Abstract
We recently reported the safety, immunological and clinical responses to a GPC3-derived peptide vaccine in a phase I clinical trial of patients with advanced hepatocellular carcinoma (HCC). We conducted a subsequent trial in advanced HCC to assess the histopathological findings before and after vaccination with the GPC3 peptide. Here, we present the clinical course and the pathological study including the autopsy of a patient with advanced HCC in the ongoing clinical trial. A 62-year old patient suffering from HCC refractory to sorafenib therapy received the GPC3 peptide vaccine. The patient had fever and remarkably impaired liver function twice after vaccination. Contrast-enhanced CT after the second vaccination showed multiple low-density areas in the liver tumor, indicating tumor necrosis. In contrast, the tumor thrombus in the right atrium increased. The patient discontinued protocol treatment due to disease progression and died 30 days after the second vaccination. An autopsy was performed to determine the main cause of death and to evaluate the antitumor effect of the vaccination. A histological examination showed central necrosis in most of the intrahepatic tumor. The main cause of death was circulatory failure due to tumor thrombus, which occupied most of the right atrium. An immunohistochemical analysis revealed infiltration of CD8-positive T cells in the residual carcinoma, but not within the cirrhotic area. Ex vivo IFN-γ enzyme-linked immunospot analysis revealed vaccine-induced immune-reactivity against the GPC3 peptide. A histopathological examination at the estimated time of a strong immunological response demonstrated a GPC3 peptide vaccination-induced cytotoxic T-lymphocyte response with an anti-tumor effect.
Keywords: :
Introduction
Cancer vaccine targeting hepatocellular carcinoma (HCC) tumor antigens have been tested in clinical trials.Citation1,Citation2 However, cancer vaccines using tumor-antigen-derived peptides have not demonstrated adequate antitumor efficacy in clinical trials for advanced HCC.Citation1-Citation3 Glypican-3 (GPC3), a carcinoembryonic antigen, is an ideal target for immunotherapy against HCC because it is overexpressed specifically in HCC (72–81%) and correlates with a poor prognosis.Citation4-Citation10 GPC3 forms a complex with Wnt molecules and promotes the growth of HCC by stimulating canonical Wnt signaling.Citation10 We identified HLA-A*24:02-restricted GPC3298–306 (EYILSLEEL) and HLA-A*02:01-restricted GPC3144-152 (FVGEFFTDV) peptides, both of which induce GPC3-reactive cytotoxic T-lymphocytes (CTLs) without inducing autoimmunity.Citation8,Citation9 We recently reported the safety, immunological, and clinical responses of a GPC3-derived peptide vaccine in a phase I clinical trial of patients with advanced HCC.Citation11 The results of that trial showed that GPC3 peptide-specific CTLs increased in peripheral blood, and that many CD8-positive T cells infiltrated the tumors in some patients, demonstrating a correlation between the CTL response and overall survival following GPC3 peptide vaccination. Based on these results, we conducted a trial in patients with advanced HCC to assess the clinical outcome and whether tumor-infiltrating lymphocytes with an antitumor effect increased. In all cases, liver biopsies were performed before and after GPC3 peptide vaccination according to the protocol. This trial was approved by the Ethics Committee of the National Cancer Center and registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR number 000005093). The patient described herein was the first case examined pathologically using autopsy specimens. Here, we present the clinical course and pathological study, including an autopsy, of a patient with advanced HCC who revealed remarkable tumor lysis immediately after the second vaccination in an ongoing clinical trial of a GPC3 peptide vaccine.
Patient presentation
A 62-year-old male had a history of asymptomatic chronic hepatitis C. In September 2009, he was diagnosed with HCC. Laboratory data disclosed no abnormalities. Abdominal CT (CT) scans showed four lesions in the liver, and the patient was treated four times with hepatic artery chemoembolization. In December 2010, CT scans revealed a new lesion indicative of a tumor thrombus extending into the inferior vena cava. The patient was treated with sorafenib. However, the sorafenib treatment was discontinued in January 2011 due to progressive multiple intrahepatic tumors.
