Abstract
Fusions of antigen presenting cells and tumor cells have been investigated in animal models and phase I/II clinical trials as candidate cancer vaccines. In animal studies there have been numerous reports of induction of protective immunity against a wide range of tumor types. Results of clinical trials have been less dramatic, but tumor-specific immune responses have been reported in many patients, with clinical responses to the vaccination in a subset. In this commentary article, I review the current status of antigen presenting cell/tumor cell fusion vaccines for cancer immunotherapy.
Introduction
There has been considerable interest in recent years in the development of immunotherapeutic approaches for treating cancer. This has been strengthened by the approval by the FDA in 2010 of the first therapeutic dendritic cell-based vaccine specifically designed for the treatment of cancer.Citation1 Many experimental cancer immunotherapy approaches are founded on the use of professional antigen presenting cells (APCs), such as dendritic cells, as stimulators of tumor-specific immune responses, and in particular in inducing tumor antigen-specific cytotoxic T-cell (CTL) responses capable of killing tumor cells in situ. One such strategy has been the development of APC/ tumor fusion cells as candidate cancer vaccines. The approach was first described by Guo and colleagues,Citation2 who showed that rats vaccinated with fusions of hepatoma cells and activated B-cells were resistant to subsequent tumor challenge, and that animals with established tumors rejected them, in a manner that was dependent on both CD4+ and CD8+ T-cells. In this commentary, I shall review the current status, future prospects and limitations of APC / tumor cell fusion vaccines as immunotherapeutic agents for treating cancer.
The Concept of APC/Tumor Fusion Cell Vaccines
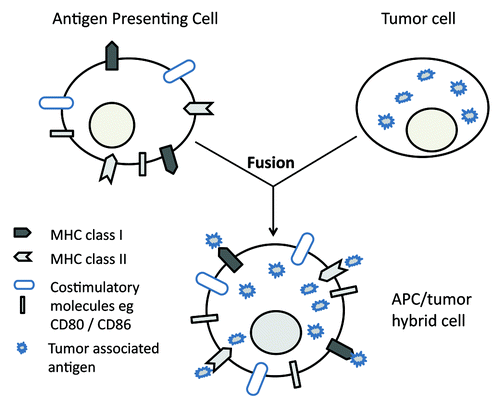
The concept of APC/tumor cell fusion vaccines is relatively simple (). Tumor cells express antigens that distinguish them from their normal somatic cell counterparts, but do not present these antigens to the host immune system in a manner that stimulates an effective tumor-specific immune response (indeed, many if not all tumors evolve mechanisms by which they evade such responsesCitation3). Mechanisms of immune evasion by tumors include downregulation of antigen processing, loss of expression of MHC molecules, and lack of expression of costimulatory molecules. Professional APCs are potent inducers of antigen specific T-cell responses, with constitutive expression of both MHC class I and MHC class II, high levels of antigen processing and presentation, and expression of relevant T-cell costimulatory molecules. By fusing tumor cells with professional APCs in vitro, we can generate fusion hybrid cells that express the relevant tumor-associated antigens (TAAs; derived from the parent tumor cells) and have the capacity to process and present these to the immune system in a manner that induces effective tumor-specific immunity (derived from the parent APC) (). Fusions of tumor cells and APCs are therefore attractive candidates for use in cancer immunotherapy, as they express multiple tumor antigens, process these through both MHC class I and class II pathways, and present the processed antigens to the host immune system in the context of effective T-cell costimulation.
Dendritic Cell/Tumor Cell Fusion Vaccines
Dendritic cells (DCs) are the most potent professional antigen presenting cells, and have been widely used as the APC partner in fusion cell vaccines. DC / tumor cell hybrids have been the subject of both animal studies and human clinical trials.
