Abstract
Objectives: To compare immunogenicity among an inactivated hepatitis A vaccine (Healive®) with one-dose and two-dose regimens, and three kinds of live attenuated vaccines in children.
Results: No significant differences were observed in seroconversion rates (seroprotection rates) among the five groups at 6 or 12 mo (p > 0.05). The geometric mean concentration (GMC) of anti-HAV IgG was significantly higher in the two-dose Healive® group than in the one-dose Healive® group and the attenuated vaccine groups at 12 mo (932.4 vs. 112.7, 135.8, 203.3, 212.8 mIU/ml, respectively, p < 0.05). In the one-dose Healive® group, the GMC was significantly lower than that in the attenuated vaccine B and C groups at 6 mo (152.6 vs. 212, 204 mIU/ml, p < 0.05) and at 12 mo (112.7 vs. 203.3, 212.8, p < 0.05), but was similar to the attenuated vaccine A group at 12 mo (112.7 vs. 135.8 mIU/ml, p > 0.05). The GMCs were significantly higher in the 1–2 y of age group than in the 3–6 y of age group for all types of vaccines except the attenuated vaccine C (p < 0.05) at 12 mo.
Methods: A single-blind, randomized, parallel-group clinical trial was conducted among healthy children aged 1.5–6 y in Xinjiang Uighur Autonomous Region, China. Subjects were randomly assigned to 5 groups. Two groups were administered one-dose or two-dose inactivated vaccine and the remaining groups were immunized with one of three kinds of attenuated vaccines, respectively. Serum samples were collected at 6- and 12-mo follow-ups. Anti-HAV IgG was measured with a microparticle enzyme immunoassay.
Conclusions: A higher GMC of anti-HAV IgG was induced in the two-dose Healive® than in the one-dose and the attenuated vaccines at 12 mo. The attenuated vaccine B or C produced higher GMCs than the one-dose Healive® at 6–12 mo after vaccination.
Introduction
Hepatitis A is a vaccine-preventable disease that imposes substantial health burden and costs on society. The vaccine against hepatitis A virus (HAV) infection has been available since 1992.Citation1 Recommendations for primary hepatitis A vaccination vary considerably between countries. In countries of intermediate endemicity, where a relatively large proportion of the adult population is susceptible to hepatitis A virus due to epidemiological shift in endemicity and hepatitis A represents a significant public health burden, a large-scale childhood vaccination should be considered as a potential supplement to health education and improved sanitation.Citation2 WHO recommends that vaccination against HAV be integrated into the national immunization schedule for children aged ≥ 1 y if indicated on the basis of incidence of acute hepatitis A, change in the endemicity from high to intermediate, and consideration of cost effectiveness.Citation3
Universal mass vaccination (UMV) of children against hepatitis A has already been shown to be a successful strategy. Following the implementation of UMV in toddlers (18–24 mo of age) in Israel in 1999, the incidence rates of hepatitis A declined from a mean of 50.4 per 100,000 (1993–1998) to 2.2 to 2.5 per 100,000 (2002–2004). Moreover, the decline in incidence was not restricted to children but was also observed across all age groups, demonstrating herd immunity.Citation4 Similar childhood vaccination programs also led to declines in hepatitis A incidence in certain regions of Italy, Spain and Australia. Using a Poisson regression, researchers estimated that more than one third of the total estimated number of hepatitis A cases among children age 2–18 y prevented by vaccination between 1995 and 2001 in the United States was the result of herd immunity.Citation5
In 1995, the Advisory Committee on Immunization Practice from the Chinese Ministry of Health recommended targeting high-risk populations, such as children age 1–15 y, medical practitioners, employees in food factories, and residents in endemic regions for hepatitis A vaccination.Citation6 With booming economy, the sanitation and hygiene have been improved markedly in the majority of regions in China for more than a decade. Many high endemic areas for HAV infection are making a transition to moderate incidence.Citation7 Substantial decreases in hepatitis A incidence have been seen in recent years, particularly in children. However, the absence of symptoms in many children means that they may remain a key source for hepatitis A transmission.Citation8
In 2008, the Chinese Ministry of Health incorporated the HAV vaccine into the Expanded Program of Immunization (EPI) in China. All infants age 18 mo are vaccinated with HAV vaccine free of charge.Citation6 Inactivated hepatitis A vaccines are widely used in the world, while the live attenuated vaccines based on the H2 and H-L-1 strains of hepatitis A virus are mainly used in China and other developing countries, such as India.Citation7
Attenuated live vaccines with one dose and inactivated vaccines with two doses are used routinely by the national immunization program to prevent hepatitis A among children in China. There are limited data on the comparative immunogenicity between one-dose and two-dose inactivated vaccines, and between inactivated and live attenuated vaccines. For inactivated vaccine, long-term protection after the second dose has only been demonstrated when the two doses are administered 6–12 mo apart. Therefore, until sufficient new data become available on long-lasting protection of a single dose, the two-dose schedule is still the recommended regimen.Citation2 The single-dose schedule has advantages over the two-dose schedule in its lower cost and ease in administration.
