Abstract
Aim: This study evaluated the immunogenicity and safety of tetravalent measles-mumps-rubella-varicella (MMRV) vaccine produced with measles and rubella monovalent bulks derived from a newly established working seed virus stock (MMRVnew WS) compared with the combined MMRV vaccine derived from the current seed virus stock, in Taiwanese and Singaporean children (NCT00892775).
Methods:Healthy children aged 11–22 mo were randomized to receive two doses of either the MMRV new WS vaccine or the MMRV vaccine. Antibody titers against measles, mumps and rubella were measured using ELISA and against varicella using an immunofluorescence assay. The primary objective was to demonstrate non-inferiority of MMRVnew WS to MMRV in terms of post-dose-1 seroconversion rates, defined as a group difference with a lower limit of the 95% confidence interval greater than -10% for each antigen. Parents/guardians recorded symptoms in diary cards for 43 d after each vaccine dose.
Results:Non-inferiority of MMRV new WS to MMRV was achieved for all vaccine antigens. The lower limits of the 95% confidence intervals for group differences (MMRVnew WS group vs. MMRV) for measles (99.4% vs 100%), mumps (89.7% vs 90.4%), rubella (99.7% vs 100%) and varicella (97.6% vs 92.9%) seroconversion rates were greater than -10%. Mild symptoms including a peak in fever between days 5 and 12, post-dose-1, was observed in both groups.
Conclusion:The immune responses elicited by the MMRV new WS vaccine were non-inferior to that elicited by the MMRV vaccine for all antigens. Both vaccines exhibited an acceptable safety profile in Taiwanese and Singaporean children.
Introduction
Measles, mumps, rubella and varicella are viral diseases associated with significant morbidity and mortality in children and sometimes linked with potentially life-threatening complications.Citation1-Citation4 Effective vaccination strategies coupled with sustained high vaccination coverage can reduce the risk of transmission of such highly infectious viruses.Citation5 The success of a two-dose measles-mumps-rubella (MMR) vaccination strategy is evident in European countries where a significant reduction in disease morbidity and mortality has been noted.Citation6 On the other hand, although varicella vaccines are licensed in many countries, they are not always included in national immunization programs and as a result the vaccine uptake is low.Citation7 Nevertheless, evidence of the impact of universal varicella vaccine coverage (80%) has been observed in the United States and Uruguay.Citation6,Citation8,Citation9
The Taiwan national immunization program incorporated two-dose MMR and one-dose varicella vaccinations as separate injections in 1992 and 2004, respectively.Citation10-Citation12 Despite high coverage rates for the first-doses of MMR (96%) and varicella (97%) vaccinations, measles and varicella outbreaks and varicella breakthrough infections have occurred.Citation11-Citation14 In Singapore, the introduction of two-dose MMR vaccination in 1999 resulted in a considerable decline in reported cases for all three diseases. While varicella vaccination has been available in Singapore since 1996, it is not included in the childhood vaccination schedule.Citation15
A combined MMR-varicella vaccine (MMRV) may achieve the higher vaccination coverage rates needed to achieve the full benefits of varicella vaccination and facilitate the inclusion of varicella into national immunization programs.Citation16 Such a vaccine has the potential to provide broad protection against four diseases in a single injection and with minimum impact on vaccination program logistics.Citation16 A tetravalent MMRV vaccine was developed based on previous experience with MMR and varicella vaccines.Citation7 Previous studies have demonstrated that the MMRV vaccine is as immunogenic as separate MMR and varicella vaccines.Citation17-Citation19 The MMRV vaccine is commercially available in Australia, Canada and several European countries as a two-dose schedule.Citation7,Citation20
Recently, production of GlaxoSmithKline’s combined MMRV vaccine was switched to measles and rubella monovalent bulks derived from a newly established working seed virus stock (MMRVnew WS) due to the depletion of the current working seed virus stock. The new working seed virus stock is one passage further than the current working seed virus stock. This study was undertaken to obtain clinical data on the immunogenicity and safety of MMRVnew WS by assessing its non-inferiority to MMRV when administered to healthy Taiwanese and Singaporean children.