Animal studies
Following on from the work of Guo et al.,Citation2 Gong and colleagues demonstrated that vaccination of mice with fusions of syngeneic DCs and murine MC38 carcinoma cells induced tumor-specific CTLs in vivo, protected against tumor cell challenge, and was associated with eradication of established tumors.Citation4 Numerous subsequent studies have confirmed the ability of DC / tumor fusion cell vaccines to induce protective tumor-specific immune responses against a wide range of tumor types, including renal, colon, lung, breast, hepatic and cervical carcinomas, melanoma, sarcoma, neurological and hematological tumors, in animal models.Citation5-Citation14 In several studies in tumor-prone mouse strains, vaccination with fusion cell vaccines was shown to protect against or delay the development of tumors.Citation7,Citation9,Citation10 Both syngeneic and allogeneic DCs have been shown to be effective as APC fusion partners in inducing tumor-specific immune responses and protection against tumor challenge, and Takeda et al. showed that DCs derived from tumor bearing mice were equally effective as DCs from healthy mice at inducing protective immunity, when used as APC partners in fusion cell vaccines.Citation15 The mechanism of protective immunity induced by DC / tumor cell fusion vaccines appears to depend on their ability to induce both CD4+ and CD8+ T-cells, with CD8+ antigen-specific CTLs representing the major mediators of tumor rejection.Citation4-Citation6,Citation8,Citation9,Citation12,Citation13
Clinical trials
The apparent efficacy of fusion cell vaccines in animal models of tumor immunity prompted translation of the approach to human studies. Several studies showed the induction of tumor-specific CTL in vitro by human DC / tumor fusion cells.Citation16-Citation20 Phase I/II clinical trials of DC/tumor cell fusion vaccines have been reported in patients with a range of tumors, including melanoma, renal and breast cancers, and multiple myeloma.Citation21-Citation29 As is often the case, the results in clinical trials of DC / tumor fusion vaccines have been much less dramatic than in animal studies. However, immunological responses have been reported in many patients, with clinical responses in a subset, and the approach has proved safe with minimal toxicity.Citation21-Citation24,Citation26,Citation28,Citation29 For example, Trefzer et al. reported one complete clinical remission, 1 partial response and 6 cases of disease stabilization in 17 patients with stage III/IV melanoma immunized with a fusion cell vaccine of autologous tumor and allogeneic dendritic cells, with 11 of 14 patients analyzed demonstrating T-cell responses to tumor-associated T-cell epitopes.Citation25 Similarly, of 21 renal cell cancer patients vaccinated with autologous tumor / allogeneic dendritic cell fusions, 2 showed partial clinical responses and 8 showed disease stabilization.Citation28 Ten of the 21 patients in this study demonstrated anti-tumor immune responses (increased CD4 and/or CD8 T-cell expression of interferon-gamma after ex vivo exposure to tumor cell lysate) following the immunization.Citation28 Using autologous DC / tumor cell fusions to immunize 23 patients with breast or renal cancer, Avigan et al. showed disease regression in 2 patients with breast cancer, and disease stabilization in 6 more of the vaccinated patients.Citation26 Finally, in a study of vaccination of 17 patients with multiple myeloma, using autologous DC / tumor cell fusions, evidence of T-cell responses to autologous tumor cells was seen in 11 patients, with disease stabilization reported in the majority of evaluable patients.Citation29 Larger, placebo controlled studies would be needed, however, to demonstrate whether these responses represent significant therapeutic benefit.
In spite of promising results in animal studies, a number of aspects of DC / tumor cell fusions may limit their wider clinical application in cancer immunotherapy. First, in vitro culture of DCs is time and labor intensive, and there is the need to generate fusion products specific for each patient (although this did not prevent the commercial development of Sipuleucel-T as a cancer vaccineCitation1). Other considerations include the optimal culture conditions for DC differentiation and maturation prior to fusion with the tumor cells, fusion efficiencies (which are often low with current methods of generating hybrid cells, necessitating separation of hybrid cells from non-fused parent cells), and the fusions themselves are short lived and have limited replicative capacity, limiting the numbers of fusion cells generated for use in vaccination. In addition, the nature of the processes of culture, isolation and cell fusion of DCs and ex vivo tumor cells limits standardisation and characterization of the fusion cells.
Non-DC fusion vaccines
While the majority of studies of fusion cell vaccines have used autologous or allogeneic DCs as the APC fusion partner, non-DC APCs have also been used in the generation of fusion cell vaccines. As mentioned above, Guo et al. used activated B-cells as APCs in their study of fusion cell vaccines against hepatocellular cancer in rats.Citation2 Several phase I clinical trials have been reported using non-DC APCs as fusion cell vaccine partners, including activated autologous B cells, activated allogeneic peripheral blood lymphocytes, and a genetically engineered melanoma cell line (FO1-12).Citation30-Citation32 As with the clinical trials using DC / tumor fusion cell vaccines, clinical or immunological responses were reported in individual patients, and the approaches were safe with minimal toxicity.
