccination of rabbits generates potentially functional anti-Aβ11 antibodies that inhibit Aβ42-mediated neurotoxicity.
Figure 6. (A) Rabbit immune sera generated after three immunizations with AV-1955 (at dilution 1:250) bound to the 40 μm brain sections of cortical tissues from a severe AD case. (B) Binding of sera to amyloid plaques was blocked by pre-absorption of the sera with 2.5 μM Aβ42 peptide. (C) Anti-Aβ MoAb, 6E10 was used as a positive control. The original magnification is 10× and the scale bar is 100 μm.
Discussion
DNA-based vaccination provides a unique method of vaccination,Citation21 exhibiting properties that may be advantageous for the development of vaccines against a variety of pathogens, as well as for human diseases including cancer, autoimmune disorders and neurological disorders, such as AD and Parkinson disease (PD). A unique property of DNA-based vaccination over peptide and recombinant protein vaccines is the ability to induce prolonged, endogenous antigen synthesis and processing within the patient’s own cells. DNA immunization has been shown to generate protective humoral and cellular immune responses against multiple viral, bacterial and tumor antigens.Citation22-Citation27 This approach also permits inactivation or removal of sequences encoding potentially toxic protein domains, while allowing the inclusion of molecular adjuvants such as cytokines to direct the appropriate T helper cell responses.Citation9,Citation28,Citation29 Previously we reported that a DNA vaccine delivered with a gene gun generated very strong antibody responses specific to N-terminus of Aβ, reduced amyloid plaques and soluble Aβ in the brains of vaccinated 3xTg-AD mice without increasing glial activation and incidence of microhemorrhages, and prevented the development of cognitive deficits in mice. Of note, the DNA vaccine did not generate Aβ-specific autoreactive T cell responses.Citation9 In this report, we demonstrated the immunogenicity and efficacy of a novel DNA-based AD vaccines that was tailored for enhanced immunogenicity over the p3Aβ11-PADRE DNA vaccine.Citation9,Citation29,Citation30 To assess the potential clinical applicability of these DNA epitope vaccines, we evaluated the responses to vaccination in rabbits, a larger animal model that is expected to be more relevant for translation to human clinical studies. Successful translation of a DNA vaccine to the clinical setting requires a suitable method for effective intracellular delivery such as gene gun and electroporation system that are currently tested in clinical trials.Citation31-Citation33 Hence we immunized rabbits with our second-generation DNA epitope vaccine using the TriGrid system, which induces significantly higher immune responses compared with immunization with conventional syringe.Citation30 However, the level of humoral immune responses induced by p3Aβ11-PADRE in rabbits () was significantly lower than in mice immunized with the same p3Aβ11-PADRE epitope vaccine via TriGrid system (data not shown). In order to enhance the immunogenicity, the third generation vaccine described in this report, AV-1955, was designed by modifying p3Aβ11-PADRE. First modification was reasoned that the immunogenicity of p3Aβ11-PADRE vaccine could be enhanced by addition of eight promiscuous Th epitopes to PADRE (). These Th epitopes were selected based on their ability to be recognized by different human MHC class II molecules and are present in the conventional vaccines used in public health programs.Citation34-Citation39 We reasoned that these new Th epitopes could enhance immune responses to the AD epitope vaccine in humans by stimulating memory responses to the foreign Th epitopes that individuals are commonly exposed to through vaccination or natural infection. Next modification was based on published reports that the free N-terminal aspartic acid of Aβ42 may be essential for induction of functional anti-Aβ humoral immune responses.Citation15-Citation17 Accordingly, we altered p3Aβ11-PADRE-Thep such that the first copy of the Aβ B-cell epitope possesses a free N-terminal aspartic acid after signal sequence cleavage (). The feasibility of AV-1955 vaccine delivered by TriGrid system was tested in rabbits and in comparison to the p3Aβ11-PADRE vaccine. Analysis of the kinetics of antibody responses after immunization of rabbits with p3Aβ11-PADRE and AV-1955 showed that AV-1955 vaccine induced significantly higher anti-Aβ42 antibodies after each immunization (). However, antibody responses declined after the third immunization in both vaccine groups. It is not surprising, because the antibody dose-response curve is a typical sigmoid curve with four phases: no immune responses, exponential growth, plateau phase and decline phase. The inhibition of antibody responses through various immunosuppressive mechanisms is important for the regulation of “uncontrolled” expansion of activated immune cells (including B cells activated after vaccination).Citation40 The level of such immunosuppression is usually correlated with the strength of antibody responses. Thus it was not unexpected that antibody responses declined steeper in the case of the more immunogenic AV-1955 vaccine than in p3Aβ11-PADRE ().
