Abstract
Oral vaccines have several attractive features; however, due to several challenges, to date, only a limited number of oral vaccines are licensed. Over the past two decades, several oral vehicle delivery systems have been developed to address these challenges and deliver antigens to the target cells in the mucosal immune system. While the size of vehicle delivery systems, the quantity of components in the vehicle formulation, the dose of administration, and even the type of animals species, are important aspects in development of a suitable oral vaccine, our results showed that entrapment of inactivated Vibrio cholera, a component in the structure of Dukoral vaccine into oral vehicle delivery systems, is able to induce a more rigorous humoral immune response in the systemic compartment. We further investigated the mechanism of Dukoral vaccine as a potential stimulator in induction of immune response by immunizing TLR-2-, TLR-4-, MyD88- and Trif-deficient mice. We are hopeful that these findings will lead to development of more precisely-designed oral vaccines in the future.
Delivery of Immunogens to Mucosal Immune System
The mucosal immune system forms a critical line of defense against pathogens, as the majority of infections are initiated through mucosal surfaces.Citation1-Citation3 Therefore, the development of mucosal vaccines, in particular oral vaccines, has been the center of attention in the scientific community over the past few decades. Oral vaccines have several advantages over injected vaccines, including the induction of immune responses in mucosal and systemic sites, ease of administration, and the ability to supply the immunization needs of individuals in developing countries, where cold chain storage is limited. However, due to several challenges including the instability of antigens against the harsh conditions of the gastrointestinal tract, as well as the large population of non-pathogenic microorganisms residing in the large intestine, the possible induction of tolerance, and limitation in the uptake of antigens by Microfold (M) cells, the lack of effective mucosal adjuvants, and a very limited understanding of the underlying mechanisms of oral vaccines, a limited number of oral vaccines are currently licensed by regulatory authorities.Citation4-Citation6
To overcome the above challenges, oral delivery systems such as bilosomes, dendrimers, multiple emulsions, immune stimulating complexes (ISCOMs), biodegradable polymers such as poly (lactide-co-glycolide acid), and chitosones have been characterized and tested.Citation7-Citation10 Some of these vehicles act as immunostimulants and while preventing the degradation of immunogens by enzymes in the gastrointestinal tract. There are also claims that some of these vehicles are able to interact with follicle-associated epithelium of Peyer’s patches, transport across the intestinal epithelial layer by M cells, and consequently deliver the immunogens to antigen presenting cells.Citation11 One of the critical challenges related to such vehicles is the size of the vesicles.Citation12,Citation13 Although some reports indicate that particles smaller than 10 μm can be taken up by M-cells of Peyer's patches,Citation14 there is no clear consensus in the scientific community regarding the optimal size for uptake by the gut mucosa and the M cells specifically. However, sizing is not the only obstacle with such oral vehicle delivery systems, the ratio and quantity of chemical components, the amount of encapsulated immunogens, the ionic surface charge, the type of associated adjuvants, and the dose of administration were also reported as other challenges. Due to these challenges on the development of efficient oral vehicle delivery systems, to date, only a limited number of orally administered vaccines have been evaluated in human trials. In an interesting phase I HIV vaccine study, HIV-seronegative volunteers were primed orally three times with a polymerized V3 peptide derived from HIV-1 isolate MN in biodegradable microspheres, followed by a systemic boosting.Citation15 However, no broad humoral or cellular immune responses were detected in immunized individuals. In another phase I study, 84 individuals were immunized with live canarypox vectors expressing HIV-1 p55, p15, gp41, and Gp120, systemically and/or mucosally via the nose, mouth, vagina, or rectum.Citation16 Similarly, no strong titers of mucosal IgG or IgA antibodies were detected.
