Abstract
Background: Routine vaccination against smallpox (variola) ceased in the US in 1976. However, in 2002 limited coverage for military personnel and some healthcare workers was reinstituted. In March 2008, ACAM2000® replaced Dryvax® as the vaccine used in the United States against smallpox. Unintentional transfer of vaccinia virus from a vaccination site by autoinoculation or contact transmission, can have significant public health implications. We summarize unintentional virus transfer AEs associated with ACAM2000® since March 2008 and compare with Dryvax®.
Results: We identified 309 reports for ACAM2000® with skin or ocular involvement, of which 93 were autoinoculation cases and 20 were contact transmission cases. The rate for reported cases of autoinoculation was 20.6 per 100,000 vaccinations and for contact transmission was 4.4 per 100,000 vaccinations. Eighteen contact transmission cases could be attributed to contact during a sporting activity (45%) or intimate contact (45%). Of the 113 unintentional transfer cases, 6 met the case definition for ocular vaccinia. The most common locations for all autoinoculation and contact cases were arm/elbow/shoulder (35/113; 31%) and face (24/113; 21%).
Methods: We reviewed 753 reports associated with smallpox in the Vaccine Adverse Event Reporting System and CDC Poxvirus consultation log, reported from March 2008 to August 2010. Reports were classified into categories based upon standard case definitions.
Conclusion: Overall, unintentional transfer events for ACAM2000® and Dryvax® are similar. We recommend continued efforts to prevent transfer events and continuing education for healthcare providers focused on recognition of vaccinia lesions, proper sample collection, and laboratory testing to confirm diagnosis.
Introduction
Though smallpox vaccination, for the prevention of variola, has been practiced in the United States since the 1800s and adverse events (AEs) have been continuously observed,Citation1,Citation2 the frequency of AEs associated with the smallpox vaccine (vaccinia virus) was not described nationally for the United States until the 1960s.Citation3-Citation6 Generalized vaccinia was recognized as the most common AE, and children less than one year experienced the largest number of all AEs. While the effort to eradicate smallpox worldwide continued, routine vaccination against smallpox ceased in the United States in 1976; however, vaccination of special groups, including some military personnel and research laboratory personnel who could be exposed to Orthopoxviruses, continued.Citation7 Then in December 2002, in response to the possibility of bioterrorism using variola virus, the US National Smallpox Vaccination Program was initiated as part of response preparedness efforts. Vaccination was offered to first responders, public health and healthcare providers designated by authorities as individuals who would investigate and care for initial cases of smallpox. Although vaccination has continued among military personnel, the vaccination program was suspended for domestic civilian healthcare response personnel after the first year due to, among other factors, a perceived unfavorable risk-to-benefit ratio. During the first four months of the program over 300,000 additional military, public health and health care personnel received smallpox vaccination with Dryvax®. At that time a Smallpox Vaccine Safety Working Group (SVS WG) was established through the Advisory Committee on Immunization Practices (ACIP) to monitor the safety of the program. Surveillance for AEs was conducted through the Vaccine Adverse Event Reporting System (VAERS), co-managed by the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC), and enhanced through the CDC’s Epidemic Information Exchange (Epi-X) secure website and clinical consultation services. Active surveillance was also conducted for more common, non-serious AEs through a telephone survey of vaccinees.Citation8
In 2008, the SVS WG published findings from the first 18 mo of the program.Citation9,Citation10 Unlike the 1960s when the population targeted for smallpox vaccination consisted mainly of children, the program begun in 2002 focused exclusively on healthy adults and primarily on military personnel. Based on the SVS WG findings the following AEs were recommended to be reported to VAERS and state health departments: unintentional transfer of vaccinia (inadvertent autoinoculation, contact transmission, ocular vaccinia), super-infection of the vaccination site or regional lymph node, generalized vaccinia, eczema vaccinatum, progressive vaccinia, erythema multiforme or Stevens-Johnson Syndrome, fetal vaccinia, post-vaccinial central nervous system diseases, myo/pericarditis and dilated cardiomyopathy.Citation11 These conditions, as well as blindness and fetal death, appear within a black box warning on the package insert of ACAM2000® (). Some of these AEs are serious and even life threatening particularly to those with pre-existing conditions such as atopic dermatitis and heart disease. Therefore, prospective vaccinees are screened for these pre-existing conditions which, if present, preclude vaccination. In the context of a defined exposure to variola virus, only severe immunodeficiency (such as would render an individual unlikely to benefit from receiving vaccination) would constitute an absolute contraindication to vaccination.Citation12
Figure 1. ACAM2000® Blackbox warning.Citation12

Smallpox vaccine is a live virus vaccine administered into the epidermis using a bifurcated needle. Vaccinia virus proliferates at the inoculation site which contains viable virus until the scab falls off and intact skin has regrown (2–4 weeks). Until this occurs, unintentional transfer of vaccinia virus to other parts of the vaccinee’s body (autoinoculation) or to others (contact) can occur. Proper site management and hand hygiene can prevent such transfer. Delayed recognition of an autoinoculation site or contact lesion could result in ongoing transmission.113 Additionally, vaccinees may have close contact with persons with pre-existing conditions, and though vaccinees are asked, they may not always be aware of conditions among family members and other close contacts that could put these individuals at increased risk of AEs following infection, including eczema vaccinatum or even death.