Results
Demographics
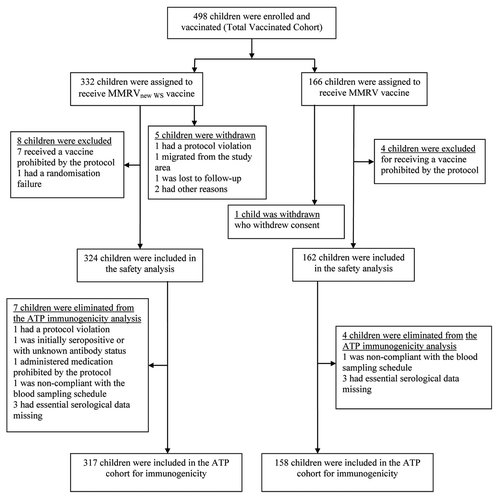
Of 498 children enrolled in the study (332 in the MMRVnew WS group; 166 in the MMRV group), 475 were included in the according-to-protocol (ATP) cohort for immunogenicity (317 in the MMRVnew WS group; 158 in the MMRV group) (). The 46 children (30 in the MMRVnew WS group; 16 in the MMRV group) who received commercially available MMRV vaccine as the second dose due to expiry of initial vaccine lots were included in the ATP cohort. The median age of children in the ATP cohort was 12 mo (range: 11–20 mo); 49.9% were female and the children were predominantly of East Asian (50.3%) and South-East Asian heritage (48.2%). Subject demographic characteristics did not differ between study groups.
Immunogenicity
The proportion of children initially seropositive for measles, mumps, rubella and varicella was < 3% in the MMRVnew WS and MMRV groups. Seroconversion rates for vaccine antigens ranged from 89.7% to 99.7% in the MMRVnew WS group and from 90.4% to 100% in the MMRV group, post-dose-1 (). In both groups, there was at least a 60 fold rise in the GMTs to varicella between doses 1 and 2. The primary non-inferiority objective was achieved for all vaccine antigens as the lower limit of the 95% CI for the group difference between MMRVnew WS and MMRV groups in terms of seroconversion rates 43 d post-dose-1 was greater than the pre-specified cut-off (-10%) ().
Table 1. Seroconversion rates and antibody GMTs in the MMRVnew WS and MMRV groups, 43 d after each dose (ATP cohort)
Safety and reactogenicity
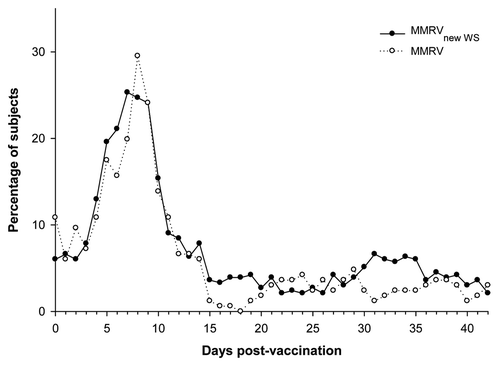
The overall incidence of symptoms (solicited and unsolicited) was 88.3% in the MMRVnew WS and 85.5% in the MMRV groups following dose-1; and 68.5% in the MMRVnew WS and 72.0% in the MMRV groups, following dose-2. Injection site redness was the most commonly reported solicited local and grade 3 symptom in both groups during the 4-d period after each dose (). Fever was the most commonly reported solicited general symptom in both groups during the 43-d period after each dose (). After dose-2, fever was reported for fewer children in both groups. A peak in the prevalence of fever between days 5 and 12 was observed post-dose-1 ().
Table 2. Incidence of solicited local symptoms reported during the 4-d follow-up period in groups MMRVnew WS and MMRV (Total vaccinated cohort)
Table 3. Incidence of solicited general symptoms reported during the 43-d follow-up period in groups MMRVnew WS and MMRV (total vaccinated cohort)
Figure 2. Prevalence of fever (any intensity) during the 43-d follow-up period after the first dose (Total vaccinated cohort). MMRVnew WS: measles-mumps-rubella-varicella vaccine derived from newly established working seed virus stock. MMRV, combined measles-mumps-rubella-varicella vaccine derived from current working seed virus stock
In the MMRVnew WS group, febrile convulsions occurring during the 43-d post-vaccination period were reported in two children (days 16 and 38 post-dose-1), accompanied by acute pharyngitis in the first child and viral infection in the second child. The second child experienced another febrile convulsion (day 18 post-dose-2) which was considered vaccine-related. In the MMRV group, a febrile convulsion accompanied by viral infection was reported for one child (day 39 post-dose-1). All cases post-dose-1 were considered unrelated to vaccination.