Over the past few years, we have been actively involved in the design and development of oral vaccine delivery systems and the entrapment of viral pathogens inside these vehicles. In these studies, various viral antigens including HIV-1, influenza, and hepatitis A viral proteins were entrapped into several oral vehicle delivery systems. Prior to the initiation of each vaccination, the inherent aspects of the delivery systems including the shape of vehicle after formulation, the particle size distribution, and the entrapment efficiency of antigens into delivery systems was determined. After the physico-chemical characterization, the immunogenicity of the candidate vaccines in mucosal and systemic sites was evaluated in animal models including mice, ferrets, and macaques. In one of our early studies, two different sizes of a bilosome-entrapped HIV-1 antigens were delivered orally in a mouse model. Bilosomes formulation is similar to liposome-like vehicles except for the presence of bile salts sodium deoxycholate in the formulation of bilosomes, making these delivery systems more stable against gastric enzymes, and providing an advantage over other conventional liposomes. We have previously showed that our candidate HIV-1 vaccine, representing hypervariable Gp120 and Gag regions, was able to induce a broad cell-mediated immune response in HLA-A2.1 mice and cynomolgus macaques against HIV-1 subtypes A–F.Citation17,Citation18 Despite a strong cell-mediated immune response, the level of mucosal immunity tended to be low in immunized animals. Therefore, we hypothesized that a multivalent HIV-1 peptide-based vaccine incorporated into a bilosome system may establish broad mucosal as well as systemic immune responses. HIV-1 antigens were entrapped into bilosomes by two different formulations (dry and wet). Prior to oral administration, the inherent aspects of bilosomes including the presence of circular lipid bilayers after formulation and the particle size distribution was determined using laser light diffraction. Both wet and dry formulations exhibited a size distribution covering a 1 to 30 µm span with the vast majority found between the 1 to15 microns range. The entrapment efficiency of the HIV-1 hypervariable regions in the bilosomes was accomplished by quantification of amino acids using the colorimetric ninhydrin assay. Interestingly, the dry bilosome preparation clearly entrapped more immunogens when compared with the wet bilosome formulation. The capabilities of both formulations to activate and mature ex-vivo generated bone marrow-derived immature dendritic cells (BMDC), were also assessed both through surface markers expression and cytokine secretion profile. Our results demonstrate that empty bilosomes have the intrinsic capacity to stimulate the maturation of ex vivo generated BMDC. Based on these results, we concluded that bilosomes appear to naturally act as immunostimulants. The presence of the HIV-1 antigens (lipidated peptides) also seems to further enhance the maturation process with a clear increase of double-positive MHCII/CD86 DCs in the bilosome preparations containing the HIV immunogens under in-vitro conditions. Dry bilosome formulations appear to drive a higher frequency of DC maturation compared with the wet formulation. As proof of concept, several groups of mice were primed/boosted orally with bilosome-entrapped HIV-1 immunogens. However, only a modest level of IgA response in the lung lavage of immunized animals was detected (data not published). No broad IgG or cell-mediated immune response was detected in peripheral sites of orally administered animals unless a systemic boost with HIV hypervariable regions was given.
Despite a tremendous effort toward reformulation of our delivery systems, including the alteration in the quantity of components in the structure of delivery systems, negative and positive charges in the surface, different incorporated amounts of antigens, and testing the administration of various doses to different animal models, the majority of developed oral vehicle delivery systems were not able to induce a broad specific humoral immune response, or even systemic IgG antibody titers, in particular. The weak immunogenicity of oral delivery systems led us to focus more on testing various adjuvants as an integral part of our newly developed oral vaccines including cholera toxin, different TLR agonists, CpG, Montanide, MPL, and Alum. Despite immunogenic outcome in some immunized groups, the data from these experiments were not consistent or repeatable.