To test if one-dose inactivated vaccine is an effective alternative to other vaccines, we conducted this single-blind, randomized, parallel-group clinical trial to compare the immunogenicity and persistence of anti-HAV antibodies between one-dose and two-dose schedules of an inactivated hepatitis A vaccine (Healive®), and also between one-dose inactivated vaccine and one-dose live attenuated vaccines. The participants of the study will be followed for more than 5 y. The objective of this paper is to present and compare the seroconversion rates (seroprotection rates) and geometric mean concentrations (GMCs) of anti-HAV antibodies among five groups of children who received different hepatitis A vaccines/regimens at one year after primary vaccination.
Results
Recruitment of subjects
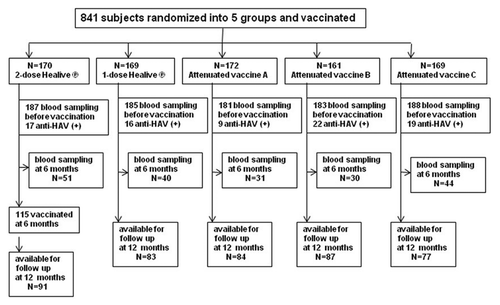
Of the 924 children recruited, after testing blood samples before vaccination, 83 participants (9.0%) were excluded from the study for being seropositive with anti-HAV IgG. The characteristics of the subjects are summarized in .The results of ANOVA and Chi-Square analysis showed that there were no significant differences in age (p = 0.391), height (p = 0.464), weight (p = 0.880), and gender ratio (p = 0.050) among the five groups for Security Analysis Set (SAS). The similar results are observed for Full Analysis Set (FAS) at 6 mo and for FAS at 12 mo.
Table 1. Demographic characteristics of subjects
Immunogenicity
At 6- and 12-mo follow-ups, seroconversion rates (seroprotection rates) and GMCs of anti-HAV IgG in the 5 vaccination groups are shown in . At 6 mo, 196 subjects were randomly selected and serum specimens were collected. The number of subjects in each group is shown in . No significant differences were found in seroconversion rates (seroprotection rates) among the five groups (98%, 92.5%, 96.8%, 100% and 100%, respectively, p > 0.05). For GMCs, the attenuated live vaccine A group was significantly lower than other groups (92.5 mIU/ml vs. 154.2, 152.6, 212, 204 mIU/ml, respectively, p < 0.05). However, the GMC was significantly higher in the attenuated live vaccine B and C groups than in the one-dose or two-dose inactivated vaccine group (212 and 204 mIU/ml vs. 152.6 and 154.2 mIU/ml, respectively, p < 0.05).
Table 2. Seroconversions (seroprotective rate) and GMCs of anti- HAV IgG at 6, 12 mo in healthy children
At 12 mo, 422 subjects (50.2% of total vaccinees) were available in the period of 2 weeks for blood sampling. Although the seroconversion rates (seroprotection rates) at 12 mo declined slightly from that at 6 mo for four of the five groups, there was still no significant difference among the groups (97.8%, 92.8%, 92.9%, 96.6% and 96.1%, respectively, p > 0.05). Post Hoc tests revealed that the GMC in the two-dose Healive® group was significantly higher than that in the one-dose Healive® group (932.4 vs. 112.7 mIU/ml, p < 0.05), and also higher than that in the one-dose attenuated live vaccine groups (932.4 vs. 135.8, 203.3, 212.8 mIU/ml, respectively, p < 0.05). For comparisons among the one-dose vaccines, the GMC in the one-dose Healive® group was significantly lower than that in the attenuated live vaccine B or C group (112.7 vs. 203.3 or 212.8 mIU/ml, respectively, p < 0.05). However, it was similar to the level in the attenuated live vaccine A group (112.7 vs. 135.8 mIU/ml, p > 0.05). Among the three attenuated vaccines, vaccine C had a significantly higher GMC than vaccine A (212.8 vs. 135.8 mIU/ml, p < 0.05).