As no established therapeutic regimens exist for this condition, he was offered participation in a clinical trial of a GPC3 peptide vaccine for advanced HCC. HLA-typing revealed an HLA-A2 phenotype. The patient had a performance status of 0, and Child-Pugh class B disease. The patient did not have active HBV infection or rapidly progressive tumor thrombus before enrollment, met the eligibility criteria, and was enrolled after providing informed consent. Early-phase contrast-enhanced CT before treatment showed a maximum 68 × 51-mm tumor with multiple intrahepatic tumors and a 44 × 30-mm tumor invading the right atrium (). Pretreatment tumor markers were as follows: α fetoprotein (AFP), 852 ng/mL and des-gamma-carboxy prothrombin (DCP), 1346 mAU/mL. A liver biopsy was performed 1 week prior to GPC3 peptide vaccination according to the protocol. In April 2011, 3 mg of HLA-A2-restricted GPC3144-152 peptide (FVGEFFTDV) (American Peptide Co.) emulsified with incomplete Freund’s adjuvant (Montanide ISA-51VG; SEPPIC) was injected intradermally as the vaccine following Good Manufacturing Practice guidelines. The patient had a low-grade fever on day 6 following the first vaccination, and inflammatory and hepatic parameters were elevated on day 12 (). The abnormal laboratory findings improved later. Therefore, he received the second vaccination on day 26 after the first vaccination. On day 9 after the second vaccination, the patient was admitted to our hospital with a high fever and general fatigue. On admission, the patient’s C-reactive protein (CRP) level (10.76 mg/dL) and laboratory hepatic parameters were elevated. One day after hospitalization, aspartate aminotransferase and alanine aminotransferase and levels were elevated to 1,580 IU/L and 1,112 IU/L, respectively. The prothrombin time-international normalized ratio increased from 1.18 to 1.51. But the patient did not have ammonemia or asterixis. As seen by early-phase contrast-enhanced CT scan, most tumors in the liver were not contrast enhanced. Findings of the CT scan indicated tumor necrosis and regression. In contrast, the size of the tumor thrombus in the right atrium increased to a maximum of 83 × 50 mm (). Levels of the tumor markers AFP and DCP decreased temporarily to 634 ng/mL and 777 mAU/mL, respectively. He was infused with a liver-supporting agent (monoammonium glycyrrhizinate, glycine, and l-cysteine hydrochloride hydrate). The inflammatory and hepatic parameters improved 1 week after hospitalization (). We did not perform a liver biopsy when the hepatic parameters were elevated because they improved promptly. Nevertheless, his status worsened gradually. Protocol treatment was discontinued due to progressive disease and he died 30 days after the second vaccination. Based on the clinical course, we could not rule out the possibility that his condition had worsened as a result of the vaccine. Therefore, an autopsy was performed to determine the main cause of death and the elevated hepatic parameters, and to evaluate the anti-tumor effect of vaccination
Figure 1. Findings of an early-phase contrast-enhanced CT (CT) scan. (A) Contrast-enhanced CT scan before vaccination shows a 68 × 51-mm tumor with multiple intrahepatic tumors (arrow) and a 44 × 30-mm tumor invading the right atrium (arrowhead). (B) Contrast-enhanced CT after the second vaccination showing multiple low-density areas in the liver, indicating extensive tumor necrosis (arrow). By contrast, a tumor thrombus in the right atrium increased to a 83 × 50-mm tumor (arrowhead). (C) Clinical course from the beginning of GPC3 peptide vaccination. Approximately 1 week after the first vaccination, the patient began reporting general fatigue and showed intermittent fever. Inflammatory and hepatic parameters were elevated (CRP: pink line, AST: red line, T-bil: green line). The abnormal laboratory parameters improved after observation. On day 9 after the second vaccination, the patient was admitted to our hospital as an emergency due to fever and general fatigue, which were similar to his previous symptoms. One day after hospitalization, the inflammatory and hepatic parameters were remarkable. Inflammatory and hepatic parameters improved 1 week after hospitalization. However, his status gradually worsened, and he died on day 30 after the second vaccination. (D) Immunological monitoring of the GPC3 peptide-specific T cell responses. Ex vivo IFN-γ enzyme-linked immunospot (ELISPOT) assays against GPC3 in 5 × 105 peripheral blood mononuclear cells (PBMCs) were performed before and after vaccination. The ∆ spot number indicates the number of GPC3 peptide-specific cytotoxic T-lymphocytes (CTLs). The number of interferon (IFN)-γ positive spots increased from 0 to 84 after the second vaccination.