A new smallpox vaccine, ACAM2000®, was approved by the FDA on August 31, 2007. On February 29, 2008, ACAM2000® replaced Dryvax® as the only smallpox vaccine available for use in the United States.Citation14 Pre-clinical and clinical trials demonstrated that ACAM2000® had similar safety and immunogenicity to Dryvax®.Citation15–Citation17 However, the vaccines differ. ACAM2000® is produced in cell culture, from a clonal virus derivative of Dryvax® and is considered a second generation vaccine. The number of percutaneous punctures used to administer ACAM2000 to persons receiving their first smallpox vaccination (15) is greater than had been used for Dryvax (3).Citation16-Citation18 A recent studyCitation19 summarized contact vaccinia transmission events from December 2002 to March 2011, a period when both Dryvax® and ACAM2000® were used. Additional case reports and investigations of sporadic contact transmission events associated with ACAM2000® have been described.Citation13,Citation20-Citation23 However, no comprehensive review of post-marketing AE has been performed for ACAM2000® to allow comparison against rates seen with Dryvax®. This analysis summarizes and describes unintentional transfer events since ACAM2000® was released.
Results
From 1 March 2008 to 31 August 2010, 450,284 (MILVAX, personal communication) ACAM2000® vaccinations were given to military members and 1,234 (CDC Drug Services, personal communication) to civilians. A total of 309 AEs were reviewed; of these 289 were vaccine recipients, the remaining were contacts of vaccinees. Of the 289 reports among vaccine recipients, 23 were classified as serious, as defined by the Code of Federal Regulations if at least one of the following was reported: death, life-threatening illness, hospitalization or prolonged hospitalization, or permanent disability.Citation24
One hundred ninety-six reports were excluded because a classification could not be made due to insufficient information (58 reports) or because they met exclusion criteria (i.e., an alternative diagnosis or clinical impression was rendered) (138 reports) (). Of the remaining 93 vaccinee reports reviewed for autoinoculation, three were classified as confirmed, 26 as suspect cases and 64 as possible (). Most autoinoculation cases (63/93) occurred in males age 18–29, which is consistent with US military demographics ().Citation25 The rate for reported suspect and confirmed autoinoculation cases was 6.4 per 100,000 vaccinations or 20.6 per 100,000 vaccinations if all reported autoinoculation cases are included (). Of the 20 non-vaccinees, 16 were confirmed and 4 were possible (). The rate of reported cases per 100,000 vaccinations was 4.4 for all contact cases (). These rates are similar to rates reported for Dryavax® ().