From 6 to 12 mo after the primary inoculation, GMCs in the attenuated vaccine A group increased from 92.5 mIU/ml to 135.8 mIU/ml; while GMCs in the one-done inactivated vaccine group decreased from 152.6 to 112.7 mIU/m. The GMCs of anti-HAV IgG between different age groups were compared and results are shown in . The GMCs were higher in the 1–2 y of age group than in the 3–6 y of age group for all five types of vaccines, and the difference was significant at 12 mo for attenuated vaccine A (192.84 vs.101.67 mIU/ml, p < 0.0.5), vaccine B (275.99 vs. 139.48 mIU/ml, p < 0.05), one-dose Healive® (148.63 vs.79.93 mIU/ml, p < 0.05), and two-dose Healive® (1269.11 vs. 660.85 mIU/ml, p < 0.05). For three attenuated vaccines, the GMCs of anti-HAV IgG increased from 6 to 12 mo in two age groups, but the exception was that the GMC level decreased significantly from 6 to 12 mo in the 3–6 y of age group for the attenuated vaccine B (202.26 vs.139.48 mIU/ml, p < 0.05).
Table3. Comparison of anti-HAV IgG GMCs between different age groups
Safety
No vaccine-related serious adverse events (SAEs) were reported within the time period of 30 min to 3 d post-injection (data not shown). In addition, there were no significant differences in adverse events among the five vaccine groups in the 1–2 y or 3–6 y of age group (data not shown). Thus, inactivated hepatitis A vaccine (Healive®) and three kinds of live attenuated hepatitis A vaccines were all well tolerated by the children.
Discussion
Some countries with intermediate endemicity of hepatitis A and rising hepatitis A-related morbidity are considering adopting programs to routinely vaccinate children, as there is accumulating evidence that such program can result in impressive reduction in overall incidence of hepatitis A, presumably because of strong herd immunity effects. As hepatitis A vaccine is used more widely, especially among young children, studying vaccine performance, including evaluating alternate schedules and monitoring long-term protection, is very important.Citation9
As hepatitis A vaccine was included in the National Immunization Program in China and was used widely in 2008, we conducted this single-blind, randomized, parallel-group clinical trial among healthy children in Xinjiang Uighur Autonomous Region to study immunogenicity and persistence of anti-HAV antibodies of one-dose inactivated vaccine, and compare it with two-dose inactivated vaccine and one-dose attenuated live vaccines produced by three different manufactures in China.