The use of EBV B-lymphoblastoid cells as APC
As an alternative to DCs, we have pioneered the use of EBV B-lymphoblastoid cells (B-LCL) as APCs in generating tumor hybrid cell lines.Citation33-Citation35 EBV B-LCL exhibit key features of professional APCs, with high constitutive expression of MHC class I and class II, and T-cell costimulatory molecules.Citation33 Unlike in vitro generated DCs, EBV B-LCL are immortalised for growth in cell culture. The LCL used (HMy2) has been modified to allow for double chemical selection of the fusion cells, facilitating the selection of stable, self-replicating LCL / tumor hybrid cell lines following fusion.Citation36 Stimulation of (allogeneic) peripheral blood T-cells from both healthy donors and tumor-bearing patients in vitro using LCL / tumor hybrid cell lines induced tumor antigen-specific CTLs that killed tumor cells presenting the relevant antigen(s), demonstrating the potential of these hybrid cell lines to induce tumor-specific immune responses in humans, in vitro at least.Citation34,Citation35 A major advantage of LCL / tumor hybrid cells is the production of stable, self-replicating hybrid cell lines, that allows for detailed characterization of the hybrid cells, and for the growth of large numbers of standardised cells for potential use as vaccines. To date, however, LCL / tumor hybrid cell vaccines have not been tested in vivo in animal models (due to the lack of a suitable animal equivalent of EBV B-LCL in mice) or in clinical trials in humans.
Potential Uses of Hybrid Vaccines in Cancer Immunotherapy
Most studies of APC / tumor fusion cells have been based on their potential use as therapeutic vaccines for active immunotherapy in tumor-bearing patients. In this context, both fully autologous (APC and tumor cells derived from the individual to be immunized) and semi-autologous (tumor cells from the patient, fused with allogeneic APCs) approaches have been tested. Indeed, in some animal studies, fusion vaccines expressing allogeneic MHC were found to induce stronger protective immunity than syngeneic vaccines.Citation11,Citation14 Animal studies suggest that the approach may be applicable to a broad range of tumor types, including both solid and hematological tumors. A number of questions relating to therapeutic cancer vaccination remain unanswered, however, including the optimal dose, route and schedule of vaccine administration, the use and choice of adjuvants or adjunct treatments (such as depletion or blockade of regulatory T cells, or of the immunosuppressive PD-1/PD-L1 pathway), and (for dendritic cell-based vaccines) the type of DC and stage of differentiation, for optimal immunization.
An alternative use of APC/tumor fusion cells in cancer immunotherapy is as in vitro stimulators of tumor-specific CTL for adoptive cellular immunotherapy.Citation20,Citation34 Adoptive CTL therapy has been used with clinical benefit in a range of tumor types, including hematological and non-hematological tumors.Citation37-Citation39 Given some of the current uncertainties of APC/ tumor cells as therapeutic cancer vaccines in humans, and the demonstrated ability of APC/ tumor hybrid cells to induce tumor antigen-specific CTL in vitro,Citation16-Citation20,Citation34,Citation35 this approach to the use of APC/ tumor hybrid cells merits further investigation. Indeed, one study (in mice) has reported that the combination of hybrid cell vaccination with hybrid cell-primed adoptive T-cell therapy, in treating a poorly immunogenic tumor (Lewis lung carcinoma), was associated with significant delay in tumor development, reduced pulmonary metastasis, and increased survival of the animals.Citation40
Summary and Future Perspectives
