Abstract
One of the problems in antigen-specific cancer immunotherapy is the low density of the tumor antigen-derived peptide endogenously presented on tumor cell surface major histocompatibility complex class I molecules. To overcome this, we are engaged in research on peptide intra-tumor injection to enhance tumor cell antigenicity. In in vivo studies using immunodeficient mice, the peptide injected into a solid mass of subcutaneous tumor was revealed to be loaded onto human leukocyte antigen class I molecules of tumor cells. In a peptide vaccine model and an adoptive cell transfer model using C57BL/6 mice, peptide intra-tumor injection was effective in terms of tumor growth inhibition and prolongation of survival time. Moreover, an antigen-spreading effect was detected after peptide intra-tumor injection. Peptide intra-tumor injection is an effective method of enhancing tumor cell antigenicity. It can induce additional peptide loading onto tumor cells, making tumor cells more antigenic for specific cytotoxic T-lymphocyte activity. Peptide intra-tumor injection may be a useful option for improvement of antigen-specific immunotherapy against solid tumors.
Introduction
Antigen-specific cancer immunotherapy is a potentially attractive cancer treatment modality because the induction of tumor-specific reactions without autoimmunity is the ideal strategy. In antigen-specific cancer immunotherapy, antigen-specific cytotoxic T lymphocytes (CTLs) recognize and destroy tumor cells that present tumor antigen-derived peptides using cell surface major histocompatibility complex (MHC) class I molecules. However, the density of the peptide endogenously presented on tumor cells is generally not sufficiently high, which may explain why most studies on antigen-specific cancer immunotherapy did not demonstrate a remarkable clinical benefit.Citation1
To develop an effective method of antigen-specific cancer immunotherapy, many researchers have fixed their attention on the effector cell, the CTL. Many reports of induction of high-avidity CTLs have been published.Citation2-Citation5 On the other hand, few reports on enhancement of tumor cell antigenicity, another attractive strategy, are extant. Here, we summarize our investigations of additional peptide loading onto tumor cells for enhancement of tumor cell antigenicity.
Peptide Intra-Tumor Injection for Enhancing Tumor Cell Antigenicity
To induce additional peptide loading onto MHC class I molecules of tumor cells, we performed peptide intra-tumor injection.Citation6
In in vivo studies using immunodeficient mice, glypican-3144–152 (FVGEFFTDV) peptide was injected into a solid subcutaneous SW620 tumor mass (xenograft) that did not express glypican-3. After several hours, the tumors were dissected and digested with collagenase. The isolated SW620 tumor cells were used as the target cells, and glypican-3144–152-specific CTLs were used as the effector cells in an interferon-γ (IFN-γ) enzyme-linked immunospot (ELISPOT) assay. Loading of the injected glypican-3144–152 peptide onto human leukocyte antigen (HLA) class I molecules of SW620 tumor cells was detected.
In a peptide vaccine model and an adoptive cell transfer model using C57BL/6 mice, intra-tumor injection of ovalbumin257–264 peptide (SIINFEKL) had significant therapeutic effects in terms of tumor growth inhibition and prolongation of survival in RMA tumors, which do not express ovalbumin. These results suggest that peptide intra-tumor injection enhanced CTL activity. Moreover, an antigen-spreading effect after peptide intra-tumor injection was revealed by a second tumor challenge test and an IFN-γ ELISPOT assay.
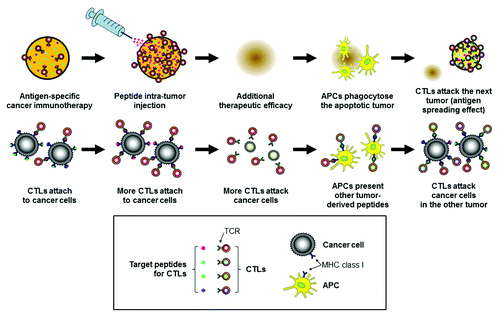
Peptide intra-tumor injection is an effective method of enhancing tumor cell antigenicity. It can induce additional peptide loading onto tumor cells, making tumor cells more antigenic for specific CTL activity. Moreover, it can induce an antigen-spreading effect, which is a great advantage of immunotherapy. Peptide intra-tumor injection may be a useful option for improvement of antigen-specific immunotherapy against solid tumors ().
Figure 1. A proposed mechanistic model of peptide intra-tumor injection for improved antigen-specific cancer immunotherapy against solid tumors. The upper row shows a general view of a tumor, and the lower row shows greater detail. The peptide injected into the tumor is shown in red in this schematic diagram. CTL, cytotoxic T lymphocyte. APC, antigen-presenting cell. TCR, T-cell receptor. MHC, major histocompatibility complex. Adapted from Nobuoka et al.Citation6
HLA Class I Expression Within and Outside of a Tumor
One limitation of peptide intra-tumor injection is that it requires the presence of MHC class I molecules. The potential loss of MHC class I expression in tumors would theoretically lead to the failure of this approach. On the other hand, if MHC class I expression is high in normal cells around the peptide-injected tumor, peptides that miss the target will be loaded onto MHC class I molecules of normal cells, which would be attacked by CTLs.