The incidence of unsolicited symptoms during the 43-d follow-up period were comparable between groups (49.7% and 44% in the MMRVnew WS and MMRV groups, respectively, post-dose-1 and 44% and 45.8%, respectively, post-dose-2). Upper respiratory tract infection was the most common unsolicited symptom post-dose-1 (15.1% and 13.3% in the MMRVnew WS and MMRV groups, respectively) and post-dose-2 (9% and 18.7% in the MMRVnew WS and MMRV groups, respectively). One or more SAEs were reported in 39 children (27 in the MMRVnew WS group; 12 in the MMRV group). Bronchiolitis, febrile convulsion and acute tonsillitis were the most commonly reported SAEs (each reported for four children in the MMRVnew WS group). In the MMRV group, bronchiolitis was the most commonly reported SAE occurring in four children. Two SAEs related to vaccination were reported in the MMRVnew WS group. One subject developed atypical Kawasaki disease (30 d post-dose-2). Another subject developed viral related encephalitis (24 d post-dose-2) accompanied by acute gastroenteritis norovirus infection and one seizure attack following hospitalization. The observed norovirus related seizure was considered unrelated to vaccination. All SAEs resolved without sequelae, except one (viral infection) in one MMRVnew WS recipient. No deaths occurred during the study.
Discussion
The depletion of the current working seed virus stock justified the need for a new working seed virus stock. This study compared the immunogenicity and safety of a first dose of MMRVnew WS vaccine to that of the MMRV vaccine when administered to healthy Taiwanese and Singaporean children. The results demonstrated that immune responses to measles, mumps, rubella and varicella post-dose-1 of MMRVnew WS vaccine were non-inferior to that elicited by the MMRV vaccine. The primary non-inferiority criterion of ruling out a 10% difference in seroconversion rates post-dose-1 of MMRVnew WS compared with MMRV was achieved for all antigens.
The observed seroconversion rates and GMTs for all vaccine antigens indicated strong immune responses after each dose of the two vaccines, suggesting enhanced protection post-dose-2. Studies have reported similar results in the assessment of the MMRV vaccine in German children aged 11–21 moCitation19 and Singaporean children aged 9 mo.Citation21 The strong increase in anti-varicella antibodies post-dose-2 in both vaccine groups is well-documented.Citation19,Citation21 This is especially encouraging as it supports the two-dose schedule for varicella vaccine in providing better long-term protection against the disease in young children.
Both vaccines were well-tolerated and had clinically acceptable safety profiles, as reported previously.Citation17-Citation19,Citation21 The incidence of fever during the 15-d period after dose-1 indicated no difference between the MMRVnew WS and MMRV vaccines. The peak prevalence in fever between days 5 and 12 post-dose-1 is characteristic of measles-containing vaccines, typically described during the second week post-vaccination.Citation22 Moreover, the majority of cases reported for fever were low grade in intensity after each vaccine dose in both groups.
A vaccine-related febrile convulsion was reported in one child from the MMRVnew WS group, post-dose-2. Previous studies have indicated an increased risk of febrile convulsions in children administered with an initial dose of combined MMRV vaccine (Merck and Co., Inc., USA) specifically between 5–12 dCitation23 or 7–10 dCitation24 post-vaccination, as compared with co-administered MMR+V vaccines. A similar risk of febrile convulsions can be expected with this study’s MMRV vaccine since a recent study has reported comparable fever profiles between GlaxoSmithKline’s and Merck’s combined MMRV vaccines.Citation25 Higher rates of fever have also been reported with the study MMRV vaccine compared with co-administration of MMR+V.Citation17-Citation19 Given this concern, the American Academy of Pediatrics recommends the use of either single dose co-administration of MMR+V or combined MMRV vaccine in children 12–47 mo of age.Citation24 In cases where MMRV usage is being considered, the benefits and risks associated with the vaccine need to be conveyed to parents/guardians.Citation26 Although the incidence of febrile convulsions is low in the present study, it is important to note that this study excluded children with a history of neurologic disease and seizures. Children with a history of febrile seizures are at high risk for recurrent febrile seizures.Citation27 Inclusion of such children may have resulted in a higher number of reported febrile seizures in this study.
