Abstract
Leukemia-associated antigens such as proteinase-3 (PR3) and Wilms´ tumor protein-1 (WT-1) are potential targets of T-cell responses, which can be monitored by T-cell assays within vaccination trials and after allogeneic stem cell transplantation (SCT). In chronic myeloid leukemia (CML) an aberrant cytokine profile of antigen-specific T-cells with predominant TNF-a secretion has previously been described. The aim of this study was to investigate whether these TNF-α+/IFN-γ- CD8+ T-cells can also be observed in AML patients after SCT. Eight HLA-A2+ AML patients at different time points after SCT were evaluated for HLA-A2-restricted CD8+ T-cell responses against PR3, WT-1 and influenza-A using pentamer staining and different cytokine-based T-cell assays. Antigen-specific T-cell immune responses against influenza-A and PR3 were observed in 4/8 patients, WT-1-specific T-cells could be detected in in 3/8 patients. Interestingly, four different cytokine secretion profiles of antigen-specific T-cells were detected:Citation1 IFN-γ+/TNF-α+,Citation2 IFN-γ+/TNF-α-,Citation3 TNF-α+/ IFN-γ- andCitation4 IFN-γ-/TNF-α-. TNF-α+/ IFN-γ- CD8+ T-cells are an interesting biological phenomenon which can obviously be observed also in AML patients. This finding has important implications for both T-cell biology and monitoring within immunotherapy trials. The functional characterization of these TNF-α+/ IFN-γ- CD8+ T-cells needs further investigations.
Keywords: :
There is increasing evidence that acute myeloid leukemia (AML) cells may be targets for immune responses within immunotherapy trials and after allogeneic stem cell transplantation (SCT). Proteinase-3 (PR3) and Wilms´ tumor protein-1 (WT-1) are leukemia-associated antigens that are overexpressed in most cases of AML. Clinical studies with therapeutic vaccines have demonstrated T-cell responses against these antigens.Citation1,Citation2 Immunomonitoring of antigen-specific T-cell responses with appropriate T-cell assays plays a pivotal role for the evaluation of these clinical immunotherapy trials. The use of both structural and cytokine-based T-cell assays is of great importance since leukemia-reactive T-cells may display different cytokine profiles reflecting different stages of activation or exhaustion/anergy.Citation3 We and others have previously reported that leukemia-reactive T-cells in chronic myeloid leukemia (CML) may show an impaired γ-interferon (IFN-γ)- release in combination with predominant tumor necrosis factor-α (TNF-α) secretion upon antigen-specific stimulation.Citation4,Citation5 However, it was not clear whether this is a characteristic feature of CML or a more general phenomenon possibly reflecting defective activation or exhaustion of T-cells. Furthermore, it was not directly demonstrated in these previous studies that CD8+ T- cells are the source of antigen-specific TNF-α secretion. The presence of antigen-specific TNF-α+/IFN-γ- T-cells has two major implications:Citation1 they may not be detected using usual functional T-cell assays which are most often based on the detection of IFN-γ, IL-2, IL-4 and IL-10;Citation2 they constitute an interesting biological phenomenon since they may represent an intermediate stage of T-cell function between activation and anergy. In the present study we asked whether TNF-α+ T-cells can also be observed in AML and whether antigen-specific release of TNF-α is in fact caused by CD8+ T-cells. We chose patients at different time points after allogeneic stem cell transplantation (SCT), since the immune milieu in these patients is particularly favorable for emerging T-cell responses.Citation1,Citation2
Immunological analyses were performed in blood samples from HLA-A2+ AML patients who had undergone allogeneic SCT after having obtained written informed consent and in accordance with local ethical guidelines. Eight patients (5 males, 3 females) were included. Six patients had been transplanted in first CR, two patients in second CR. In three cases the donor was a HLA-identical sibling, in five cases an unrelated HLA-identical donor. The conditioning regimen consisted of 6 × 2 Gy TBI + cyclophosphamide in 6 patients and fludarabine/busulfane/ATG in two patients.