One of a few licensed oral vaccines worldwide is Dukoral developed against Vibrio cholera. It is comprised of inactivated whole-cell Vibrio cholerae serotype 01, along with recombinant cholera toxin B-subunit (CTB).Citation19 Despite the protective efficacy of this vaccine, the correlates of immune responses have not been characterized. CTB is recognized as one of the best mucosal adjuvants as its receptor (ganglioside GM1) is present on all intestinal epithelial cell surfaces.Citation20 Therefore, CTB in the Dukoral formulation might enhance immune responses even though we have never been able to distinguish a strong humoral immune response in orally immunized mice with entrapped viral antigens and CTB alone. An interesting study by Hase et al.,Citation21 has shown that glycoprotein 2 (GP2) expressed on the apical plasma membrane of M cells is able to bound to FimH, which is a component of type I pili on the bacterial outer membrane. We have decided to examine the abilities of Vibrio cholerae after entrapment of viral antigens and Dukoral into an oral vehicle delivery system. We hypothesized that inactivated Vibrio cholerae in the Dukoral formulation is able to target M cell receptors such as GP2; therefore, by entrapment of Dukoral and the antigen of our interest in an oral vehicle delivery system, antigens might enter through the M cells and be directed toward antigen presenting cells. Different formulation of chitosan nanospheres (composed of chitosan and sodium triphosphate pentabasic at different ratios) loaded with HIV-1 Gp120 proteins MN, CM, LAN in the presence or absence of Dukoral. The candidate vaccines were administered to mice orally. The primary oral administration was followed by two more oral immunizations with two week interval between each administration. Sera and fecal pallets were collected and stored based on standard procedures. The kinetics of specific antibody induction was evaluated in each group and interestingly, the group that received Dukoral in its formulation not only had an IgA response in fecal samples but a strong specific IgG response in the serum after the last administration (). The ability of Dukoral to improve humoral immune response in systemic sites was further evaluated after the entrapment of Dukoral and other virus proteins into oral delivery systems. The groups that received Dukoral in their oral vaccine formulation demonstrated broad specific IgG in their sera, regardless of their oral delivery systems. No strong humoral response was detected in systemic sites of animals which received CTB in their oral vaccine formulation. It appears that Vibrio cholerae might be the key component in Dukoral that delivers the antigens into mucosal target cells.
Figure 1. HIV-1 specific serum IgG (A) and HIV-1 specific fecal IgA (B) antibody titers following oral immunization. Mice (n = 6) were immunized orally on days 0, 14 and 28 with chitosan nanospheres loaded with HIV-1 Gp120 proteins MN, CM, LAN and Dukoral. Sera and fecal samples were collected after each vaccination and HIV-1 specific serum IgG (A) and HIV-1 specific fecal IgA (B) antibodies were measured by ELISA. Results are shown as the mean O.D 450 nm ± SEM. The arrows indicate the day of vaccine administration.
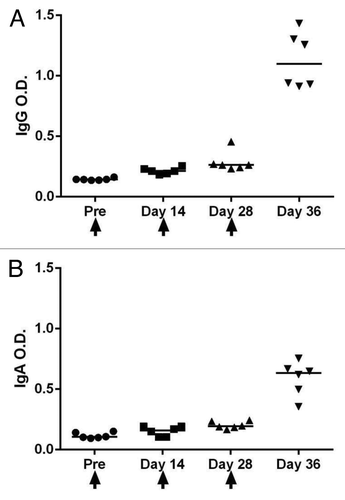
These results led us to investigate the mechanisms responsible for the induction of immune responses to the Dukoral vaccine. The initiation of immune response to pathogens might occur through receptors known as pattern-recognition receptors.Citation22,Citation23 Therefore, the involvement of TLR receptors, one of the important PRRs, in the induction of humoral or cell-mediated immune responses were evaluated after oral administration of Dukoral to TLR-2-, TLR-4-, MyD88- and Trif-deficient mice. Our results have demonstrated that antibody production following oral immunization with the Dukoral, occurs in a TLR-independent manner, whereas cell-mediated immune responses to the Dukoral were largely TLR-dependent. A peculiar finding was that humoral immune responses in MyD88−/− animals were comparable to those seen in wild type animals despite the fact that MyD88−/− DCs were impaired in their ability to mature. These findings suggest that humoral responses to the Dukoral vaccine are able to occur despite deficiencies in TLR signaling, DC functions, and possibly also cell-mediated immune responses (papers in preparation). It is possible that the components of Dukoral are able to directly activate T cell-independent B cell responses. Two comprehensive research articles are currently under preparation with regards to these results.