The antibodies generated in response to AV-1955 vaccination bound to different species of Aβ42 peptide: the affinity of binding with oligomers (KD = 7.04 × 10−8M) was higher than binding to monomers (KD = 2.22 × 10−7) or fibrils (KD = 2.03 × 10−7) (). Currently, the consensus is that Aβ oligomers of various sizes are the most pathologic forms of Aβ42 peptide responsible for disrupting neuronal functions and inducing cognitive decline in AD.Citation41-Citation44 Thus, anti-Aβ11 antibodies could be effective for prevention of Aβ42 aggregate formation or their removal from the brains regardless of nature of the aggregated species.
An important feature of anti-Aβ42 antibody is inhibition of cytotoxic effects of Aβ42 oligomers and fibrils on a human neuroblastoma cells and the ex vivo binding to β-amyloid plaques in AD human brain tissues. Here, we showed the therapeutic potential of anti-Aβ antibodies purified from immune rabbit sera in a neurotoxicity assay performed with SH-SY5Y neuroblastoma cell line. As expected based on published results,Citation18 Aβ42 fibrils and oligomers were cytotoxic and pre-incubation of these toxic forms of Aβ42 with antibodies rescued SH-SY5Y cells viability (). Thus, our data demonstrate that the AV-1955 vaccine induces production of antibodies in rabbits that are capable of neutralizing the toxicity of Aβ-oligomers and fibrils in in vitro cellular assay. Next, we demonstrated that immune sera from rabbits immunized with AV-1955 vaccine are capable of binding to amyloid plaques in the brain sections of an AD case (). Importantly, this binding was specific to Aβ since it was completely blocked by their pre-absorption of immune sera with Aβ42 peptide ().
Collectively, the data presented in this report demonstrated that the AV-1955 vaccine delivered by the TriGrid system induced rapid and robust anti-Aβ42 antibody production in rabbits and these antibodies have therapeutic potential as indicated in ex vivo and in vitro assays. Accordingly, based on these results, our multidisciplinary team is currently evaluating the AV-1955 epitope vaccine delivered by EP in Rhesus macaques with the aim to begin a DNA vaccine clinical trial in AD patients.
Limitations
One important question is associated with the safety of our AV-1955 vaccine. The entire concept of an epitope AD vaccine is based on a simple hypothesis: pro-inflammatory immune responses cannot be harmful to humans if they are not directed to a self-antigen (for example to Aβ in AN1792 trial).Citation45,Citation46 Effector T cells specific to epitopes incorporated into our third-generation DNA vaccine are specific to foreign antigens from TT, Flu, HBV or to synthetic peptide, PADRE, and therefore no autoreactive cellular immune responses could be generated. Of note in this study we did not attempt to detect cellular immune responses to amyloid in rabbits immunized with AV-1955 or p3Aβ11-PADRE DNA vaccines due to the absence of IL-4 or/and IFNγ-ELISPOT kits for rabbits. However, we should mention that we recently tested the AV-1955 vaccine in monkeys and preliminary data suggest that this vaccine induced robust Th cell responses specific only to Th epitopes incorporated into the vaccine design. Thus, we believe that the AV-1955 vaccine will not induce harmful autoreactive Th cells in humans. Nevertheless, the safety of the AV-1955 vaccine should be directly assessed in clinical trials; additionally, there should be an opportunity to learn more about safety and efficacy of similar types of vaccines from Novartis, Merck, United Biomedical and Wyeth that are currently being tested in AD patients (http://clinicaltrials.gov)
Materials and Methods
Rabbits
Female New Zealand white rabbits weighting between 3.0 and 3.5 kg were utilized for these studies and were housed at Absorption Systems (San Diego). These experiments were approved by Absorption Systems’ Institutional Animal Care and Use Committee according to NIH guidelines.