For the evaluation of CD8+ T-cell responses by means of ELISpot, cytokine bead array (CBA) and intracytoplasmatic cytokine staining (ICS) by flow cytometry the following HLA-A2-restricted peptides were used: VLQELNVTV (proteinase-3), RMFPNAPYL (WT-1), GILGFVFTL (influenza-A virus) and an irrelevant HLA-A2-restricted HIV control peptide. All peptides were generated by FMOC-peptide synthesis with a purity of 98% (Wita GmbH, Teltow, Germany). T cell assays were performed as previously described.Citation6
Briefly, for ELISpot assays 9 × 105 or 106 PBMC were seeded into 9 respectively 10 wells (105 PBMC/well) on antibody (anti-IFN-γ and anti-TNF-α) pre-coated ELISpot plates (Multiscreen). Peptides were added at a concentration of 10 μg/ml. Incubation was performed overnight and ELISpot plates were developed according to the manufacturer’s instructions. Evaluation was performed by means of an AID ELISpot reader system (AID, Strassberg, Germany). Peptide-specific responses were defined as havingCitation1 a ratio of specific peptide: control > 2 andCitation2 an absolute number of spots > 10. Results were expressed as „spots per number of PBMC“.
Cytometric bead array (CBA) assays permit simultaneous cytometric quantitation of multiple cytokines in solution by capturing these to spectrally distinct beads. The cytokine pattern measured by the CBA used (CBA, Becton and Dickinson) consists of IFN-γ, IL-2, TNF-α, IL-4, IL-10 and IL-5. 1 × 105 PBMC were cultured overnight in complete medium (RPMI-1640, Gibco, Eggenstein, Germany) in the presence of 10% human AB serum and 10 μg/ml peptide. Culture supernatant was frozen at -20°C until cytokine analysis was performed according to the manufacturer’s instructions. A peptide-specific response was defined as having a ratio “cytokine secretion of specific peptide: cytokine secretion of control peptide” ≥ 2 and mean fluorescence intensity (MFI) of specific peptide: MFI of control peptide > 2.
Intracytoplasmatic cytokine staining (ICS) was performed by using the MACS secretion assay (Miltenyi, Bergisch Gladbach, Germany) for the staining procedure. Briefly, fresh heparinized blood was stimulated in an incubator for 1 h with PR3, WT-1 and influenza-A virus peptide. PBMC pulsed with an irrelevant control peptide were used as a negative control. PHA- stimulated PBMC served as a positive control. Analysis was performed after exclusion of dead cells by propidium iodide (PI) staining (live gate). The gate of interest was further defined by light scatter properties (lymphocyte gate) and CD3 and/or CD8 staining (anti CD3-APC, anti CD-8-FITC, both BD) in order to restrict the analysis on CD8+ T-cells in the lymphocyte gate. IFN-γ- or TNF-α-positive CD8+ T-cells were considered as being peptide-specific, if their frequency was at least twice of that measured after stimulation with control peptide.
Pentamer staining was performed according to the manufacturer’s instructions (ProImmune) using pentamers specific for HLA-A2-restricted peptides from PR3, WT-1 and influenza-A virus (see above). The gate of interest was defined by light scatter properties (lymphocyte gate) and staining with anti CD3 and anti CD8 antibodies (anti CD3-APC, anti CD-8-FITC, both BD). Usually more than 1 × 105 cells were analyzed. T-cells were only considered pentamer-positive, if a distinct pentamerbright population ≥ 0,01% of CD8+ T-cells could be identified in the gate of interest in which no specific events were observed after control staining. All events observed in the gate of interest were “backgated” into the lymphocyte gate in order to get additional information on their specificity. Flowcytometric analyses were performed on a FACS Calibur (Beckton and Dickinson) using CellQuest software.
Eight HLA-A2+ patients after SCT for AML were evaluated. Median age was 47 y (range 20–64 y). 6/8 patients had received SCT in first CR, 2/8 patients in second CR. T-cell assays were performed at different time points after SCT (range 1–15 mo). Since our study was aimed at detecting the cytokine secretion profile of low frequency leukemia-reactive T cells, in vitro restimulation or in vitro expansion of T cells had to be avoided in order to maintain the original cytokine pattern upon antigenic stimulation. Under these circumstances, T-cell frequencies close to the lower detection limit of standard T-cell assays had to be assumed. Therefore, we used a combination of a structural T-cell assay (pentamer staining) with different cytokine-based assays [intracytoplasmatic cytokine staining (ICS), cytokine bead array (CBA), Enzyme-linked Immuno Spot Assay (ELISpot)] aiming at a confirmation of these very small T- cell populations by a second complementary assay. In some patients it was not possible to perform all T-cell assays due to a limited number of PBMC obtained (). The use of ICS enabled us to show that TNF-α or IFN-γ were in fact produced by CD8+ T-cells since this assay allows to exclude non-CD8+ T cells from analysis (see gating strategy described in the methods section). TNF-α-ELISpot was abandoned after the use in some patients because the analysis was severely impaired by high background staining (data not shown).