In summary, numerous animal studies over the past 20 years have demonstrated the efficacy of APC/tumor cell fusion vaccines in protecting against tumor cell challenge, in eradicating established tumors, and in preventing tumor development in tumor-prone animals. Phase I/II clinical trials have demonstrated the safety and tolerability of the approach, although efficacy in human studies has been much less dramatic than that demonstrated in animal models, and there are a number of outstanding questions regarding optimization of the approach for use in humans. Further investigation of the approach, to address these questions in relation to APC/tumor hybrid cells as cancer vaccines, is warranted. In addition to their potential as therapeutic cancer vaccines, APC/tumor fusion cells may have a role as inducers of tumor antigen-specific CTL in vitro, for potential use in adoptive T-cell cancer immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Sims RB. Development of sipuleucel-T: autologous cellular immunotherapy for the treatment of metastatic castrate resistant prostate cancer. Vaccine 2012; 30:4394 - 7; http://dx.doi.org/10.1016/j.vaccine.2011.11.058; PMID: 22122856
- Guo Y, Wu M, Chen H, Wang X, Liu G, Li G, et al. Effective tumor vaccine generated by fusion of hepatoma cells with activated B cells. Science 1994; 263:518 - 20; http://dx.doi.org/10.1126/science.7507262; PMID: 7507262
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3:991 - 8; http://dx.doi.org/10.1038/ni1102-991; PMID: 12407406
- Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med 1997; 3:558 - 61; http://dx.doi.org/10.1038/nm0597-558; PMID: 9142127
- Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol 1998; 161:5516 - 24; PMID: 9820528
- Gong J, Koido S, Chen D, Tanaka Y, Huang L, Avigan D, et al. Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12. Blood 2002; 99:2512 - 7; http://dx.doi.org/10.1182/blood.V99.7.2512; PMID: 11895787
- Chen D, Xia J, Tanaka Y, Chen H, Koido S, Wernet O, et al. Immunotherapy of spontaneous mammary carcinoma with fusions of dendritic cells and mucin 1-positive carcinoma cells. Immunology 2003; 109:300 - 7; http://dx.doi.org/10.1046/j.1365-2567.2003.01656.x; PMID: 12757626
- Siders WM, Vergilis KL, Johnson C, Shields J, Kaplan JM. Induction of specific antitumor immunity in the mouse with the electrofusion product of tumor cells and dendritic cells. Mol Ther 2003; 7:498 - 505; http://dx.doi.org/10.1016/S1525-0016(03)00044-3; PMID: 12727113
- Xia J, Tanaka Y, Koido S, Liu C, Mukherjee P, Gendler SJ, et al. Prevention of spontaneous breast carcinoma by prophylactic vaccination with dendritic/tumor fusion cells. J Immunol 2003; 170:1980 - 6; PMID: 12574367
- Iinuma T, Homma S, Noda T, Kufe D, Ohno T, Toda G. Prevention of gastrointestinal tumors based on adenomatous polyposis coli gene mutation by dendritic cell vaccine. J Clin Invest 2004; 113:1307 - 17; PMID: 15124022
- Suzuki T, Fukuhara T, Tanaka M, Nakamura A, Akiyama K, Sakakibara T, et al. Vaccination of dendritic cells loaded with interleukin-12-secreting cancer cells augments in vivo antitumor immunity: characteristics of syngeneic and allogeneic antigen-presenting cell cancer hybrid cells. Clin Cancer Res 2005; 11:58 - 66; PMID: 15671528
- Tanaka Y, Koido S, Ohana M, Liu C, Gong J. Induction of impaired antitumor immunity by fusion of MHC class II-deficient dendritic cells with tumor cells. J Immunol 2005; 174:1274 - 80; PMID: 15661883
- Savai R, Schermuly RT, Schneider M, Pullamsetti SS, Grimminger F, Seeger W, et al. Hybrid-primed lymphocytes and hybrid vaccination prevent tumor growth of lewis lung carcinoma in mice. J Immunother 2006; 29:175 - 87; http://dx.doi.org/10.1097/01.cji.0000197096.38476.fc; PMID: 16531818