We performed immunohistochemical staining of HLA class I using EMR 8–5, a monoclonal anti-pan HLA class I heavy-chain antibody, in resected specimens of various cancers, including hepatocellular carcinoma, intrahepatic cholangiocarcinoma, carcinoma of the ampulla of Vater, gallbladder cancer, pancreatic cancer, esophageal cancer, colorectal cancer and breast cancer. Although previous reports have shown HLA class I downregulation, particularly in breast cancer,Citation7-Citation9 our results revealed that most of the tumors exhibited high expression of HLA class I molecules and that the expression within the tumor area was higher than that outside (unpublished data).
We also performed a peptide-loading test using a fresh tissue sample from a patient who underwent hepatectomy for hepatocellular carcinoma. The tumor and nontumorous areas were divided and digested with collagenase. The obtained cancer cells and nontumorous hepatocytes were pulsed with cytomegalovirus341–349 (QYDPVAALF) peptide. To compare the peptide loading levels, an IFN-γ ELISPOT assay was performed using these peptide-pulsed human tissue samples as the target cells. The results revealed that less cytomegalovorus341–349 peptide had been loaded onto HLA class I molecules of nontumorous hepatocytes compared with cancer cells (unpublished data). These results suggest that CTLs are less likely to harm normal cells around the peptide-injected tumor because expression of HLA class I molecules in normal cells is low in most patients.
Development of another Method for Enhancing Tumor Cell Antigenicity
Another limitation of peptide intra-tumor injection is its delivery method. A small tumor is not applicable because it cannot be detected by imaging modalities and cannot be approached using a needle. A large tumor is also not applicable because the injected peptide cannot spread throughout the tumor. Establishment of a novel selective peptide delivery method to tumor cells by a systemic route may in future occur due to advances in drug-delivery technologies. Such a development would result in enhancement of tumor cell antigenicity becoming more suitable for clinical application.
| Abbreviations: | ||
| CTL | = | cytotoxic T lymphocyte |
| MHC | = | major histocompatibility complex |
| IFN-γ | = | interferon-γ |
| ELISPOT | = | enzyme-linked immunospot |
| HLA | = | human leukocyte antigen |
| APC | = | antigen-presenting cell |
| TCR | = | T cell receptor |
Acknowledgments
This work was supported in part by Health and Labor Science Research Grants for Research on Hepatitis, Clinical Research, and Third Term Comprehensive Control Research from the Ministry of Health, Labour and Welfare, Japan.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Chang CC, Campoli M, Ferrone S. HLA class I antigen expression in malignant cells: why does it not always correlate with CTL-mediated lysis?. Curr Opin Immunol 2004; 16:644 - 50; http://dx.doi.org/10.1016/j.coi.2004.07.015; PMID: 15342012
- Zeh HJ 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol 1999; 162:989 - 94; PMID: 9916724
- Oh S, Hodge JW, Ahlers JD, Burke DS, Schlom J, Berzofsky JA. Selective induction of high avidity CTL by altering the balance of signals from APC. J Immunol 2003; 170:2523 - 30; PMID: 12594278
- Pudney VA, Metheringham RL, Gunn B, Spendlove I, Ramage JM, Durrant LG. DNA vaccination with T-cell epitopes encoded within Ab molecules induces high-avidity anti-tumor CD8+ T cells. Eur J Immunol 2010; 40:899 - 910; http://dx.doi.org/10.1002/eji.200939857; PMID: 20039301
- Yoshikawa T, Nakatsugawa M, Suzuki S, Shirakawa H, Nobuoka D, Sakemura N, et al. HLA-A2-restricted glypican-3 peptide-specific CTL clones induced by peptide vaccine show high avidity and antigen-specific killing activity against tumor cells. Cancer Sci 2011; 102:918 - 25; http://dx.doi.org/10.1111/j.1349-7006.2011.01896.x; PMID: 21281401
- Nobuoka D, Yoshikawa T, Takahashi M, Iwama T, Horie K, Shimomura M, et al. Intratumoral peptide injection enhances tumor cell antigenicity recognized by cytotoxic T lymphocytes: a potential option for improvement in antigen-specific cancer immunotherapy. Cancer Immunol Immunother 2012; In press http://dx.doi.org/10.1007/s00262-012-1366-6; PMID: 23143746
- Kaneko K, Ishigami S, Kijima Y, Funasako Y, Hirata M, Okumura H, et al. Clinical implication of HLA class I expression in breast cancer. BMC Cancer 2011; 11:454; http://dx.doi.org/10.1186/1471-2407-11-454; PMID: 22014037
- Lee HW, Min SK, Ju YS, Sung J, Lim MS, Yang DH, et al. Prognostic significance of HLA class I expressing in gastric carcinoma defined by monoclonal anti-pan HLA class I antibody, EMR8-5. J Gastrointest Surg 2011; 15:1336 - 43; http://dx.doi.org/10.1007/s11605-011-1545-3; PMID: 21512844
- Torigoe T, Asanuma H, Nakazawa E, Tamura Y, Hirohashi Y, Yamamoto E, et al. Establishment of a monoclonal anti-pan HLA class I antibody suitable for immunostaining of formalin-fixed tissue: unusually high frequency of down-regulation in breast cancer tissues. Pathol Int 2012; 62:303 - 8; http://dx.doi.org/10.1111/j.1440-1827.2012.02789.x; PMID: 22524657