Table 1. T cell assays detecting antigen-specific T cells: Overview
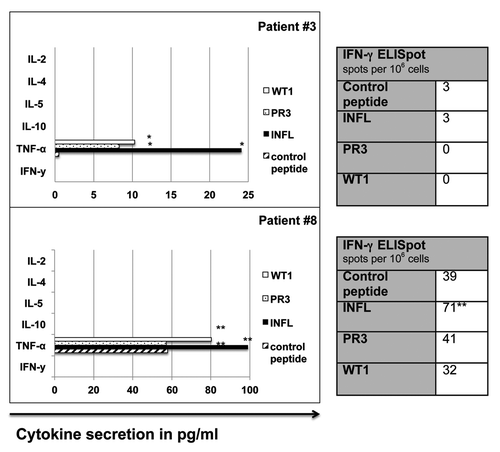
In our cohort, 4/8 patients showed immune responses against influenza-A and PR3. WT-1- specific immune responses were documented in 3/8 patients (see and ). Interestingly, different cytokine secretion profiles could be observed in antigen- specific T-cells within our patient cohort (): IFN-γ+/TNF-α+ T-cells recognizing influenza-A were detected in only one patient (# 5) and IFN-γ+/TNF-α- T-cells recognizing PR3 in two patients (# 2 and 5). Interestingly, TNF-α+/ IFN-γ- T-cells recognizing influenza-A, PR3 and WT-1 were detected in two patients (# 3 and 8, ). In these two patients, TNF-α was the only cytokine, which was released upon peptide stimulation as measured by CBA (). Finally, IFN-γ-/TNF-α-/pentamer+ T-cells against WT-1 (#4) and WT-1 and influenza-A (#2) were detected in two patients. An antigen-specific T-cell response fulfilling the predefined level of specificity (see material and methods) against one or more of the three antigens tested (influenza-A, PR3, WT-1) could be detected more frequently by pentamer staining or ICS as compared with CBA and ELISpot (). Furthermore, T-cell responses detected by CBA/ELISpot were mostly directed against influenza-A, which is a viral antigen and thus more immunogenic. Results of the different T-cell assays are depicted in . summarizes the different cytokine profiles of T-cells recognizing a particular antigenic peptide in the patients studied.
Table 2. Cytokine secretion profile of antigen-specific T cells: Overview
Figure 1. Intracytoplasmatic cytokine staining (ICS). Intracytoplasmatic cytokine staining (IFN-γ and TNF-α) of CD8+ T cells, after in vitro stimulation with peptide- pulsed PBMC (patient # 8). Control, irrelevant HLA-A2+ peptide.
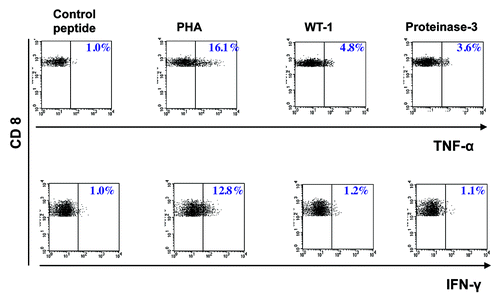
Figure 2. Cytometric bead array (CBA) and IFN-γ ELISpot in patients with TNF-α+/ IFN-γ- CD8+ T cells. TNF-α+/ IFN-γ- antigen-specific T cell responses in patients #3 and #8 as measured by CBA and IFN-γ ELISpot. *, peptide-specific cytokine secretion; **, detectable over background, but not meeting our predefined cut-off for specificity. INFL, Influenza-A; IFN-γ, Interferon-γ; PR3, proteinase-3; TNF-α, Tumor necrosis factor-α; WT1, Wilms´ tumor protein- 1.