One-year follow-up data showed that there was no difference in seroconversion rates (seroprotection rates) among the five groups at 6 and 12 mo. The GMC was significantly higher in the two-dose Healive® group than in the one-dose Healive® group at 12 mo post-immunization. This is consistent with the previous study which showed that the two-dose regimen has advantage over the one-dose regimen in the short-term.Citation10 In addition, the GMC of the one-dose of Healive® was lower than that of the attenuated vaccines (B or C), but similar to that of vaccine A in the short-term. Finally, we found a higher GMC at 12 mo than at 6 mo for the live attenuated vaccine groups in this study, while previous studies found the opposite results.Citation11,Citation12
As for long-term protection, a 17 y antibody persistence study of the two-dose inactivated hepatitis A vaccine showed that subjects who became seronegative at year 16 mounted an anamnestic response after receiving a challenge dose of HAV vaccine within 12 mo.Citation13 Therefore, based on current scientific evidence, protection is considered to be life-long after a complete hepatitis A vaccination schedule (two doses).Citation13 Previous studies also demonstrated that three kinds of live attenuated hepatitis A vaccines licensed in China and used in this study have good immunogenicity in the long-term of more than 10 y.Citation10 In addition, a strong anamnestic response also observed in vaccinated children who became anti-HAV seronegative at 6-y time-point after immunization with the attenuated vaccine A when a challenge dose of same vaccine was administered.Citation14 There are limited data on the long-term protective effect of a single-dose inactivated vaccine. A study found that among 54 adult Swedish travelers administered the second dose 4–8 y after the primary dose, 35 (65%) still had levels of anti-HAV greater than 10 IU/l prior to their second dose. An excellent response to the delayed second dose was observed in all subjects, with GMCs reaching 2160 IU/l. The results indicated that a single dose of inactivated hepatitis A vaccine induced a long-term proliferative T-cell response in addition to anti-HAV antibodies.Citation15
Additional evidence of a potential long-term protective effect of the one-dose regimen came from Argentina, where a universal hepatitis A vaccination with a single dose of inactivated vaccine at 12 mo of age was implemented in 2005. Surveillance data showed a large decline in hepatitis A incidence rate from 2005 to 2007, when it reached the record low in the past 12 y. It is important to note that this decline has been unprecedented in magnitude and has been observed in all age groups and regions, showing a marked herd immunity effect. A caveat in the case of Argentina is that about 20% of children in the program also received a second-dose from private providers, which may have complicated the finding.Citation16
Our current study will continue for more than five years. The findings of this study will help answer the questions related to the long-term persistence of anti-HAV antibody of one-dose inactivated vaccine and the comparative efficacy and costs of different vaccines with different dose regimens. This is the first study of its kind, which is likely to have important practical implications in guiding the national immunization program to achieve success and reduce cost. A limitation of the study is the loss of some participants in the follow-up. However, there is still enough sample size to achieve sufficient statistical power for our analysis.
One hundred ninety six subjects (23.3% of total vaccinees) were randomly selected by each group at 6 mo and serum specimens were collected for immunogenicity monitoring. At 12 mo, 422 subjects (50.2% of total vaccinees) were available during the period of 2 weeks for blood sampling. Approximately 50% participants could not come back for first year follow-up. There have some reasons for this circumstance. First, a large number of children could not come back during the period of 2 weeks when the blood sampling was performed at 12-mo time-point of follow-up, since their parents were busy or went to cities for temporary jobs and could not bring them to be bled, So most of them were not lost for follow-up, and just simply were not followed. They would come back for next serial follow-ups. Second, a small part of children were not bled due to their parents refused to have them bled again. Finally, some children have moved to urban areas for living, and could not be followed up since then.
In conclusion, our study did not find differences in seroconversion rates (seroprotection rates) among the attenuated hepatitis A vaccines and inactivated vaccine with one- or two-dose schedule at 6 and 12 mo. With respect to the GMC, a significantly higher level at 12 mo was observed in the two-dose inactivated vaccine (Healive®) group than in the one-dose group and the attenuated vaccine groups. In addition, the GMCs of the attenuated vaccines (B or C) were higher than that of one-dose of Healive® in the short-term. The long-term immunogenicity and persistence of anti-HAV antibodies among the five groups will be evaluated in the future.
Materials and Methods
Vaccines
The inactivated hepatitis A vaccine (Healive®) used in this study was the pediatric formulation with 250 U/ml of hepatitis A antigen (batch number 2008030801, Sivovac Biotech Co .Ltd, Beijing, China), and the vaccine was administrated through the intramuscular route in the deltoid region. Live attenuated vaccines were produced by three manufactures: Pukang Biotechnological Co. Zhejiang, China (A: batch number 20080103B); Kunming Institute of Biological Products, Yunnan, China (B: batch number 2008040203); Changchun Institute of Biological Products, Jilin, China (C: batch number 20071154-1). Attenuated vaccines with a titer of 6.5 log TCID50 (tissue culture infecting dose) were given subcutaneously in the deltoid region. The inactivated hepatitis A vaccine (Healive®) and three kinds of live attenuated vaccine were supplied in coded, identical-appearing single-dose vials. All kinds of vaccines used in this clinical trial were stored at 4°C in refrigerators and transported with cold chain. The inactivated and three live attenuated hepatitis A vaccines used in this clinical trial are licensed by State food and drug administration (SFDA, China) for routine use in humans.