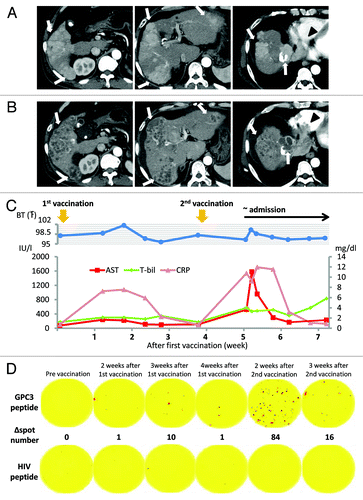
Results
Immunological analysis and autopsy
Generally, CTLs specific for tumor antigens cannot be detected directly ex vivo; they can be detected only after expansion by repeated in vitro stimulation with the antigenic peptide in conjunction with appropriate antigen-presenting cells. This is attributed to the sensitivity of the assay and the low frequency of tumor-antigen-specific CTLs.Citation12 GPC3 peptide-specific CTLs in PBMCs, which can be detected directly ex vivo without in vitro stimulation, can provide strong immunological evidence. An ex vivo IFN-γ ELISPOT assay was performed, as described previously.Citation13 The number of GPC3 peptide-specific CTLs increased from 0 to 84 in 5 × 105 PBMCs after the second vaccination (). This result led us to anticipate a good clinical response because the increased number of CTLs and the specific CTL number correlated with the clinical response in a previous trial of the GPC3 peptide vaccine.Citation11
A liver biopsy was performed before vaccination according to protocol. Histological examination of the specimen revealed well-differentiated HCC. Immunohistochemical staining showed expression of GPC3 and HLA class I in the cytoplasm and membranes of the carcinoma cells and a few CD8-positive T cells in the carcinoma tissue before vaccination ().
Figure 2. (A) Pathological findings of liver biopsy specimens before vaccination. A microscopy image of a hematoxylin and eosin (H&E)-stained section shows well-differentiated hepatocellular carcinoma (HCC). Immunohistochemical staining for GPC3 and HLA class I showed positivity in the cytoplasm and membranes of carcinoma cells, respectively. No CD8-positive T cells were observed in carcinoma tissue before vaccination. (B) Macroscopic findings of the liver and heart before formalin fixation at the time of autopsy. Most liver tumors had a necrotic area (arrow). A tumor thrombus occupied most of the right atrium (arrow). (C) Pathological findings of the autopsy specimen. (a) Microscopic images of H&E-stained sections showing central necrosis of carcinoma tissue, whereas a cirrhotic nodule adjacent to the carcinoma tissue was not necrotic. (b) Magnified image of the area enclosed within the white box in (a) showing that cancer cells exhibited a morphology (left) different from that of cirrhotic cells (right). (c) CD8-positive T cells (brown) infiltrated the carcinoma cells accompanied by necrosis. In contrast, no infiltration of CD8-positive T cells was detected within the cirrhotic nodule. (d) Magnified image of the area enclosed within the red box in (a) showing necrosis and viable carcinoma cells. (e) Positive immunohistochemical GPC3 staining was observed in only the cytoplasm of carcinoma cells. (f) CD8-positive T cells infiltrated the necrotic area and carcinoma tissue.