Concluding Remarks
The majority of currently licensed vaccines induce specific serum IgG which proposes the theory that a high level of specific IgG in systemic compartments is required to confer protective immunity against pathogens. Our results have demonstrated that the Vibrio cholerae component of the Dukoral vaccine is able to induce systemic IgG antibody titers. Although questions with regards to the mechanistic details governing the induction of immune response by the Dukoral vaccine remain unanswered, the initiation of immune response might occur through interactions between Vibrio cholerae outer membranes with potential M cell receptors. A better understanding of the mechanisms of Dukoral and other licensed oral vaccines is needed so that more effective oral vaccines can be developed and perhaps sooner than later.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
AA thank the Ontario HIV Treatment Network for support (grant no. ROGB G116) for support.
References
- McGhee JR. A mucosal gateway for vaccines. Nat Biotechnol 2011; 29:136 - 8; http://dx.doi.org/10.1038/nbt.1766; PMID: 21301439
- McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine 1992; 10:75 - 88; http://dx.doi.org/10.1016/0264-410X(92)90021-B; PMID: 1539467
- McGhee JR, Kiyono H. Mucosal immunity to vaccines: current concepts for vaccine development and immune response analysis. Adv Exp Med Biol 1992; 327:3 - 12; http://dx.doi.org/10.1007/978-1-4615-3410-5_2; PMID: 1295349
- Azizi A, Ghunaim H, Diaz-Mitoma F, Mestecky J. Mucosal HIV vaccines: a holy grail or a dud?. Vaccine 2010; 28:4015 - 26; http://dx.doi.org/10.1016/j.vaccine.2010.04.018; PMID: 20412879
- Azizi A, Kumar A, Diaz-Mitoma F, Mestecky J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog 2010; 6:e1001147; http://dx.doi.org/10.1371/journal.ppat.1001147; PMID: 21085599
- Parr EL, Parr MB. A comparison of antibody titres in mouse uterine fluid after immunization by several routes, and the effect of the uterus on antibody titres in vaginal fluid. J Reprod Fertil 1990; 89:619 - 25; http://dx.doi.org/10.1530/jrf.0.0890619; PMID: 2401988
- Katz DE, DeLorimier AJ, Wolf MK, Hall ER, Cassels FJ, van Hamont JE, et al. Oral immunization of adult volunteers with microencapsulated enterotoxigenic Escherichia coli (ETEC) CS6 antigen. Vaccine 2003; 21:341 - 6; http://dx.doi.org/10.1016/S0264-410X(02)00613-8; PMID: 12531630
- Frey A, Giannasca KT, Weltzin R, Giannasca PJ, Reggio H, Lencer WI, et al. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med 1996; 184:1045 - 59; http://dx.doi.org/10.1084/jem.184.3.1045; PMID: 9064322
- Italia JL, Kumar MN, Carter KC. Evaluating the potential of polyester nanoparticles for per oral delivery of amphotericin B in treating visceral leishmaniasis. J Biomed Nanotechnol 2012; 8:695 - 702; http://dx.doi.org/10.1166/jbn.2012.1414; PMID: 22852479
- Mann JF, Scales HE, Shakir E, Alexander J, Carter KC, Mullen AB, et al. Oral delivery of tetanus toxoid using vesicles containing bile salts (bilosomes) induces significant systemic and mucosal immunity. Methods 2006; 38:90 - 5; http://dx.doi.org/10.1016/j.ymeth.2005.11.002; PMID: 16414269
