Abstract
Any therapeutic vaccination approach against HIV-1 must induce CTL and Th1 cells. But, therapeutic vaccination is more than that. For extensive application of a therapeutic vaccine several questions need to be solved in advance to achieve a global impact. In this commentary some of them are addressed. We analyze the epidemiology, sociology, economy and immunopathology related to the HIV/AIDS disease. Also, important technical issues and real possibilities to overcome at least some of the major limitation of the antiretroviral treatments in the pursuit of an effective vaccine are considered. From the integration of previous analyses some conclusions are drawn. Because it is just a commentary some arguments are not unveiled into their full extension. At the end, we discuss some issues in relation to the development of the vaccine candidate TERAVAC-HIV-1 as a case study.
According to the last report of the UNAIDS an estimated of 31.4–35.9 million people are living with HIV worldwide.Citation1 Although many nations reported a decline in their numbers, there are some others where the epidemic is still growing. Unfortunately, an uncontrolled spiral of war conflicts is still in motion and statistic in conflict areas is missing or misleading. Sexual assaults of women and even children has been reported in some conflict areasCitation2 and the number of refugees that have been displaced and forced to leave their homes because of war or climate changeCitation3 impacts is taking catastrophic proportions in Africa and Asia. All these facts might fuel the HIV pandemic.
It is worthy of remark that around 69% of all people infected with HIV lived in the poorest countries of the sub-Saharan Africa. Currently, antiretroviral treatment (ART) is the only effective tool against persistent HIV-1 infection, its use has significantly increase the quality of life and prevented the death of many people from AIDS-related causes. But ART coverage is no enough and only reached to 8 million people worldwide by the end of 2011. Although in low-and middle-income countries women coverage grew up to 68%, 47% of men and 28% of children,Citation1 such low coverage for men and particularly children are shocking. That is mainly explained because prices for therapies are still out of the economic possibilities of those who most need the treatments. Financial resources given by international organizations, foundations and foreign governments are critical to sustain ART access in many low-income countries with a high prevalence of HIV. In this regard, it is expected that the global economic crisis will negatively impact on the sustainability of the national programs when the demand on financial aid would increase due to a higher number of seropositive persons. Furthermore, the emergence of multi-drug-resistant viruses to generic drugs will change the ART landscape to a new scenario where second- and third-line regimens would be the solution. Then, considering the higher cost of these drugs if prices are not significantly reduced the HIV programs for populations at higher risk will be compromised in the future.
The Achilles' heel of the ART is the viral reservoir.Citation4 Because the virus is not cleared, ART is a life-long therapy and retention on treatment or adherence is a real problem. It is close related to the appearance of drug-resistant viruses.Citation5 In many areas nearly 50% of people enrolled in ART are no longer under treatment 2–5 y later.Citation1 There are several causes to explain that situation and one of them is the toxicity of drugs causing long-term metabolic disorders making it necessary to interrupt the treatment. When this time comes, no other therapeutic alternative exists and the disease inevitably progresses, leading to death. An alternative option is the development of immunotherapies to clear the viral infection or to control the infection (functional cureCitation4) if the virus cannot be eradicated. Anti-HIV immunotherapies are intended to substitute ART (if possible) or to allow some control on the viral load during some time off therapy (vacation) in order to decrease the side-effect associated with its continued use.
When we consider the huge number of seropositive patients, the epidemiological risk associated with wars and climate changes, the cost of therapies and it side-effects, it is obvious that a therapeutic vaccine produced at a low cost will be a great aid in the fight against the HIV/AIDS pandemic. Otherwise, if the limitations of the ART are solved there would be no room for therapeutic vaccination. In that scenario, financial resources would be expended in effective ARTs and research in therapeutic vaccines might be discouraged.
Is Possible to Attain a Therapeutic Effect Considering the Immunopathological Events in Chronic HIV Infection?