One vaccine-related SAE occurred: norovirus infection coupled with seizure. However, norovirus gastroenteritis has previously been reported to be a significant cause of diarrhea-associated benign infant seizures.Citation28
Our study results demonstrated non-inferiority of the immune responses elicited by the MMRVnew WS vaccine to that elicited by the MMRV vaccine. The MMRVnew WS vaccine was found to be generally well tolerated in Taiwanese and Singaporean children.
Materials and Methods
Study design and children
This phase II, randomized, double-blind study (NCT00892775) was conducted at multiple centers in Singapore and Taiwan between June 2009 and December 2010. Healthy children aged 11–22 mo were randomized (2:1) to receive two doses of either the MMRV vaccine derived from new working seed viruses (MMRVnew WS group) or the MMRV vaccine derived from current working seed viruses (MMRV group). Due to expiry of the initial vaccine lots, 46 children (30 in the MMRVnew WS group; 16 in the MMRV group) received commercially available MMRV vaccine as a second dose.
Children were excluded from participation if they had received any investigational drug/vaccine 30 d before the study vaccine or immunosuppressive medication/immunoglobulins/blood products six months before the study. A history of allergy likely to be aggravated by any of the vaccine constituents, neurological disease or seizures, chronic illness or family history of immunodeficiency or symptoms of acute illness at the time of enrolment were reasons for exclusion. Vaccination was postponed for children with a rectal temperature ≥ 38.0°C or an axillary temperature ≥ 37.5°C. Finally, children were excluded if they lived in a household with newborn infants, pregnant women with a negative history of chickenpox or immunodeficient people.
The study was conducted according to Good Clinical Practice, the Declaration of Helsinki and applicable rules and regulations of Taiwan and Singapore. Each participating center’s Independent Ethics Committee reviewed and approved all study-related documents. Parents/guardians provided written informed consent before executing any study-related procedures.
Study vaccines
The study vaccines, MMRVnew WS and MMRV, were manufactured by GlaxoSmithKline, Belgium (). The vaccines were supplied in monodose vials, each containing a lyophilized pellet which was reconstituted with the diluent (provided in a pre-filled syringe) as a 0.5 ml dose before subcutaneous injection in the upper arm (deltoid region).
Table 4. Composition of study vaccines
Immunogenicity assessment
Blood samples were collected at pre-vaccination and 43 d after each dose. Antibody titers were measured using commercial enzyme-linked immunosorbent assays (ELISA) – (Enzygnost™, Dade Behring, Marburg, Germany) with cut-off values of 150 mIU/mL, 231 U/mL and 4 IU/mL for measles, mumps and rubella, respectively. For varicella, antibody titers were measured using an immunoflorescence assay (IFA) – (Virgo™, Hemagen Diagnostics, Columbia, MD, USA) (cut-off value of 4 dilution−1). The primary endpoint was based on immune responses following dose-1 of both vaccines. Additionally, antibody titers against varicella using ELISA (cut-off = 25 mIU/mL) were measured.
Safety/Reactogenicity assessment
Parents/guardians used diary cards to record the occurrence of solicited local symptoms (pain, redness and swelling) at the injection site for 4 d after each dose and solicited general symptoms [fever (axillary temperature ≥ 37.5°C/rectal temperature ≥ 38.0°C), rash/exanthem, parotid/salivary gland swelling and any suspected signs of meningism, including febrile convulsions] for 43 d after each dose. Body temperature was measured daily via the rectal/axillary route for the first 15 d after each vaccination. Between days 15 and 43, the presence of fever was assessed using a temperature-sensitive pad, and if fever was suspected, an accurate measurement of temperature was performed with a thermometer. Unsolicited symptoms were recorded for 43 d after each dose and serious adverse events (SAEs) were recorded throughout the study.