Subjects and study design
A single-blinded, randomized, parallel-group clinical trial was conducted among children in rural areas of Changji city in Xinjiang Uighur Autonomous Region, China. The Ethics Committee of Peking University Health Science Center approved the trial protocol. A total of 924 children age 1.5–6 y were enrolled in the study in 2008. Informed written consents were obtained from parents or guardians before inclusion of their children in the study. Exclusion criteria for the trial included the history of liver disease, chronic diseases, treatment with an extracted growth hormone, therapy with immunosuppressive drugs, immunodeficiency, treatment with immune globulin or blood-derived products in the previous six months, allergy to the vaccine’s components, vaccination in 30 d before enrollment and anti-HAV seropositivity.
The 841 subjects were randomly assigned to five groups. Two groups were administered the inactivated hepatitis A vaccine (Healive®) with one-dose or two-dose schedule. At the same time, each of the remaining three groups was vaccinated with one-dose of one of the three kinds of live attenuated vaccines. The first screening blood samples were collected before immunization. At 6 mo, 196 subjects (23.3% of total vaccinees) were randomly selected by each group and serum specimens were collected. At 12 mo, 422 subjects (50.2% of total vaccinees) were available in the period of 2 weeks for blood sampling. The serum specimens were collected among all available subjects. The flow diagram of the study is shown in .
Randomization and blinding
The randomization code was prepared by a statistician from the Sivovac Biotech Co.Ltd, Beijing, China, using SAS software (version 9.0) based on the predefined block size. Randomization-code numbers were assigned to subjects in chronological order by the investigators. During the study, participants and investigators were blinded to vaccination allocations. The Centers for Disease Control and Prevention of Changji city performed subject enrollment, vaccine inoculation and serum sample collection. The Centers for Disease Control and Prevention of Xinjiang Uighur Autonomous Region collected and stored the raw data and files.
Laboratory tests
The quantitative testing of anti-HAV IgG was performed using a microparticle enzyme immunoassay (MEIA) (HAVAB 2.0, Abbott Laboratories, Chicago, USA) in a laboratory at Department of Microbiology and Center of Infectious Disease, School of Basic Medicine, Peking University Health Science Center. An anti-HAV IgG value ≥ 20 mIU/ml was considered protective, and also as seropositive (seroconversion) after vaccination.Citation17-Citation19 Therefore, the term “seroconversion” is meaning of “seroprotection.”
Statistical analysis
The number of subjects excluded from analysis was presented in . Seroconversion rates and GMCs of anti-HAV IgG at 6 and 12 mo after the initial injection and their 95% confidence intervals (CIs) were calculated. Pearson Chi-Square was used to compare anti-HAV IgG seroconversion rates and ANOVA was used to compare GMCs among the five vaccination groups. A p-value < 0.05 (two-tailed) was considered statistically significant. SPSS 18.0 (SPSS Inc., Chicago, IL, USA) statistical software was used to perform above analyses.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgments
This work was funded by the grants of Sinovac Biotech Co., Ltd (Beijing, China).The authors are grateful to Dr. Shumei Yun and Dr. Sherri Homan from Missouri Department of Health and Senior Services, Jefferson City, MO. USA for proofreading and editing the manuscript.