Our results show that T-cell responses against common viral antigens such as influenza-A as well as leukemia-associated antigens can be observed very soon after SCT in AML patients. Antigen- specific immune responses could be detected more often by ICS and pentamer staining, even in cases where ELISpot and CBA were negative ( and ). This is very likely to be explained by the flowcytometric gating strategy that allows for exclusion of non-CD8+T-cells from the analysis, thereby lowering non-specific background, which precludes the detection of very small T-cell populations. Therefore, ICS and pentamer staining seem to be particularly appropriate for the detection of small antigen-specific T-cell populations, which is in line with previous reports Citation7,Citation8. Since structural T-cell assays such as pentamer staining –although rather sensitive- give no information on the functional status and consequently the biological role of T-cells, the cytokine secretion profile is of particular interest. Interestingly, antigen-specific T-cells, which only secreted TNF-α could also be detected in AML patients, showing that this finding is not restricted to CML and is therefore very likely to be a more general immunological phenomenon. Our data clearly demonstrate that there are in fact CD8+ T-cells which respond to antigenic stimulation with TNF-α release without IFN-γ secretion. In fact, T-cell responses in our study could be divided into four groups according to pentamer staining and IFN-γ/TNF-α secretion profile: IFN-γ/TNF-α double positive, IFN-γ single positive, TNF-α single positive and pentamer-positive/cytokine negative T-cells (). With regard to the latter group, it can neither be excluded that these cells secrete another cytokine which was not included in our analysis nor that another cytokine was produced at levels which were below the sensitivity of the assays used. Nevertheless, TNF-α+/IFN-γ- antigen-specific T-cells are an interesting biological finding, which so far has not been sufficiently recognized. The number of patients in our study is small and our findings need confirmation in a larger cohort of patients. and functional characterization (i.e., concerning effector functions of TNF-α+/IFN-γ- T- cells), however, we report on a possibly important finding which might broaden our understanding of different stages of T-cell tolerance and anergy. There is general consensus that the cytokine pattern secreted by T-cells reflects their functional status, however, most studies on T-cell activation and T-cell exhaustion/ T-cell anergy have been performed in chronic viral infection. Since T-cell activation and anergy are very likely to follow the same basic rules in infection and malignancy, it is worthwhile to consider the basic concepts of T-cell activation and exhaustion derived from chronic viral infection.Citation9-Citation12 Basically, these models suggest that CD8+ T-cell exhaustion and anergy result from a persistently high antigen load in combination with low CD4+ T-cell help beyond the expansion phase of the T-cell response. Whereas fully activated T-cells display CTL effector function together with IFN-γ, TNF-α and IL-2 secretion, exhausted T-cells sequentially lose their ability to produce IL-2, followed by TNF-α and finally IFN-γ. A fully exhausted T-cell has neither effector (CTL) function nor does it secrete IL-2, TNF-α or IFN-γ. This kind of T-cell is then finally deleted. In human tumors, particularly in melanoma, T-cell exhaustion at least bears a striking similarity to these viral models and seems to be mediated in part by inhibitory receptors.Citation13-Citation15 In a mouse model for AML, Zhou et al. recently showed that coexpression of T-cell immunoglobulin domain 3 (Tim-3) and programmed cell death protein 1 (PD-1) on CD8+ T-cells identifies an exhaustion phenotype which has lost its ability to produce cytokines.Citation16 The lack of functional characterization of TNF-α+/IFN-γ- T-cells is an obvious limitation of our study. However, T-cells secreting TNF-α alone have never been described in all of these viral models, in contrast to T-cells secreting IFN-γ and TNF-α or IFN-γ alone which are frequently observed and seem to represent two consecutive functional stages during development of full T-cell exhaustion. Therefore, our results are interesting and deserve further investigation in order to decipher the functional role of TNF-α+/IFN-γ- T-cells and to determine whether they are a specific feature of tumor-specific T-cells. Leukemia blast-induced T-cell anergy has previously been demonstrated Citation17 and might be supported by immunosuppressive drugs in the setting of SCT. Functional studies including assessment of the CTL activity of the TNF-α+/IFN-γ- CD8+ T-cells will have to be performed in order to shed more light onto this important issue. Our current study does not allow to assign a clear biological function to this T-cell phenotype. In conclusion, to the best of our knowledge this is the first report on leukemia antigen-specific TNF-α+/IFN-γ- T-cells in AML patients after allogeneic SCT and it possibly reveals a new type of functionally impaired tumor-specific T-cells. Our results have important implications for the design of immunomonitoring within clinical trials and warrant further functional characterization of TNF-α-secreting T-cells in leukemia and solid tumors.