DNA constructs
The construction strategy of p3Aβ11-PADRE was described previously.Citation9,Citation28 In this study, 3Aβ11-PADRE coding regions were sub-cloned into the pVAX1 vector (Life Technologies; ). Additionally, we prepared 2 new constructs outlined in . A polynucleotide encoding multiple T helper epitopes separated by GS linkers (Thep, ) was synthesized by GenScript Company and ligated with the 3Aβ11-PADRE minigene. The region coding the extra amino acids localized between signal sequence cleavage site and the first copy of Aβ11 peptide was removed using an overlapping PCR technique exactly as described previously.Citation9,Citation28
Immunizations
Vaccine delivery was performed by intramuscular administration of 0.5 ml (1mg/ml) plasmid DNA using Ichor's TDS-IM technology as previously reported.Citation47 Rabbits were immunized four times biweekly and blood was collected 12–14 d after each immunization.
Detection of anti-Aβ antibody responses by ELISA
The concentrations of anti-Aβ antibodies were determined by ELISA as described.Citation29,Citation48 Plates were coated with monomeric Aβ42 peptide (2.5µM; American Peptide Company) and HRP-conjugated anti-rabbit IgG (1:5000; Pierce) was used as a secondary antibody. The optical density (OD) was read at 450 nm (Biotek), and antibody concentrations in serially diluted sera (1:100, 1:500, 1:2500 and 1:12500) were calculated using a calibration curve (ranged from 0.15 to 200 ng) generated with purified rabbit polyclonal antibody recognizing N-terminal region (aa 1–17) of Aβ (GenScript). The concentration of antibody was determined using the dilution that gave OD in the linear portion of concentration curve. The isotypes of anti-Aβ antibodies were detected in sera from experimental rabbits at dilution 1:200 to be able to detect even low titers of given isotype. HRP-conjugated anti-rabbit IgG and IgM (both from Bethyl Laboratories, Inc.) as secondary antibodies at the dilution 1:10,000 were used.
Additional ELISA was performed to detect the binding of antibodies to Aβ1–12 (DAEFRHDSGYEV; Genscript) and Aβ-2–10 (KMDAEFRHDSGY; GenScript) peptides. Plates were coated with 10µg of each peptide. Diluted sera from immunized rabbits containing 1µg antibody in a volume of 100µl (concentration of antibodies was determined as described above) were added into wells. HRP-conjugated anti-rabbit IgG (1:5000; Pierce) was used for detection of bound anti-Aβ antibodies.
ELISAs were repeated three times. The inter-assay variability of ELISA was 5–8%. Data from one ELISA was presented.
Immunoprecipitation and western blotting
The expression of generated constructs and secretion of the proteins were detected in the supernatant of transiently transfected CHO cells by IP/WB as described previously.Citation9,Citation28 Proteins were immunoprecipitated from the conditioned medium (CM) using 6E10 monoclonal antibody (Covance) specific to 3–8 aa of Aβ peptide, separated by 10% Bis-Tris gel electrophoresis (Life Technologies) and transferred onto a nitrocellulose membrane. Proteins were visualized by incubating with monoclonal antibody 6E10 followed by HRP-conjugated anti-mouse IgG (Santa Cruz Biotechnology) or rabbit antibody specific to the free N-terminus of Aβ peptide followed by HRP-conjugated anti-rabbit IgG (Santa Cruz Biotechnology). Antibody specific to the Aβ free N-terminus was generated in rabbits and affinity purified by Dr. Cribbs’ group at UCI. This antibody was specific to Aβ1–15 and Aβ1–42 but did not bind to peptides with hidden or truncated aspartic acid (data not shown).