References
- Wasley A, Fiore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev 2006; 28:101 - 11; http://dx.doi.org/10.1093/epirev/mxj012; PMID: 16775039
- Hepatitis A vaccination. World Health Organization. Accessed at http://www.who.int/vaccines/en/hepatitisa.shtml
- WHO position paper on hepatitis A vaccines – June 2012. Wkly Epidemiol Rec 2012; 87:261 - 76; PMID: 22905367
- Dagan R, Leventhal A, Anis E, Slater P, Ashur Y, Shouval D. Incidence of hepatitis A in Israel following universal immunization of toddlers. JAMA 2005; 294:202 - 10; http://dx.doi.org/10.1001/jama.294.2.202; PMID: 16014594
- Samandari T, Bell BP, Armstrong GL. Quantifying the impact of hepatitis A immunization in the United States, 1995-2001. Vaccine 2004; 22:4342 - 50; http://dx.doi.org/10.1016/j.vaccine.2004.04.014; PMID: 15474727
- Fangcheng Z, Xuanyi W, Mingding C, Liming J, Jie W, Qi J, et al. Era of vaccination heralds a decline in incidence of hepatitis A in high-risk groups in China. Hepat Mon 2012; 12:100 - 5; http://dx.doi.org/10.5812/hepatmon.4907; PMID: 22509186
- Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L, Hepatitis A. Hepatitis A: Epidemiology and prevention in developing countries. World J Hepatol 2012; 4:68 - 73; http://dx.doi.org/10.4254/wjh.v4.i3.68; PMID: 22489258
- Koslap-Petraco MB, Shub M, Judelsohn R. Hepatitis A: disease burden and current childhood vaccination strategies in the United States. J Pediatr Health Care 2008; 22:3 - 11; http://dx.doi.org/10.1016/j.pedhc.2006.12.011; PMID: 18174084
- Hollinger FB, Bell B, Levy-Bruhl D, Shouval D, Wiersma S, Van Damme P. Hepatitis A and B vaccination and public health. J Viral Hepat 2007; 14:Suppl 1 1 - 5; http://dx.doi.org/10.1111/j.1365-2893.2007.00924.x; PMID: 17958635
- Zheng H, Cui FQ. [The immunogenicity and impact factors of hepatitis A attenuated live vaccine and inactivated vaccine]. Zhongguo Yi Miao He Mian Yi 2009; 15:371 - 4; PMID: 20077742
- Wang XY, Xu ZY, Ma JC, von Seidlein L, Zhang Y, Hao ZY, et al. Long-term immunogenicity after single and booster dose of a live attenuated hepatitis A vaccine: results from 8-year follow-up. Vaccine 2007; 25:446 - 9; http://dx.doi.org/10.1016/j.vaccine.2006.08.004; PMID: 16949710
- Wang XY, Xu Z, Yao X, Tian M, Zhou L, He L, et al. Immune responses of anti-HAV in children vaccinated with live attenuated and inactivated hepatitis A vaccines. Vaccine 2004; 22:1941 - 5; http://dx.doi.org/10.1016/j.vaccine.2003.11.007; PMID: 15121306
- Van Herck K, Crasta PD, Messier M, Hardt K, Van Damme P. Seventeen-year antibody persistence in adults primed with two doses of an inactivated hepatitis A vaccine. Hum Vaccin Immunother 2012; 8:323 - 7; PMID: 22327499
- Zhuang FC, Jiang Q, Gong YP, Me SH, Xin YJ, Qian W, et al. Epidemiological effects of live attenuated hepatitis A vaccine (H2 strain); results of 10-year observation. Chin J Epidemiol 2001; 22:188 - 90
- Iwarson S, Lindh M, Widerström L. Excellent booster response 4 to 8 years after a single primary dose of an inactivated hepatitis A vaccine. J Travel Med 2004; 11:120 - 1; http://dx.doi.org/10.2310/7060.2004.17079; PMID: 15109480
- Vacchino MN. Incidence of Hepatitis A in Argentina after vaccination. J Viral Hepat 2008; 15:Suppl 2 47 - 50; http://dx.doi.org/10.1111/j.1365-2893.2008.01029.x; PMID: 18837834
- André F, Van Damme P, Safary A, Banatvala J. Inactivated hepatitis A vaccine: immunogenicity, efficacy, safety and review of official recommendations for use. Expert Rev Vaccines 2002; 1:9 - 23; http://dx.doi.org/10.1586/14760584.1.1.9; PMID: 12908508
- Van Herck K, Van Damme P. Prevention of hepatitis A by Havrix: a review. Expert Rev Vaccines 2005; 4:459 - 71; http://dx.doi.org/10.1586/14760584.4.4.459; PMID: 16117704
- Wu JY, Liu Y, Chen JT, Xia M, Zhang XM. Review of 10 years of marketing experience with Chinese domestic inactivated hepatitis A vaccine Healive®. Hum Vaccin Immunother 2012; 8:1 - 9; http://dx.doi.org/10.4161/hv.21909; PMID: 22251991