Figure 2. Case definitions adapted from the smallpox vaccine adverse reactions surveillance guidelinesCitation11 and Brighton Collaboration Vaccinia Virus Vaccine Adverse Events Working Group.Citation31aExamples include: erythema multiforme, urticaria, non-viral pustulosis, viral exanthem. bConjunctivitis: inflamed membrane that lines the inner surface of the eyelid and exposed surface of the eyeball excluding the cornea with serous or mucopurulent discharge and the presence of vesicles, ulcerations, or “moist appearing” white lesions. cBlepharitis: pustules, edema, hyperemia of eyelid +/− lymphadenopathy (periauricular or submandibular), cellulitis, fever. dPeriocular area: papules, vesicles or pustules above the brow or below the inferior occipital rim, but not involving adnexa, lids, lid margins or canthi
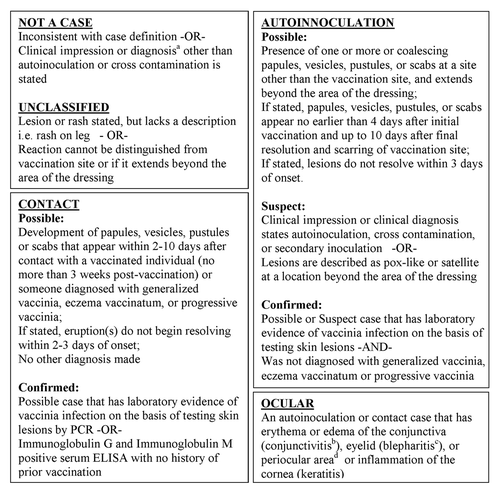
Figure 3. Flow diagram illustrating selection, exclusion, and classification of VAERS reports that were reviewed as potential cases of unintentional transfer of vaccinia.
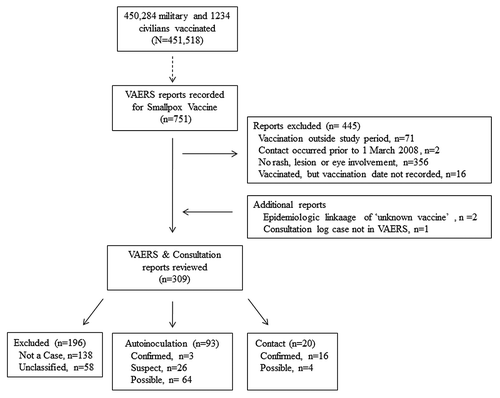
Table 1. Characteristics of persons report vaccinated between 1 March 2008 and 31 August 2010 with ACAM2000® vaccinees with a VAERS adverse event report that included signs or symptoms consistent with a rash, lesion or eye involvement.
Table 2. Rate of unintentional transfer per 100,000 vaccinations by vaccine type, study year, and age group vaccinated.
Similar to the autoinoculation cases, the majority of individuals who met the case definition for contact transmission and had possible or confirmed contact infections were aged 18–29, but unlike the autoinoculation cases (83% male), 50% of the individuals who were inadvertently infected were female (). Most contact infections could be attributed to exposures during either recreational contact sports—such as wrestling (45%)—or during intimate contact (e.g., embracing, coitus) (45%) (). Exposures at two sporting venues resulted in a total of 8 contact infections, 2 of which were the result of tertiary spread (ie., a downstream transmission event in which the initial contact case is the source).
Table 3. Characteristics of persons with contact vaccinia associated with persons vaccinated with ACAM2000® between 1 March 2008 and 31 August 2010 reported to VAERS or recorded in CDC’s consultation log.
For 9 of the 16 confirmed contact infections the date of lesion onset and date of first provider visit was documented; the average time for an individual to seek care upon noticing a lesion was 4 d (range 3–6). In 9 other instances, where a differential diagnosis was stated for the initial exam, vaccinia was not suspected. The most common non-vaccinia diagnoses considered were herpes (5; 56%) and methicillin-resistant Staphylococcus aureus (4; 44%). Molluscum contagiosum, impetigo and viral exanthem were also considered.
The location of vaccinia lesions provides clues as to how the virus transmission event may have occurred (e.g., rubbing eyes after touching vaccination site) and can also influence treatment decisions (e.g., use of vaccinia immunoglobulin). Of the 93 autoinoculation cases and 20 contact vaccinia cases, 6 (5%) met the case definition for ocular vaccinia (4 autoinoculation, 2 contact). Most unintentional transfer lesions were located on the arm/elbow/shoulder (35;31%), face (24;21%), and torso (16;14%) (). This observation remained consistent when considering autoinoculations independent of contact inoculations. In contrast, when considering only contact vaccinia, genital lesions (5; 25%) were more common than those on the torso (1;5%) Lesion constellations described as “diffuse” as well as lesions on the abdomen, chest, or joints were reported only in possible autoinoculation cases ().