Purification of anti-Aβ11 antibodies
Anti-Aβ11 antibodies were purified from sera of rabbits immunized with the AV-1955 epitope vaccine by an affinity column (SulfoLink, Pierce) using an immobilized Aβ18-C peptide (GenScript) as we previously described.Citation18 Purified antibodies were analyzed via 10% Bis-Tris gel (Life Technologies), and the concentrations were determined using a BCA protein assay kit (Pierce).
Surface Plasmon Resonance (SPR) analysis
Binding studies were performed on the BIAcore 3000 SPR platform (GE Healthcare) as described previously.Citation18 Monomeric, oligomeric and fibrillar forms of Aβ42 peptides were prepared as described previously and confirmed by Electron Microscopy (EM) and binding of these peptides to 6E10, A11 and OC antibodies by dot blot.Citation18-Citation20 All forms of Aβ42 peptides were immobilized to the surface of biosensor chip CM5 (GE Healthcare) via an amine coupling to carboxyl groups in the dextran matrix of the chip. Serial dilutions of purified rabbit anti-Aβ11 antibody or irrelevant rabbit IgG (666.6, 222.2, 74.1, 24.7, 8.23, 2.74 nM) in the running buffer containing 10 mM HEPES, 150 mM NaCl, 0.05% surfactant P20, pH 7.4 (GE Healthcare), were injected at 20 μl/min over each immobilized form of peptide, and the kinetics of binding/dissociation was measured as change of the SPR signal (in resonance units (RU)). Each injection was followed by a regeneration step of a 25 sec pulse of 1 M NaCl, 50 mM NaOH. Fitting of experimental data was done with BIAevaluation 4.1.1 software using 1:1 interaction model to determine apparent binding constants.
Neurotoxicity assay
A cell culture MTT assay was performed exactly as described previously,Citation49 except that purified rabbit anti-Aβ11 antibodies were used, incubation time of cells with peptide and peptide/Abs complex was 48h and final concentrations of peptide and antibodies were 2.5 μM and 0.5 μM, respectively.
Detection of Aβ plaques in human brain tissues
Sera from immunized rabbits were screened for the ability to bind to human Aβ plaques using 40 μm brain sections of formalin-fixed cortical tissue from an AD case (received from the BBT Repository, MIND, UCI) using immunohistochemistry as we previously described.Citation29,Citation46 As a secondary antibody, anti-rabbit IgG was used (Jackson ImmunoResearch Laboratories). A digital camera (Olympus) was utilized to capture images of the plaques at a 10× magnification. The binding of anti-Aβ11 sera to the amyloid plaques was blocked by 2.5 μM of Aβ42 peptide as we described.Citation29,Citation46
Statistical analysis
Statistical parameters [mean, standard deviation (s.d.), significant differences] were calculated using Prism 5.01 software (GraphPad Software, Inc.). Statistically significant differences were examined using a two-tailed t-test or analysis of variance (ANOVA) and Tukey's multiple comparisons post-test (a p value of less than 0.05 was considered significant).