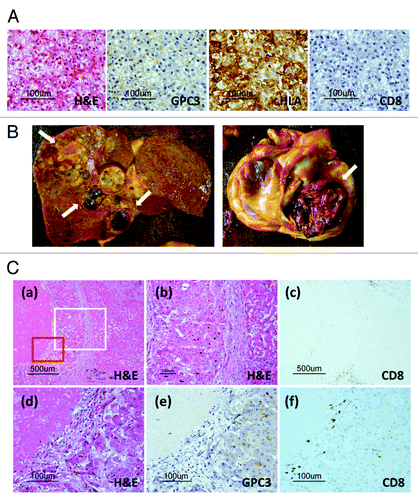
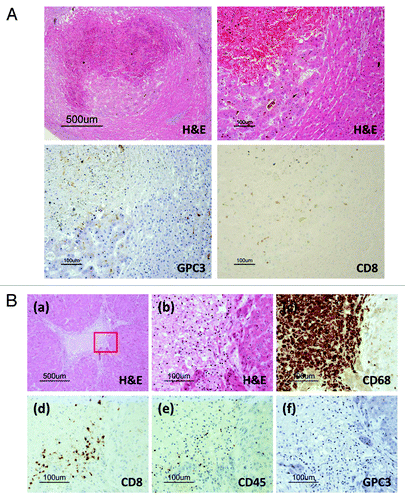
A general autopsy (with the exception of the brain) was performed 2 h following death. Macroscopic findings of the liver revealed multiple macro-nodular lesions with central necrosis mainly in the right lobe (, left). As the tumor occupied most of the right atrium, the main cause of death was circulatory failure due to progressive tumor thrombus (, right). We judged that his condition had worsened as a result of the tumor thrombus. A histological examination showed central necrosis in most of the tumor in the right lobe, and viable carcinoma cells remained around the necrotic tissue, whereas a cirrhotic nodule adjacent to the carcinoma tissue was not necrotic ( and ). Immunohistochemical staining revealed GPC3-positive carcinoma cells (). There was infiltration of CD8-positive T cells (brown) in the residual carcinoma, but not within the cirrhotic area ( and ). We did not detect degeneration or necrosis of the hepatocytes in the non-tumor liver parenchyma of the left lobe. These findings suggest that the elevated hepatic parameters in our patient were due to an antitumor effect. We diagnosed that the cause of death was unlikely to be related to vaccine-induced liver injury. We focused on the necrotic area around the cirrhotic nodules, in which CD68-positive macrophages (brown) aggregated (). CD8-positive T cells also infiltrated the marginal zone between the necrotic area and noncancerous cirrhotic nodule, suggesting that carcinoma cells were attacked by CD8-positive T cells, which may have resulted in necrosis (). The histology of the tumor thrombus in the right atrium was similar to that of the intrahepatic tumor. However, viable tumor cells remained in half of the tumor thrombus and little infiltration of CD8-positive T cells was detected (data not shown).
Figure 3. Pathological findings in the autopsy specimen. (A) Carcinoma in a cirrhotic nodule. CD8-positive T cells (brown) infiltrated only the carcinoma area, accompanied by necrosis. No infiltration of CD8-positive T cells was detected in the cirrhotic nodule. Only carcinoma cells were GPC3-positive by immunohistochemical staining. (B) Necrotic area surrounded by cirrhotic nodules. (a) Necrosis was surrounded by viable cirrhotic cells. (b) The margin between the necrosis and the cirrhotic nodule. This portion is enclosed by the red box in (a). (c) CD68-positive macrophages (brown) aggregated in the necrotic area around the cirrhotic nodule. (d) CD8-positive T cells (brown) infiltrated the necrotic area but not the cirrhotic nodule. (e) CD45-positive lymphocytes infiltrated the necrotic area. Based on the image in (d), most of the lymphocytes were CD8-positive T cells. (f) Cirrhotic cells did not express GPC3.
Discussion
To date, the time to CTL induction and subsequent tumor response has been prolonged in cancer vaccine trials.Citation14 By contrast, no discrepancy regarding the time between CTL induction and tumor response was observed in our phase I trial of a GPC3 peptide vaccine.Citation11 In this case, central necrosis of each intra-hepatic tumor was observed at the time of a strong immunological response against the GPC3 peptide, immediately after the second vaccination.
We did not perform a liver biopsy when the hepatic parameters were elevated. A biopsy may be necessary to rule out vaccine-induced liver injury when the hepatic parameters are elevated. However, the clinical course and autopsy results suggested that the elevated hepatic parameters in our patient were due to an antitumor effect.