Table 4. Locationa of lesions or rash for all possible, suspect, and confirmed cases of inadvertent transfer of vaccinia virus reported to VAERS or recorded n CDC’s consultation log associated with persons vaccinated with ACAM2000® between 1 March 2008 and 31 August 2010 by inoculation type.
Discussion
The objectives of this study were to determine if autoinoculation and contact vaccinia rates differ between ACAM2000® and Dryvax® and to summarize these events in a contemporary context. Our findings suggest that overall unintentional transfer events for ACAM2000® and Dryvax® are similar (). In general, these results are consistent with the state surveys performed in the 1960s evaluating AEs associated with Dryvax®. These studies were performed with the intent to capture less severe clinical illness.Citation4,Citation6 However, the contact vaccinia rate observed in this study is somewhat higher than was observed in the 1960s. This might reflect the current robust efforts to investigate all suspected instances of contact transmission or could in part stem from contemporary deficits in vaccine-derived immunity in the general population. When comparing our findings to the Neff, et al. study performed in 2004,Citation9 we find a nominally higher rate of autoinoculation (20.6 per 100,000) with ACAM2000® than Dryvax® when all case categories are included (i.e., confirmed, suspect, possible). However, when imposing more stringent criteria for case inclusion (only suspect and confirmed cases) the rate drops to 6.4 per 100,000 vaccinations, which is lower than the 2004 rate of 7 per 100,000 for Dryvax®. A cautious interpretation would suggest that the actual rate might lie somewhere between and that autoinoculation rates should be viewed as having remained essentially unchanged since ACAM2000® was released in March 2008.
Similar to reports published in 2004 and 2011, we found that contact vaccinia was associated primarily with sports and intimate contact.Citation9,Citation19,Citation26 However, the previous studies found that females were primarily affected by contact transmission while we found an even distribution between males and females. This is most likely a function of the proportion of sporting event clusters represented in our study, most of which involved men. We also found that many contacts waited an average of 4 d from lesion onset to seek care, and many clinicians did not consider vaccinia in the differential diagnosis at the patient’s first exam. Delayed consideration of vaccinia as a possible diagnosis could lead to additional transmission events, through either direct or indirect (i.e., bed linens or towels) transmission modalities. Just as the vaccination site can shed the virus up to 4 weeks after vaccination so do contact vaccinia lesions. In this series of reports we found that delays in identifying contact infections may have contributed to two tertiary transmission events. Ensuring the cessation of ongoing spread ultimately required the coordinated efforts of multiple State-based and Federal agencies, academic institutions, providers and vaccinees.Citation20,Citation21
Because vaccinia can be transferred to any location on the body, the lesion location can serve as a clue as to how transmission might have occurred (i.e., if fomites were involved). This information can be useful to aid in providing recommendations to patients about reducing risks. In the 1960s, the eye was the most common location of unintentional transfer, accounting for approximately 80% of all autoinoculations,Citation4-Citation6,Citation27 whereas in 2004, they accounted for 13%Citation9 and only 5% in our study. This disparity could be attributable to the age of vaccinees, as children (prominent among vaccinees in the 1960s) may be somewhat more prone than adults to touching their eyes without first ensuring hand hygiene was performed vs. differences in the vaccines used. In our study, the most common location for unintentional transfer lesions was the arm/elbow/shoulder suggesting that transfer events may be a result of either poor hand hygiene during bandage changes or improper bandaging. The face was also a common location, unsurprising in light of the frequency with which humans touch their faces.Citation28,Citation29
Although our findings are consistent with previous smallpox vaccine safety studies, the frequencies of unintentional transfer of vaccinia adverse events associated with ACAM2000® presented are estimates. VAERS is a passive surveillance system, reporting of adverse events relies on both the clinical recognition of the adverse event and its reporting. Thus, the rates presented here may actually be underestimates of the true incidence of inadvertent transmission events. VAERS reports are often subjective and may contain incomplete information. Laboratory testing is the only way to confirm that a rash or lesion is due to vaccinia. Unfortunately, most VAERS reports summarized in this study did not include laboratory testing of the lesion(s); therefore, most cases were classified based on clinical impression or rash description. Also, most VAERS reports did not include the level of detail outlined in surveillance guidelinesCitation11 for suspect unintentional transfer. As a result, reports might have been misclassified thereby affecting estimated rates.