Acknowledgments
We would like to thank A. Poghosyan, B. Ellefsen, M. Valenzuela, T. Marquez and L. Chau for technical help. We also thank Dr Annette Marleau, Dr Claire F. Evans and Drew Hannaman for help with editing and valuable comments. This work was supported by funding from NIH (NS-50895, NS-065518, AG-20241 and NS-057395). H.D. and N.M. were supported by NIA T32 training grant (AG000096). Additional support for AD case tissues was provided by University of California, Irvine Alzheimer Disease Research Center Grant P50 AG16573.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med 2003; 9:448 - 52; http://dx.doi.org/10.1038/nm840; PMID: 12640446
- Ferrer I, Boada Rovira M, Sánchez Guerra ML, Rey MJ, Costa-Jussá F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol 2004; 14:11 - 20; http://dx.doi.org/10.1111/j.1750-3639.2004.tb00493.x; PMID: 14997933
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003; 61:46 - 54; http://dx.doi.org/10.1212/01.WNL.0000073623.84147.A8; PMID: 12847155
- Town T, Tan J, Flavell RA, Mullan M. T-cells in Alzheimer’s disease. Neuromolecular Med 2005; 7:255 - 64; http://dx.doi.org/10.1385/NMM:7:3:255; PMID: 16247185
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al, AN1792(QS-21)-201 Study Team. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005; 64:1553 - 62; http://dx.doi.org/10.1212/01.WNL.0000159740.16984.3C; PMID: 15883316
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 2008; 372:216 - 23; http://dx.doi.org/10.1016/S0140-6736(08)61075-2; PMID: 18640458
- Candore G, Balistreri CR, Colonna-Romano G, Grimaldi MP, Lio D, Listi’ F, et al. Immunosenescence and anti-immunosenescence therapies: the case of probiotics. Rejuvenation Res 2008; 11:425 - 32; http://dx.doi.org/10.1089/rej.2008.0662; PMID: 18442326
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 2008; 46:1078 - 84; http://dx.doi.org/10.1086/529197; PMID: 18444828
- Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, et al. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy. PLoS One 2008; 3:e2124; http://dx.doi.org/10.1371/journal.pone.0002124; PMID: 18461171
- Bureau MF, Gehl J, Deleuze V, Mir LM, Scherman D. Importance of association between permeabilization and electrophoretic forces for intramuscular DNA electrotransfer. Biochim Biophys Acta 2000; 1474:353 - 9; http://dx.doi.org/10.1016/S0304-4165(00)00028-3; PMID: 10779687
- Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol 2000; 164:4635 - 40; PMID: 10779767
- Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, et al. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci U S A 1999; 96:6417 - 22; http://dx.doi.org/10.1073/pnas.96.11.6417; PMID: 10339602
- Evans C, Hannaman D, eds. Current status of electroporation technologies for vaccine delivery. New York, NY: Springer.
- Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One 2011; 6:e19252; http://dx.doi.org/10.1371/journal.pone.0019252; PMID: 21603651
- Lee M, Bard F, Johnson-Wood K, Lee C, Hu K, Griffith SG, et al. Abeta42 immunization in Alzheimer’s disease generates Abeta N-terminal antibodies. Ann Neurol 2005; 58:430 - 5; http://dx.doi.org/10.1002/ana.20592; PMID: 16130106
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, et al. Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol 2004; 165:283 - 97; http://dx.doi.org/10.1016/S0002-9440(10)63296-8; PMID: 15215183
- Cribbs D, Head E, Glabe C, Vasilevko V. Conformational and liner specific antibodies in aged beagles after prolonged vaccination with aggregated Abeta formulated in Alum. 9th International Conferance AD/PD. Prague, Czech Republic, 2009, March 11-15.