Treatment-induced necrosis is included in the modified RECIST assessment for HCC.Citation15 Therefore, a positive radiographic response following vaccination, suggesting tumor necrosis, could be evaluated as a treatment response.
Necrosis was found in the center of each tumor; therefore, the central necrosis caused by ischemia, in addition to CD8-positive T cells attacking tumor cells, may have led to tumor necrosis. Three findings support the hypothesis that tumor necrosis was caused by CD8-positive T cells, as follows: (1) the necrotic changes determined by CT after vaccination, accompanied by clinical laboratory data; this was consistent with an immune response, although no tumor necrosis was evident on the CT before vaccination; (2) no necrosis was evident in the left lobe (no tumors) of the autopsy liver specimen, but it was present in the right liver lobe (tumors present); and (3) CD8-positive T cells infiltrated residual viable tumor cells. The analyses used in this study may contribute to identifying the pathological state after vaccination.
We detected infiltration of CD8-positive T cells into the hepatic tumors, but little infiltration of CD8-positive T cells into the tumor thrombus. This discrepancy may have been caused by the heterogeneity associated with immune-escape mechanisms in tumor cells.
This case report of central necrosis in a patient with HCC might be regarded as spontaneous regression correlated with circulatory failure due to a massive tumor embolism. It was not known whether the tumor necrosis was induced by CTLs, ischemia, or other factors. However, the infiltration of CD8-positive T cells into tumor cells supports immune-related necrosis.
The rate of spontaneous partial regression among patients with HCC is 0.406% compared with the control arm of a randomized clinical trial.Citation16 In contrast, three of 33 patients who received GPC3 peptide vaccination in the phase I trial had suspicious tumor necrosis on CT scans. In one report, massive infiltration of CD8-positive T cells in the remaining liver tumor and tumor necrosis were identified by histological examination of a biopsy specimen after vaccination.Citation11 Indeed, on-going clinical trials of the GPC3 peptide vaccine will provide additional information and further demonstrate the antitumor effect.Citation17,Citation18 Histological results at the estimated time of a strong GPC3-specific CTL response suggest that GPC3 peptide vaccination may be a promising approach to treat HCC.
Materials and Methods
Ex vivo interferon-γ (IFN-γ) enzyme-linked immunospot assay
An ex vivo IFN-γ enzyme-linked immunospot (ELISPOT) assay was performed to evaluate the antigen-specific CTL response, as described previously.Citation13 Briefly, peripheral blood (30 mL) was obtained from the patient before the first vaccination and 2 weeks after each vaccination and centrifuged on a Ficoll–Paque gradient. Non-cultured peripheral blood mononuclear cells (PBMCs) (5 × 105/well) were added to plates in the presence of 10 µg/mL peptide antigens and incubated for 20 h. The GPC3 antigen used was the HLA-A2-restricted GPC3144–152(FVGEFFTDV) peptide. PBMCs with the HLA-A2-restricted HIV19–27 (TLNAWVKVV) peptide (ProImmune) were used as negative controls. Assays were performed in duplicate.
Immunohistochemical analysis
Immunohistochemical staining with monoclonal antibodies against GPC3 (clone, 1G12; Biomosaics), HLA class I (clone, EMR8/5; Hokudo), CD8 (clone, 1A5; Novocastra), CD45 (cloned 2B11 and PD7/26; Ventana), and CD68 (clone, KP-1; Ventana) was performed according to the manufacturer’s protocol.
| Abbreviations: | ||
| GPC3 | = | glypican-3 |
| HCC | = | hepatocellular carcinoma |
| HLA | = | human leukocyte antigen |
| CTL | = | cytotoxic T-lymphocyte |
| IFN-γ | = | interferon-γ |
| PBMC | = | peripheral blood mononuclear cells |
| AFP | = | α-fetoprotein |
| DCP | = | des-γ-carboxy prothrombin |
Authors’ Disclosure of Potential Conflicts of Interest
The authors have no potential conflicts of interest to declare with regard to this study.