- Yasuda T, Kamigaki T, Kawasaki K, Nakamura T, Yamamoto M, Kanemitsu K, et al. Superior anti-tumor protection and therapeutic efficacy of vaccination with allogeneic and semiallogeneic dendritic cell/tumor cell fusion hybrids for murine colon adenocarcinoma. Cancer Immunol Immunother 2007; 56:1025 - 36; http://dx.doi.org/10.1007/s00262-006-0252-5; PMID: 17131118
- Takeda A, Homma S, Okamoto T, Kufe D, Ohno T. Immature dendritic cell/tumor cell fusions induce potent antitumour immunity. Eur J Clin Invest 2003; 33:897 - 904; http://dx.doi.org/10.1046/j.1365-2362.2003.01194.x; PMID: 14511362
- Gong J, Koido S, Kato Y, Tanaka Y, Chen D, Jonas A, et al. Induction of anti-leukemic cytotoxic T lymphocytes by fusion of patient-derived dendritic cells with autologous myeloblasts. Leuk Res 2004; 28:1303 - 12; http://dx.doi.org/10.1016/j.leukres.2004.03.018; PMID: 15475072
- Raje N, Hideshima T, Davies FE, Chauhan D, Treon SP, Young G, et al. Tumour cell/dendritic cell fusions as a vaccination strategy for multiple myeloma. Br J Haematol 2004; 125:343 - 52; http://dx.doi.org/10.1111/j.1365-2141.2004.04929.x; PMID: 15086415
- Klammer M, Waterfall M, Samuel K, Turner ML, Roddie PH. Fusion hybrids of dendritic cells and autologous myeloid blasts as a potential cellular vaccine for acute myeloid leukaemia. Br J Haematol 2005; 129:340 - 9; http://dx.doi.org/10.1111/j.1365-2141.2005.05477.x; PMID: 15842657
- Imura K, Ueda Y, Hayashi T, Itoh T, Shimizu K, Tamai H, et al. Induction of cytotoxic T lymphocytes against human cancer cell lines using dendritic cell-tumor cell hybrids generated by a newly developed electrofusion technique. Int J Oncol 2006; 29:531 - 9; PMID: 16865268
- Rosenblatt J, Wu Z, Vasir B, Zarwan C, Stone R, Mills H, et al. Generation of tumor-specific T lymphocytes using dendritic cell/tumor fusions and anti-CD3/CD28. J Immunother 2010; 33:155 - 66; http://dx.doi.org/10.1097/CJI.0b013e3181bed253; PMID: 20145548
- Trefzer U, Weingart G, Chen Y, Herberth G, Adrian K, Winter H, et al. Hybrid cell vaccination for cancer immune therapy: first clinical trial with metastatic melanoma. Int J Cancer 2000; 85:618 - 26; http://dx.doi.org/10.1002/(SICI)1097-0215(20000301)85:5<618::AID-IJC4>3.0.CO;2-Z; PMID: 10699939
- Krause SW, Neumann C, Soruri A, Mayer S, Peters JH, Andreesen R. The treatment of patients with disseminated malignant melanoma by vaccination with autologous cell hybrids of tumor cells and dendritic cells. J Immunother 2002; 25:421 - 8; http://dx.doi.org/10.1097/00002371-200209000-00006; PMID: 12218780
- Märten A, Renoth S, Heinicke T, Albers P, Pauli A, Mey U, et al. Allogeneic dendritic cells fused with tumor cells: preclinical results and outcome of a clinical phase I/II trial in patients with metastatic renal cell carcinoma. Hum Gene Ther 2003; 14:483 - 94; http://dx.doi.org/10.1089/104303403321467243; PMID: 12691613
- Barbuto JA, Ensina LF, Neves AR, Bergami-Santos P, Leite KR, Marques R, et al. Dendritic cell-tumor cell hybrid vaccination for metastatic cancer. Cancer Immunol Immunother 2004; 53:1111 - 8; http://dx.doi.org/10.1007/s00262-004-0551-7; PMID: 15185011
- Trefzer U, Herberth G, Wohlan K, Milling A, Thiemann M, Sherev T, et al. Vaccination with hybrids of tumor and dendritic cells induces tumor-specific T-cell and clinical responses in melanoma stage III and IV patients. Int J Cancer 2004; 110:730 - 40; http://dx.doi.org/10.1002/ijc.20191; PMID: 15146563
- Avigan D, Vasir B, Gong J, Borges V, Wu Z, Uhl L, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res 2004; 10:4699 - 708; http://dx.doi.org/10.1158/1078-0432.CCR-04-0347; PMID: 15269142