- Mamikonyan G, Necula M, Mkrtichyan M, Ghochikyan A, Petrushina I, Movsesyan N, et al. Anti-A beta 1-11 antibody binds to different beta-amyloid species, inhibits fibril formation, and disaggregates preformed fibrils but not the most toxic oligomers. J Biol Chem 2007; 282:22376 - 86; http://dx.doi.org/10.1074/jbc.M700088200; PMID: 17545160
- Kayed R, Canto I, Breydo L, Rasool S, Lukacsovich T, Wu J, et al. Conformation dependent monoclonal antibodies distinguish different replicating strains or conformers of prefibrillar Aβ oligomers. Mol Neurodegener 2010; 5:57; http://dx.doi.org/10.1186/1750-1326-5-57; PMID: 21144050
- Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener 2007; 2:18; http://dx.doi.org/10.1186/1750-1326-2-18; PMID: 17897471
- Donnelly JJ, Liu MA, Ulmer JB. Antigen presentation and DNA vaccines. Am J Respir Crit Care Med 2000; 162:S190 - 3; PMID: 11029393
- Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature 1992; 356:152 - 4; http://dx.doi.org/10.1038/356152a0; PMID: 1545867
- Agadjanyan MG, Ugen K, Wang B, Villafana T, Merva M, Petrushina I, et al. DNA inoculation with an HTLV-I envelope DNA construct elicits immune responses in rabbits. In: Chanock RM, Ginsberg, H.S., Brown, F., Lerner, R.A., ed. Vaccines '94: Modern Approaches to New Vaccines Including Prevention of AIDS. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1994:47-53.
- Pardoll DM, Beckerleg AM. Exposing the immunology of naked DNA vaccines. Immunity 1995; 3:165 - 9; http://dx.doi.org/10.1016/1074-7613(95)90085-3; PMID: 7648389
- Wang B, Ugen KE, Srikantan V, Agadjanyan MG, Dang K, Refaeli Y, et al. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A 1993; 90:4156 - 60; http://dx.doi.org/10.1073/pnas.90.9.4156; PMID: 8483929
- Boyer JD, Ugen KE, Wang B, Agadjanyan M, Gilbert L, Bagarazzi ML, et al. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. [see comments] Nat Med 1997; 3:526 - 32; http://dx.doi.org/10.1038/nm0597-526; PMID: 9142121
- Biragyn A, Belyakov IM, Chow YH, Dimitrov DS, Berzofsky JA, Kwak LW. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood 2002; 100:1153 - 9; http://dx.doi.org/10.1182/blood-2002-01-0086; PMID: 12149191
- Movsesyan N, Mkrtichyan M, Petrushina I, Ross TM, Cribbs DH, Agadjanyan MG, et al. DNA epitope vaccine containing complement component C3d enhances anti-amyloid-beta antibody production and polarizes the immune response towards a Th2 phenotype. J Neuroimmunol 2008; 205:57 - 63; http://dx.doi.org/10.1016/j.jneuroim.2008.08.016; PMID: 18838175
- Davtyan H, Mkrtichyan M, Movsesyan N, Petrushina I, Mamikonyan G, Cribbs DH, et al. DNA prime-protein boost increased the titer, avidity and persistence of anti-Abeta antibodies in wild-type mice. Gene Ther 2010; 17:261 - 71; http://dx.doi.org/10.1038/gt.2009.140; PMID: 19865176
- Davtyan H, Ghochikyan A, Movsesyan N, Ellefsen B, Petrushina I, Cribbs DH, et al. Delivery of a DNA vaccine for Alzheimer’s disease by electroporation versus gene gun generates potent and similar immune responses. Neurodegener Dis 2012; 10:261 - 4; http://dx.doi.org/10.1159/000333359; PMID: 22301697
- Jones S, Evans K, McElwaine-Johnn H, Sharpe M, Oxford J, Lambkin-Williams R, et al. DNA vaccination protects against an influenza challenge in a double-blind randomised placebo-controlled phase 1b clinical trial. Vaccine 2009; 27:2506 - 12; http://dx.doi.org/10.1016/j.vaccine.2009.02.061; PMID: 19368793
- van Drunen Littel-van den Hurk S, Hannaman D. Electroporation for DNA immunization: clinical application. Expert Rev Vaccines 2010; 9:503 - 17; http://dx.doi.org/10.1586/erv.10.42; PMID: 20450325