Our findings suggest that inadvertent inoculation rates for ACAM2000® are unchanged from those seen in 2004 when Dryvax® was used to vaccinate a similarly aged population. Our study highlights potential risks—certain adult behaviors and delays in diagnosis—that can be mitigated through proper vaccination site bandaging, good hand hygiene and increased awareness among providers and vaccinees. Despite current measures in place to mitigate these risks through instructions from trained vaccinators at the time of vaccination and providing each vaccinee with the FDA-approved Medication Guide (which outlines proper care of the vaccination site and items in contact with the site, frequent hand washing, what to avoid after vaccination and information about serious side effects including contact transmission), cases of inadvertent inoculation still occur. An evaluation of the understanding and behavior of vaccinees with regard to risks of the vaccine might help identify gaps in the educational process and could contribute to reducing cases of inadvertent transmission further. We also recommend continuing education for military and civilian physicians (particularly for those civilian physicians who practice in communities near military installations) focused on recognition of vaccinia lesions, proper sample collection, and the importance of laboratory testing to confirm diagnosis. Consultative services are available through the Centers for Disease Control and Prevention and the Department of Defense’s Vaccine Healthcare Centers Network.
Methods
VAERS receives reports of AE from health care providers, vaccine recipients, vaccine manufacturers, and other interested parties. Because passive surveillance systems like VAERS are subject to many limitations such as underreporting, biased reporting, and incomplete information, it is usually not possible to verify causal associations between vaccines and AEs from VAERS reports.Citation30
A query performed on 29 November 2010 of Smallpox Vaccine VAERS reports from March 2008 until September 2010 yielded 751 reports (). Data fields queried included the following: VAERS ID, Gender Code, Age, Vaccine, State/Territory of Report, Month Reported, Month Vaccinated and Adverse Event Description. A total of 445 reports were excluded. Seventy-one reports were excluded because the date of vaccination did not occur during the study period, 1 March 2008 and 31August 2010. Two reports were excluded because they described instances of contact transmission that occurred prior to March 2008. The AE description was reviewed for the remaining 678. Of these, 356 were excluded because they did not contain a description of signs or symptoms consistent with a rash, lesion or eye involvement and an additional 16 were excluded the vaccination date was not reported. Two additional reports, listed under “unknown vaccine,” were identified through epidemiologic linkage with another report in the data set.
CDC Poxvirus and Rabies Branch personnel provide consultations for clinicians suspecting poxvirus infections; an internal record of such consultations was reviewed to identify additional events or information not reported to VAERS. Confirmed laboratory results from the consultation log were matched to a VAERS report based on clinical description and limited demographic information and added to the data set. One additional contact case was identified after reviewing the consultation log bringing the total number of reports reviewed to 309. Additionally, 15 confirmed contact cases were matched to a VAERS report. Clinical and demographic information not provided in the VAERS report was included in the data set to provide a more comprehensive description of the cases.
Due to the variability in the information provided in VAERS reports, current case definitions provided by the smallpox vaccine adverse reactions surveillance guidelinesCitation11 and Brighton Collaboration Vaccinia Virus Vaccine Adverse Events Working GroupCitation31 were modified specifically for this report to optimize the information available and designate the level of certainty associated with classification ().The textual descriptions of the AE were reviewed and individual cases were classified based on these modified definitions. Cases identified as possible, suspect, or confirmed autoinoculation or contact vaccinia cases were reviewed further to identify if they met the case definition of ocular vaccinia. For instances in which multiple symptoms occurred consistent with rash, skin lesion or ocular involvement, the case was classified based on the category for which the most clinical information was recorded.
Laboratory testing for human Orthopoxvirus infections is performed in State and Federal government reference laboratories. Laboratory confirmation consists of quantitative PCR techniques, except where active lesions are not available for testing, serologic specimens are tested.Citation32,Citation33 Human subject procedures were reviewed and determined not to meet the definition of research on human subjects.
| Abbreviations: | ||
| AE | = | adverse event |
| SVS WG | = | Smallpox Vaccine Safety Working Group |
| ACIP | = | Advisory Committee on Immunization Practices |
| VAERS | = | Vaccine Adverse Event Reporting System |
| FDA | = | Food and Drug Administration |
| CDC | = | Centers for Disease Control and Prevention |
| MILVAX | = | Military Vaccine Agency of the Department of Defense |
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, Department of Defense, or Food and Drug Administration.