Conflicts of Interest and Financial Disclosures
The authors declare that there are no conflicts of interest with regard to this work.
References
- Barrett AJ, Le Blanc K. Immunotherapy prospects for acute myeloid leukaemia. Clin Exp Immunol 2010; 161:223 - 32; PMID: 20529084
- Anguille S, Van Tendeloo VF, Berneman ZN. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia 2012; 26:2186 - 96; http://dx.doi.org/10.1038/leu.2012.145; PMID: 22652755
- Siebert JC, Walker EB. Monitoring cytokine profiles during immunotherapy. Immunotherapy 2010; 2:799 - 816; http://dx.doi.org/10.2217/imt.10.76; PMID: 21091113
- Westermann J, Lessen A, Schlimper C, Baskaynak G, le Coutre P, Dörken B, et al. Simultaneous cytokine analysis by cytometric bead array for the detection of leukaemia-reactive T cells in patients with chronic myeloid leukaemia. Br J Haematol 2006; 132:32 - 5; http://dx.doi.org/10.1111/j.1365-2141.2005.05844.x; PMID: 16371017
- Gannagé M, Abel M, Michallet AS, Delluc S, Lambert M, Giraudier S, et al. Ex vivo characterization of multiepitopic tumor-specific CD8 T cells in patients with chronic myeloid leukemia: implications for vaccine development and adoptive cellular immunotherapy. J Immunol 2005; 174:8210 - 8; PMID: 15944330
- Westermann J, Flörcken A, Willimsky G, van Lessen A, Kopp J, Takvorian A, et al. Allogeneic gene-modified tumor cells (RCC-26/IL-7/CD80) as a vaccine in patients with metastatic renal cell cancer: a clinical phase-I study. Gene Ther 2011; 18:354 - 63; http://dx.doi.org/10.1038/gt.2010.143; PMID: 21068778
- Whiteside TL, Zhao Y, Tsukishiro T, Elder EM, Gooding W, Baar J. Enzyme-linked immunospot, cytokine flow cytometry, and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clin Cancer Res 2003; 9:641 - 9; PMID: 12576430
- Tassignon J, Burny W, Dahmani S, Zhou L, Stordeur P, Byl B, et al. Monitoring of cellular responses after vaccination against tetanus toxoid: comparison of the measurement of IFN-gamma production by ELISA, ELISPOT, flow cytometry and real-time PCR. J Immunol Methods 2005; 305:188 - 98; http://dx.doi.org/10.1016/j.jim.2005.07.014; PMID: 16157348
- Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492 - 9; http://dx.doi.org/10.1038/ni.2035; PMID: 21739672
- Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology 2010; 129:474 - 81; http://dx.doi.org/10.1111/j.1365-2567.2010.03255.x; PMID: 20201977
- Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol 2004; 78:5535 - 45; http://dx.doi.org/10.1128/JVI.78.11.5535-5545.2004; PMID: 15140950
- Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 2009; 106:8623 - 8; http://dx.doi.org/10.1073/pnas.0809818106; PMID: 19433785
- Baitsch L, Baumgaertner P, Devêvre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8⁺ T cells in metastases from melanoma patients. J Clin Invest 2011; 121:2350 - 60; http://dx.doi.org/10.1172/JCI46102; PMID: 21555851
- Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Liénard D, Lejeune F, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res 2004; 64:2865 - 73; http://dx.doi.org/10.1158/0008-5472.CAN-03-3066; PMID: 15087405
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114:1537 - 44; http://dx.doi.org/10.1182/blood-2008-12-195792; PMID: 19423728
- Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011; 117:4501 - 10; http://dx.doi.org/10.1182/blood-2010-10-310425; PMID: 21385853
- Narita M, Takahashi M, Liu A, Nikkuni K, Furukawa T, Toba K, et al. Leukemia blast-induced T-cell anergy demonstrated by leukemia-derived dendritic cells in acute myelogenous leukemia. Exp Hematol 2001; 29:709 - 19; http://dx.doi.org/10.1016/S0301-472X(01)00636-1; PMID: 11378266