- Shukla A, Katare OP, Singh B, Vyas SP. M-cell targeted delivery of recombinant hepatitis B surface antigen using cholera toxin B subunit conjugated bilosomes. Int J Pharm 2010; 385:47 - 52; http://dx.doi.org/10.1016/j.ijpharm.2009.10.027; PMID: 19835938
- Shukla A, Khatri K, Gupta PN, Goyal AK, Mehta A, Vyas SP. Oral immunization against hepatitis B using bile salt stabilized vesicles (bilosomes). J Pharm Pharm Sci 2008; 11:59 - 66; PMID: 18445364
- Rescia VC, Takata CS, de Araujo PS, Bueno da Costa MH. Dressing liposomal particles with chitosan and poly(vinylic alcohol) for oral vaccine delivery. J Liposome Res 2011; 21:38 - 45; http://dx.doi.org/10.3109/08982101003735988; PMID: 20470223
- van der Lubben IM, Verhoef JC, van Aelst AC, Borchard G, Junginger HE. Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer’s patches. Biomaterials 2001; 22:687 - 94; http://dx.doi.org/10.1016/S0142-9612(00)00231-3; PMID: 11246962
- Lambert JS, Keefer M, Mulligan MJ, Schwartz D, Mestecky J, Weinhold K, et al. A Phase I safety and immunogenicity trial of UBI microparticulate monovalent HIV-1 MN oral peptide immunogen with parenteral boost in HIV-1 seronegative human subjects. Vaccine 2001; 19:3033 - 42; http://dx.doi.org/10.1016/S0264-410X(01)00051-2; PMID: 11311997
- Wright PF, Mestecky J, McElrath MJ, Keefer MC, Gorse GJ, Goepfert PA, et al, National Institutes of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. Comparison of systemic and mucosal delivery of 2 canarypox virus vaccines expressing either HIV-1 genes or the gene for rabies virus G protein. J Infect Dis 2004; 189:1221 - 31; http://dx.doi.org/10.1086/382088; PMID: 15031791
- Azizi A, Anderson DE, Torres JV, Ogrel A, Ghorbani M, Soare C, et al. Induction of broad cross-subtype-specific HIV-1 immune responses by a novel multivalent HIV-1 peptide vaccine in cynomolgus macaques. J Immunol 2008; 180:2174 - 86; PMID: 18250424
- Ghunaim H, Kumar A, Torres J, Diaz-Mitoma F, Azizi A. An immunological comparison between lipidated and non-lipidated multivalent HIV-1 peptides representing Gp120 and Gag hypervariable regions. Vaccine 2011; 29:5950 - 8; http://dx.doi.org/10.1016/j.vaccine.2011.06.047; PMID: 21718739
- Jelinek T, Kollaritsch H. Vaccination with Dukoral against travelers’ diarrhea (ETEC) and cholera. Expert Rev Vaccines 2008; 7:561 - 7; http://dx.doi.org/10.1586/14760584.7.5.561; PMID: 18564011
- Bunyakul N, Edwards KA, Promptmas C, Baeumner AJ. Cholera toxin subunit B detection in microfluidic devices. Anal Bioanal Chem 2009; 393:177 - 86; http://dx.doi.org/10.1007/s00216-008-2364-6; PMID: 18777170
- Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 2009; 462:226 - 30; http://dx.doi.org/10.1038/nature08529; PMID: 19907495
- Bauer S, Müller T, Hamm S. Pattern recognition by Toll-like receptors. Adv Exp Med Biol 2009; 653:15 - 34; http://dx.doi.org/10.1007/978-1-4419-0901-5_2; PMID: 19799109
- Kirschning CJ, Bauer S. Toll-like receptors: cellular signal transducers for exogenous molecular patterns causing immune responses. Int J Med Microbiol 2001; 291:251 - 60; http://dx.doi.org/10.1078/1438-4221-00128; PMID: 11680785