Intensity of symptoms was graded on a scale of 0–3. Grade 3 solicited symptoms were defined as: pain: when limb was moved or a spontaneously painful limb; redness and swelling: injection site surface diameter > 20 mm; fever: axillary temperature > 39°C or rectal temperature > 39.5°C; rash: > 150 lesions. Unsolicited symptoms (including SAEs) were defined as grade 3 when preventing normal daily activity.
Statistical analyses
All statistical analyses were performed using Statistical Analysis Software (SAS) version 9.2, and 95% confidence intervals (CI) were calculated using Proc StatXact 8.1. The sample size was estimated taking into consideration the primary non-inferiority objective. Non-inferiority was achieved if the lower limit of the two-sided standardized asymptotic 95% CI for the difference in seroconversion rates between the two groups (MMRVnew WS minus MMRV) was above -10% for each vaccine antigen, post-dose-1. A sample size of 498 children (332 in the MMRVnew WS group; 166 in the MMRV group), considering approximately a 15% drop-out rate was planned. To meet the primary objective, 420 evaluable children (280 in the MMRVnew WS group; 140 in the MMRV group) were adequate to achieve a power of at least 93.4%. A randomized (2:1) blocking scheme ensured that the balance between treatments was maintained by providing a unique treatment number that identified the vaccine dose to be administered to the children.
The immunogenicity analysis was performed on the ATP cohort, which included all children for whom pre- and post-dose-1 vaccination serology results were available, who were seronegative for at least one vaccine antigen before vaccination and who complied with study procedures. Seroconversion rates and geometric mean titers (GMTs) were calculated with exact 95% CIs for antibodies against each vaccine antigen after each dose. Seroconversion was defined as the appearance of antibodies (i.e., antibody concentration/titer ≥ cut-off value) in the serum of children who were seronegative before vaccination. The 95% CIs for the GMTs were obtained by exponential transformation of the 95% CI for the mean of log-transformed titer.
The safety analysis was performed on the total vaccinated cohort which included all vaccinated children. Solicited and unsolicited symptoms reported within their respective post-vaccination periods were tabulated with exact 95% CI. All SAEs reported throughout the study were described.
| Abbreviations: | ||
| ATP | = | according-to-protocol |
| CCID50 | = | median cell culture infective dose (50%) |
| CI | = | confidence interval |
| ELISA | = | enzyme-linked immunosorbent assay |
| GMT | = | geometric mean titer |
| IFA | = | immunofluorescense assay |
| mIU/mL | = | milli international unit per milliliter |
| MMR | = | measles-mumps-rubella vaccine |
| MMRV | = | measles-mumps-rubella-varicella vaccine |
| MMRVnew WS | = | measles-mumps-rubella-varicella vaccine manufactured with measles and rubella monovalent bulks derived from a newly established working seed virus |
| SAE | = | serious adverse event |
| SAS® | = | Statistical Analysis System |
Acknowledgments
The authors thank all the investigators involved in this clinical trial conducted in Taiwan and Singapore. The authors also thank the study nurses and other staff members involved and the parents and children who participated in the study. The authors acknowledge Christopher da Costa (ex-employee of GSK Vaccines) for his contributions on the study design; Bruce Innis for his critical contributions to the overall study design and interpretation of the data; Pierre Cambron for serology testing; Ann Delforge for study coordination; Valerie Balosso for clinical data coordination; Ashmita Ravishankar for manuscript writing and K Manjula for publication management (all employees of GSK Vaccines).
Trademarks
Virgo is a registered trademark of Hemagen Diagnostics, Columbia, MD, USA. Enzygnost is a registered trademark of Dade Behring, Marburg, Germany.
Disclosure of Potential Conflicts of Interest
L.M.H. and L.B.W. declare to have received payment for consultancy for being on the advisory board and their institutions received grants to conduct clinical trials, authors (L.M.H., L.B.W. and C.P.C.) declare either they/institution received payment for lectures including service on speakers bureaus and support for travel to meetings for the study or other purposes. M.P. and O.H. are employed by the GlaxoSmithKline group of companies.