Potential conflicts of interest
All authors report no conflicts of interest.
Acknowledgments
We would like to thank the Christy Hughes, Whitni Davidson, Ginny Emerson, Andrea McCollum at CDC Poxvirus and Rabies Branch and Lori Hall at CDC drug services for their support and guidance with this project.
References
- Greenberg M. Complications of vaccination against smallpox. Am J Dis Child 1948; 76:492 - 502; PMID: 18108416
- Leake JP. Question and answers on smallpox vaccination. Public Health Rep 1927; 42:221 - 38; http://dx.doi.org/10.2307/4578155; PMID: 19315071
- Neff JM, Lane JM, Pert JH, Moore R, Millar JD, Henderson DA. Complications of smallpox vaccination. I. National survey in the United States, 1963. N Engl J Med 1967; 276:125 - 32; http://dx.doi.org/10.1056/NEJM196701192760301; PMID: 4381041
- Neff JM, Levine RH, Lane JM, Ager EA, Moore H, Rosenstein BJ, et al. Complications of smallpox vaccination United States 1963. II. Results obtained by four statewide surveys. Pediatrics 1967; 39:916 - 23; PMID: 4381735
- Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968. N Engl J Med 1969; 281:1201 - 8; http://dx.doi.org/10.1056/NEJM196911272812201; PMID: 4186802
- Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis 1970; 122:303 - 9; http://dx.doi.org/10.1093/infdis/122.4.303; PMID: 4396189
- Rotz LD, Dotson DA, Damon IK, Becher JA, Advisory Committee on Immunization Practices. Vaccinia (smallpox) vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2001. MMWR Recomm Rep 2001; 50:RR-10 1 - 25, quiz CE1-7; PMID: 15580803
- Centers for Disease Control and Prevention. Notice to Readers: Smallpox Vaccine Adverse Events Monitoring and Response System for the First Stage of the Smallpox Vaccination Program MMWR. MMWR Morb Mortal Wkly Rep 2003; 52:88 - 99
- Neff J, Modlin J, Birkhead GS, Poland G, Robertson RM, Sepkowitz K, et al, Advisory Committee on Immunization Practices, Armed Forces Epidemiological Board. Monitoring the safety of a smallpox vaccination program in the United States: report of the joint Smallpox Vaccine Safety Working Group of the advisory committee on immunization practices and the Armed Forces Epidemiological Board. Clin Infect Dis 2008; 46:Suppl 3 S258 - 70; http://dx.doi.org/10.1086/524749; PMID: 18284367
- Casey CG, Iskander JK, Roper MH, Mast EE, Wen XJ, Török TJ, et al. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA 2005; 294:2734 - 43; http://dx.doi.org/10.1001/jama.294.21.2734; PMID: 16333009
- Casey C, Vellozzi C, Mootrey GT, Chapman LE, McCauley M, Roper MH, et al, Vaccinia Case Definition Development Working Group, Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep 2006; 55:RR-1 1 - 16; PMID: 16456528
- Sanofi Pasteur. ACAM2000 Package Insert. September 2009. Available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm180810.htm. Accessed 13 April 2012.