- Trefzer U, Herberth G, Wohlan K, Milling A, Thiemann M, Sharav T, et al. Tumour-dendritic hybrid cell vaccination for the treatment of patients with malignant melanoma: immunological effects and clinical results. Vaccine 2005; 23:2367 - 73; http://dx.doi.org/10.1016/j.vaccine.2005.01.081; PMID: 15755630
- Avigan DE, Vasir B, George DJ, Oh WK, Atkins MB, McDermott DF, et al. Phase I/II study of vaccination with electrofused allogeneic dendritic cells/autologous tumor-derived cells in patients with stage IV renal cell carcinoma. J Immunother 2007; 30:749 - 61; http://dx.doi.org/10.1097/CJI.0b013e3180de4ce8; PMID: 17893567
- Rosenblatt J, Vasir B, Uhl L, Blotta S, Macnamara C, Somaiya P, et al. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood 2011; 117:393 - 402; http://dx.doi.org/10.1182/blood-2010-04-277137; PMID: 21030562
- Moviglia GA. Development of tumor B-cell lymphocyte hybridoma (TBH) autovaccination. Results of a phase I-II clinical trial. Transfus Sci 1996; 17:643 - 9; PMID: 10168565
- Kugler A, Seseke F, Thelen P, Kallerhoff M, Müller GA, Stuhler G, et al. Autologous and allogenic hybrid cell vaccine in patients with metastatic renal cell carcinoma. Br J Urol 1998; 82:487 - 93; http://dx.doi.org/10.1046/j.1464-410X.1998.00794.x; PMID: 9806175
- Newton DA, Acierno PM, Metts MC, Baron PL, Brescia FJ, Gattoni-Celli S. Semiallogeneic cancer vaccines formulated with granulocyte-macrophage colony-stimulating factor for patients with metastatic gastrointestinal adenocarcinomas: a pilot phase I study. J Immunother 2001; 24:19 - 26; http://dx.doi.org/10.1097/00002371-200101000-00003; PMID: 11211145
- Dunnion DJ, Cywinski AL, Tucker VC, Murray AK, Rickinson AB, Coulie P, et al. Human antigen-presenting cell/tumour cell hybrids stimulate strong allogeneic responses and present tumour-associated antigens to cytotoxic T cells in vitro. Immunology 1999; 98:541 - 50; http://dx.doi.org/10.1046/j.1365-2567.1999.00912.x; PMID: 10594686
- Mohamed YS, Dunnion D, Teobald I, Walewska R, Browning MJ. Long-lived fusions of human haematological tumour cells and B-lymphoblastoid cells induce tumour antigen-specific cytotoxic T-cell responses in vitro. Immunobiology 2012; 217:719 - 29; http://dx.doi.org/10.1016/j.imbio.2011.12.001; PMID: 22305004
- Mohamed YS, Dunnion D, Teobald I, Walewska R, Browning MJ. In vitro evaluation of human hybrid cell lines generated by fusion of B-lymphoblastoid cells and ex vivo tumour cells as candidate vaccines for haematological malignancies. Vaccine 2012; 30:6578 - 87; http://dx.doi.org/10.1016/j.vaccine.2012.08.032; PMID: 22939910
- Edwards PA, Smith CM, Neville AM, O’Hare MJ. A human-hybridoma system based on a fast-growing mutant of the ARH-77 plasma cell leukemia-derived line. Eur J Immunol 1982; 12:641 - 8; http://dx.doi.org/10.1002/eji.1830120804; PMID: 7140810
- Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010; 115:925 - 35; http://dx.doi.org/10.1182/blood-2009-08-239186; PMID: 19880495
- Warren EH, Fujii N, Akatsuka Y, Chaney CN, Mito JK, Loeb KR, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood 2010; 115:3869 - 78; http://dx.doi.org/10.1182/blood-2009-10-248997; PMID: 20071660
- Montagna D, Turin I, Schiavo R, Montini E, Zaffaroni N, Villa R, et al. Feasibility and safety of adoptive immunotherapy with ex vivo-generated autologous, cytotoxic T lymphocytes in patients with solid tumor. Cytotherapy 2012; 14:80 - 90; http://dx.doi.org/10.3109/14653249.2011.610303; PMID: 21942841
- Savai R, Schermuly RT, Pullamsetti SS, Schneider M, Greschus S, Ghofrani HA, et al. A combination hybrid-based vaccination/adoptive cellular therapy to prevent tumor growth by involvement of T cells. Cancer Res 2007; 67:5443 - 53; http://dx.doi.org/10.1158/0008-5472.CAN-06-3677; PMID: 17545626