This study was sponsored and funded by GlaxoSmithKline Biologicals S.A., Rixensart, Belgium. GlaxoSmithKline Biologicals S.A. was involved in all stages of the study conduct and analysis; and also took charge of all costs associated with the development and the publishing of the manuscript.
References
- Measles vaccines: WHO position paper. Wkly Epidemiol Rec 2009; 84:349 - 60; PMID: 19714924
- Mumps virus vaccines. WHO position paper. Wkly Epidemiol Rec 2007; 82:49 - 60
- Rubella vaccines: WHO position paper. Wkly Epidemiol Rec 2011; 86:301 - 16; PMID: 21766537
- Varicella vaccines. WHO position paper. Wkly Epidemiol Rec 1998; 73:241 - 8; PMID: 9715106
- Centers for Disease Control and Prevention (CDC). Progress in reducing measles mortality--worldwide, 1999-2003. MMWR Morb Mortal Wkly Rep 2005; 54:200 - 3; PMID: 15744229
- Vesikari T, Sadzot-Delvaux C, Rentier B, Gershon A. Increasing coverage and efficiency of measles, mumps, and rubella vaccine and introducing universal varicella vaccination in Europe: a role for the combined vaccine. Pediatr Infect Dis J 2007; 26:632 - 8; http://dx.doi.org/10.1097/INF.0b013e3180616c8f; PMID: 17596807
- Czajka H, Schuster V, Zepp F, Esposito S, Douha M, Willems P. A combined measles, mumps, rubella and varicella vaccine (Priorix-Tetra): immunogenicity and safety profile. Vaccine 2009; 27:6504 - 11; http://dx.doi.org/10.1016/j.vaccine.2009.07.076; PMID: 19665608
- Quian J, Rüttimann R, Romero C, Dall’Orso P, Cerisola A, Breuer T, et al. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997-2005. Arch Dis Child 2008; 93:845 - 50; http://dx.doi.org/10.1136/adc.2007.126243; PMID: 18456699
- Marin M, Zhang JX, Seward JF. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics 2011; 128:214 - 20; http://dx.doi.org/10.1542/peds.2010-3385; PMID: 21788222
- Lin CC, Yang CY, Shih CT, Chen BH, Huang YL. Rubella seroepidemiology and catch-up immunization among pregnant women in Taiwan: comparison between women born in Taiwan and immigrants from six countries in Asia Am J Trop. Med Hyg (Geneve) 2010; 82:40 - 4; http://dx.doi.org/10.4269/ajtmh.2010.09-0302
- Chang LY, Huang LM, Chang IS, Tsai FY. Epidemiological characteristics of varicella from 2000 to 2008 and the impact of nationwide immunization in Taiwan. BMC Infect Dis 2011; 11:352; http://dx.doi.org/10.1186/1471-2334-11-352; PMID: 22176638
- Huang WC, Huang LM, Chang IS, Tsai FY, Chang LY. Varicella breakthrough infection and vaccine effectiveness in Taiwan. Vaccine 2011; 29:2756 - 60; http://dx.doi.org/10.1016/j.vaccine.2011.01.092; PMID: 21315697
- Chen JH, Tsou TP, Liu DP. Measles resurgence in Taiwan---lessons learned. J Formos Med Assoc 2009; 108:267 - 9; http://dx.doi.org/10.1016/S0929-6646(09)60065-6; PMID: 19369172
- Cheng WY, Yang CF, Hou YT, Wang SC, Chang HL, Chiu HY, et al. Imported measles and implications for its elimination in Taiwan. Emerg Infect Dis 2011; 17:1523 - 6; PMID: 21801641
- Jean-Jasmin LM, Lynette SP, Stefan M, Kai CS, Chew FT, Wah LB. Economic burden of varicella in Singapore--a cost benefit estimate of implementation of a routine varicella vaccination. Southeast Asian J Trop Med Public Health 2004; 35:693 - 6; PMID: 15689089
- Rentier B, Gershon AA, European Working Group on Varicella. Consensus: varicella vaccination of healthy children--a challenge for Europe. Pediatr Infect Dis J 2004; 23:379 - 89; http://dx.doi.org/10.1097/01.inf.0000122606.88429.8f; PMID: 15131459