- Montgomery JR, Carroll RB, McCollum AM. Ocular vaccinia: a consequence of unrecognized contact transmission. Mil Med 2011; 176:699 - 701; PMID: 21702392
- Centers for Disease Control and Prevention. Notice to readers: newly licensed smallpox vaccine to replace old smallpox vaccine. MMWR 2008; 57:207 - 8
- Frey SE, Newman FK, Kennedy JS, Ennis F, Abate G, Hoft DF, et al. Comparison of the safety and immunogenicity of ACAM1000, ACAM2000 and Dryvax in healthy vaccinia-naive adults. Vaccine 2009; 27:1637 - 44; http://dx.doi.org/10.2147/DDDT.S3687; PMID: 19071184
- Artenstein AW, Johnson C, Marbury TC, Morrison D, Blum PS, Kemp T, et al. A novel, cell culture-derived smallpox vaccine in vaccinia-naive adults. Vaccine 2005; 23:3301 - 9; http://dx.doi.org/10.1016/j.vaccine.2005.01.079; PMID: 15837236
- Nalca A, Zumbrun EE. ACAM2000TM: The new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther 2010; 25:71 - 9; http://dx.doi.org/10.1016/j.vaccine.2008.11.079; PMID: 19071184
- Qin L, Upton C, Hazes B, Evans DH. Genomic analysis of the vaccinia virus strain variants found in Dryvax vaccine. J Virol 2011; 85:13049 - 60; http://dx.doi.org/10.1128/JVI.05779-11; PMID: 21976639
- Wertheimer ER, Olive DS, Brundage JF, Clark LL. Contact transmission of vaccinia virus from smallpox vaccinees in the United States, 2003-2011. Vaccine 2012; 30:985 - 8; http://dx.doi.org/10.1016/j.vaccine.2011.12.049; PMID: 22192851
- Young GE, Hidalgo CM, Sullivan-Frohm A, Schult C, Davis S, Kelly-Cirino C, et al. Secondary and tertiary transmission of vaccinia virus from US military service member. Emerg Infect Dis 2011; 17:718 - 21; http://dx.doi.org/10.3201/eid1704.101316; PMID: 21470470
- Hughes CM, Blythe D, Li Y, Reddy R, Jordan C, Edwards C, et al. Vaccinia virus infections in martial arts gym, Maryland, USA, 2008. Emerg Infect Dis 2011; 17:730 - 3; http://dx.doi.org/10.3201/eid1704.101010; PMID: 21470473
- Kroeker DA, Hinkle JL, Jones JT, Scrafford DA, Rohr MK, Wittler RR. Picture of the month. Contact vaccinia. Arch Pediatr Adolesc Med 2011; 165:177 - 8; http://dx.doi.org/10.1001/archpediatrics.2010.281-a; PMID: 21300659
- CDC. Vaccinia virus infection after sexual contact with a military smallpox vaccinee—Washington, 2010. MMWR Mortal Wkly Rep 2010; 25:773 - 5.21
- Food and Drug Administration. 21 CFR Part 600.80. Postmarketing reporting of adverse experiences. Washington, DC. Fed Regist 2012; 7:5 - 21
- Office of the Secretary of Defense. Population representation in military service FY 2008 report. Available at http://prhome.defense.gov/MPP/ACCESSION%20POLICY/PopRep2009/. Accessed 25 May 2011.
- Centers for Disease Control and Prevention (CDC). Secondary and tertiary transfer of vaccinia virus among U.S. military personnel--United States and worldwide, 2002-2004. MMWR Morb Mortal Wkly Rep 2004; 53:103 - 5; PMID: 14961003
- Ruben FL, Lane JM. Ocular vaccinia. An epidemiologic analysis of 348 cases. Arch Ophthalmol 1970; 84:45 - 8; http://dx.doi.org/10.1001/archopht.1970.00990040047012; PMID: 5423606
- Hendley JO, Wenzel RP, Gwaltney JM Jr.. Transmission of rhinovirus colds by self-inoculation. N Engl J Med 1973; 288:1361 - 4; http://dx.doi.org/10.1056/NEJM197306282882601; PMID: 4350527
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg 2008; 5:347 - 52; http://dx.doi.org/10.1080/15459620802003896; PMID: 18357546
- Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J 2004; 23:287 - 94; http://dx.doi.org/10.1097/00006454-200404000-00002; PMID: 15071280
- Wenger P, Oleske JM, Kohl KS, Fisher MC, Brien JH, Graham PL, et al, Brighton Collaboration Vaccinia Virus Adverse Event Working Group for Inadvertent Inoculation. Inadvertent inoculation as an adverse event following exposure to vaccinia virus: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2007; 25:5754 - 62; http://dx.doi.org/10.1016/j.vaccine.2007.02.087; PMID: 17537553
- Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol 2006; 36:194 - 203; http://dx.doi.org/10.1016/j.jcv.2006.03.012; PMID: 16731033
- Karem KL, Reynolds M, Braden Z, Lou G, Bernard N, Patton J, et al. characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol 2005; 12:867 - 72; PMID: 16002637