- Huang CF, Monie A, Weng WH, Wu TC. DNA vaccines for cervical cancer. Am J Transl Res 2010; 2:75 - 87; PMID: 20182584
- Baraldo K, Mori E, Bartoloni A, Petracca R, Giannozzi A, Norelli F, et al. N19 polyepitope as a carrier for enhanced immunogenicity and protective efficacy of meningococcal conjugate vaccines. Infect Immun 2004; 72:4884 - 7; http://dx.doi.org/10.1128/IAI.72.8.4884-4887.2004; PMID: 15271954
- Demotz S, Barbey C, Corradin G, Amoroso A, Lanzavecchia A. The set of naturally processed peptides displayed by DR molecules is tuned by polymorphism of residue 86. Eur J Immunol 1993; 23:425 - 32; http://dx.doi.org/10.1002/eji.1830230219; PMID: 7679644
- Alexander J, Fikes J, Hoffman S, Franke E, Sacci J, Appella E, et al. The optimization of helper T lymphocyte (HTL) function in vaccine development. Immunol Res 1998; 18:79 - 92; http://dx.doi.org/10.1007/BF02788751; PMID: 9844827
- Alexander J, del Guercio MF, Maewal A, Qiao L, Fikes J, Chesnut RW, et al. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J Immunol 2000; 164:1625 - 33; PMID: 10640784
- Greenstein JL, Schad VC, Goodwin WH, Brauer AB, Bollinger BK, Chin RD, et al. A universal T cell epitope-containing peptide from hepatitis B surface antigen can enhance antibody specific for HIV gp120. J Immunol 1992; 148:3970 - 7; PMID: 1376346
- James EA, Bui J, Berger D, Huston L, Roti M, Kwok WW. Tetramer-guided epitope mapping reveals broad, individualized repertoires of tetanus toxin-specific CD4+ T cells and suggests HLA-based differences in epitope recognition. Int Immunol 2007; 19:1291 - 301; http://dx.doi.org/10.1093/intimm/dxm099; PMID: 17906339
- O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med 2004; 10:801 - 5; http://dx.doi.org/10.1038/nm0804-801; PMID: 15286781
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006; 440:352 - 7; http://dx.doi.org/10.1038/nature04533; PMID: 16541076
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci 2005; 8:79 - 84; http://dx.doi.org/10.1038/nn1372; PMID: 15608634
- Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, et al. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem 2006; 281:4292 - 9; http://dx.doi.org/10.1074/jbc.M511018200; PMID: 16361260
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, et al. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med 2005; 11:556 - 61; http://dx.doi.org/10.1038/nm1234; PMID: 15834427
- Ghochikyan A. Rationale for peptide and DNA based epitope vaccines for Alzheimer’s disease immunotherapy. CNS Neurol Disord Drug Targets 2009; 8:128 - 43; http://dx.doi.org/10.2174/187152709787847298; PMID: 19355933
- Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, et al. Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol 2005; 174:1580 - 6; PMID: 15661919
- Luxembourg A, Hannaman D, Ellefsen B, Nakamura G, Bernard R. Enhancement of immune responses to an HBV DNA vaccine by electroporation. Vaccine 2006; 24:4490 - 3; http://dx.doi.org/10.1016/j.vaccine.2005.08.014; PMID: 16140436
- Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, et al. Alzheimer’s disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Abeta species in amyloid precursor protein transgenic mice. J Neurosci 2007; 27:12721 - 31; http://dx.doi.org/10.1523/JNEUROSCI.3201-07.2007; PMID: 18003852
- Davtyan H, Ghochikyan A, Cadagan R, Zamarin D, Petrushina I, Movsesyan N, et al. The immunological potency and therapeutic potential of a prototype dual vaccine against influenza and Alzheimer’s disease. J Transl Med 2011; 9:127; http://dx.doi.org/10.1186/1479-5876-9-127; PMID: 21806809