- Nolan T, McIntyre P, Roberton D, Descamps D. Reactogenicity and immunogenicity of a live attenuated tetravalent measles-mumps-rubella-varicella (MMRV) vaccine. Vaccine 2002; 21:281 - 9; http://dx.doi.org/10.1016/S0264-410X(02)00459-0; PMID: 12450703
- Knuf M, Habermehl P, Zepp F, Mannhardt W, Kuttnig M, Muttonen P, et al. Immunogenicity and safety of two doses of tetravalent measles-mumps-rubella-varicella vaccine in healthy children. Pediatr Infect Dis J 2006; 25:12 - 8; http://dx.doi.org/10.1097/01.inf.0000195626.35239.58; PMID: 16395096
- Schuster V, Otto W, Maurer L, Tcherepnine P, Pfletschinger U, Kindler K, et al. Immunogenicity and safety assessments after one and two doses of a refrigerator-stable tetravalent measles-mumps-rubella-varicella vaccine in healthy children during the second year of life. Pediatr Infect Dis J 2008; 27:724 - 30; http://dx.doi.org/10.1097/INF.0b013e318170bb22; PMID: 18600190
- Rümke HC, Loch HP, Hoppenbrouwers K, Vandermeulen C, Malfroot A, Helm K, et al. Immunogenicity and safety of a measles-mumps-rubella-varicella vaccine following a 4-week or a 12-month interval between two doses. Vaccine 2011; 29:3842 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.02.067; PMID: 21382484
- Goh P, Lim FS, Han HH, Willems P. Safety and immunogenicity of early vaccination with two doses of tetravalent measles-mumps-rubella-varicella (MMRV) vaccine in healthy children from 9 months of age. Infection 2007; 35:326 - 33; http://dx.doi.org/10.1007/s15010-007-6337-z; PMID: 17710370
- Centers for disease control and prevention: Measles. In: Atkinson W, Hamborsky J, McIntyre L, Wolfe S (eds.): Epidemiology and prevention of vaccine-preventable diseases (9th edn). Public Health Foundation, Washington DC, 2006;125-44.
- Jacobsen SJ, Ackerson BK, Sy LS, Tran TN, Jones TL, Yao JF, et al. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine 2009; 27:4656 - 61; http://dx.doi.org/10.1016/j.vaccine.2009.05.056; PMID: 19520201
- Klein NP, Fireman B, Yih WK, Lewis E, Kulldorff M, Ray P, et al, Vaccine Safety Datalink. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics 2010; 126:e1 - 8; http://dx.doi.org/10.1542/peds.2010-0665; PMID: 20587679
- Blatter MM, Klein NP, Shepard JS, Leonardi M, Shapiro S, Schear M, et al. Immunogenicity and safety of two tetravalent (measles, mumps, rubella, varicella) vaccines coadministered with hepatitis a and pneumococcal conjugate vaccines to children twelve to fourteen months of age. Pediatr Infect Dis J 2012; 31:e133 - 40; http://dx.doi.org/10.1097/INF.0b013e318259fc8a; PMID: 22622699
- Committee on Infectious Diseases. Policy statement—Prevention of varicella: update of recommendations for use of quadrivalent and monovalent varicella vaccines in children. Pediatrics 2011; 128:630 - 2; http://dx.doi.org/10.1542/peds.2011-1968; PMID: 21873692
- Berg AT, Shinnar S, Hauser WA, Alemany M, Shapiro ED, Salomon ME, et al. A prospective study of recurrent febrile seizures. N Engl J Med 1992; 327:1122 - 7; http://dx.doi.org/10.1056/NEJM199210153271603; PMID: 1528207
- Chen SY, Tsai CN, Lai MW, Chen CY, Lin KL, Lin TY, et al. Norovirus infection as a cause of diarrhea-associated benign infantile seizures. Clin Infect Dis 2009; 48:849 - 55; http://dx.doi.org/10.1086/597256; PMID: 19239351