Financial Support
This study was supported in part by Health and Labor Science Research Grants for Clinical Research and Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labor, and Welfare, Japan.
References
- Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res 2006; 12:2817 - 25; http://dx.doi.org/10.1158/1078-0432.CCR-05-2856; PMID: 16675576
- Greten TF, Forner A, Korangy F, N’Kontchou G, Barget N, Ayuso C, et al. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer 2010; 10:209; http://dx.doi.org/10.1186/1471-2407-10-209; PMID: 20478057
- Greten TF, Manns MP, Korangy F. Immunotherapy of hepatocellular carcinoma. J Hepatol 2006; 45:868 - 78; http://dx.doi.org/10.1016/j.jhep.2006.09.004; PMID: 17046096
- Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun 2003; 306:16 - 25; http://dx.doi.org/10.1016/S0006-291X(03)00908-2; PMID: 12788060
- Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003; 125:89 - 97; http://dx.doi.org/10.1016/S0016-5085(03)00689-9; PMID: 12851874
- Shirakawa H, Kuronuma T, Nishimura Y, Hasebe T, Nakano M, Gotohda N, et al. Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer. Int J Oncol 2009; 34:649 - 56; PMID: 19212669
- Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 2009; 100:1403 - 7; http://dx.doi.org/10.1111/j.1349-7006.2009.01206.x; PMID: 19496787
- Nakatsura T, Komori H, Kubo T, Yoshitake Y, Senju S, Katagiri T, et al. Mouse homologue of a novel human oncofetal antigen, glypican-3, evokes T-cell-mediated tumor rejection without autoimmune reactions in mice. Clin Cancer Res 2004; 10:8630 - 40; http://dx.doi.org/10.1158/1078-0432.CCR-04-1177; PMID: 15623647
- Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, et al. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res 2006; 12:2689 - 97; http://dx.doi.org/10.1158/1078-0432.CCR-05-2267; PMID: 16675560
- Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 2005; 65:6245 - 54; http://dx.doi.org/10.1158/0008-5472.CAN-04-4244; PMID: 16024626
- Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res 2012; 18:3686 - 96; http://dx.doi.org/10.1158/1078-0432.CCR-11-3044; PMID: 22577059
- Romero P, Cerottini JC, Speiser DE. Monitoring tumor antigen specific T-cell responses in cancer patients and phase I clinical trials of peptide-based vaccination. Cancer Immunol Immunother 2004; 53:249 - 55; http://dx.doi.org/10.1007/s00262-003-0473-9; PMID: 14704832
- Yoshikawa T, Nakatsugawa M, Suzuki S, Shirakawa H, Nobuoka D, Sakemura N, et al. HLA-A2-restricted glypican-3 peptide-specific CTL clones induced by peptide vaccine show high avidity and antigen-specific killing activity against tumor cells. Cancer Sci 2011; 102:918 - 25; http://dx.doi.org/10.1111/j.1349-7006.2011.01896.x; PMID: 21281401
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10:909 - 15; http://dx.doi.org/10.1038/nm1100; PMID: 15340416
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30:52 - 60; http://dx.doi.org/10.1055/s-0030-1247132; PMID: 20175033
- Oquiñena S, Guillen-Grima F, Iñarrairaegui M, Zozaya JM, Sangro B. Spontaneous regression of hepatocellular carcinoma: a systematic review. Eur J Gastroenterol Hepatol 2009; 21:254 - 7; http://dx.doi.org/10.1097/MEG.0b013e328324b6a2; PMID: 19279469
- Sawada Y, Sakai M, Yoshikawa T, Ofuji K, Nakatsura T. A glypican-3-derived peptide vaccine against hepatocellular carcinoma. Oncoimmunology 2012; 1:1448 - 50; http://dx.doi.org/10.4161/onci.21351; PMID: 23243625
- Nobuoka D, Yoshikawa T, Sawada Y, Fujiwara T, Nakatsura T. Peptide vaccines for hepatocellular carcinoma. Hum Vaccin Immunother 2013; 9:210 - 2; PMID: 23442593