When people are infected with the HIV-1 they become chronic carrier of the virus. There is not a single case scientifically documented of a person that spontaneously cleared the virus so far. Sexual intercourse and intravenous drug use cover most of the global virus transmissionCitation1 and it is known from studies with the SIV, SHIV models and humans that independently of the route of acquisition, mucosalCitation6,Citation7 or parenteral,Citation6,Citation8 a profound depletion of CD4+ occurs in lymphoid tissue of the gastrointestinal tract.Citation9 Furthermore, this profound depletion persists in untreated patients and it is in this area where an important viral reservoir is established.Citation9 It is important to highlight that even with MegaART (5 drugs) this reservoir is not fully destroyed.Citation10
The clinical course of HIV infection in untreated people may follows three types of progression depends on the way of infection (transmission), the viral load of the inoculum, virulence of the transmitted isolate(s), age of the person, HLA-1 haplotype, genetic polymorphism associated with resistance and susceptibility and other factors not well known. In most cases seropositive patients will follow a classical progression course and they will develop AIDS symptoms in 5–10 y after infection, fast progressors may develop AIDS as soon as one year and they account for approximately 2% of the infections globally and finally long-term non progressors, less than 7% of cases, who remain AIDS-free for more than 10 y. In the last group there are two subgroups: 1, virologic controllers who maintain the viral load below 2,000 RNA copies/mL and 2, elite controllers who have undetectable viral load or below 50 RNA copies/mL. In circumstances where patients have not access to ART fast progressors will not be susceptible to therapeutic vaccination. However, when all patients can be treated they all become “long-term non-progressors.” In that scenario, in principle, all of them might be susceptible to therapeutic vaccination. Nevertheless, it is known that only a subset of patients with a nadir of CD4 cell counts higher than 350–400 cell/μL and suppressed viral load preserve some immunocompetence,Citation11 the Th2-Th1 shift is not impairedCitation12 and new HIV-specific CTL and Th clones (TCR specificities) can be elicited after vaccination.Citation13 Because of that in practical terms the time for effective therapeutic vaccination expands from the acute phase to a point in the chronic phase where CD4 counts are still above 350–400 cells/mL. As explained before, with ART access this period of time might last much more years than expected without treatment and it becomes wider the window of opportunity for therapeutic vaccination . Unfortunately, the current threshold for initiating treatment in resources-limited countries with moderate or high incidence of HIV infection is CD4 count less than 350 cells/mL. In addition, a high proportion of patients are diagnosed late with AIDS symptoms.Citation14 In this setting there is not any window of opportunity for therapeutic vaccination unless additional measures are taken to ensure an effective immune restoration. In this regard it will be very important to improve the public health systems to warrant early diagnosis.
Several therapeutic vaccine candidates tested in clinical trials had showed a significant but transient reduction in the viral load vs. control or placebo groups during several weeks. But, perhaps the best evidence that the HIV-specific immunity is able to control the viral replication was obtained very recently in a therapeutic clinical trial. In this blinded placebo-controlled trial a significant reduction in the viral load was still verified a year after three infusions of dendritic cells (DC) pulsed with heat-inactivated autologous virus.Citation15
What Points Should be Considered to Succeed in Therapeutic Vaccination?
Even in patients on ART with nadir of CD4 counts higher than 350 cells/mL long-term immunization schedules are require to elicit HIV-specific polyfunctional T cell responses.Citation16,Citation17 Additionally, as previously explained higher immune responses are achieved with total suppression of the viral load. Consequently, schedules with six or more immunizations time doses under suppressive ART might be necessary and even periodic rounds of immunization to boost the immune response and sustain the therapeutic effect.
The aforementioned immunopathological evidence suggests that therapeutic immunization schedules must ensure effective immune control over the viral reservoir in the gut and other tissues in the systemic compartment. It is known that mucosal-parenteral coadministration schedules elicit higher immune responses than single route schedules.Citation18,Citation19 Consequently, to elicit a mucosal and systemic HIV-specific immune response mucosal-parenteral coadministration schedules must be considered.
Moreover, chronic HIV infection induces immune exhaustion or immunosenescenceCitation20 and long-term ART is needed to achieve a near-complete immune restoration in the gastrointestinal mucosa observed in a few number of patients.Citation21 On the other side, studies in mice suggest than immunesenescence occurs first in the mucosal compartment compared with the systemic compartment and previously in GALT than in NALT.Citation22 Since nasal immunization stimulates immune responses in the systemic compartment, lungs and distal mucosal sites in the gut and vaginaCitation23 it is this route the preferable mucosal route in mucosal-parenteral coadministration schedules. In the case of parenteral immunization the systemic response might be stimulated with IM, ID or SC immunizations depends on the physico-chemical nature and adjuvant properties of the therapeutic vaccine. In mucosal-parenteral coadministration schedules, it is expected that cell trafficking, rather than local cell proliferation, contributes to the CD4+ and CD8+ T-cell response in the gut following therapeutic vaccination in chronic infected patients.
One of the possible benefits of the therapeutic vaccination might be to counterbalance the progressive Th1 to Th2/Th0 cytokine-profile shift observed in chronic HIV infection to allow viral replication control under a Th1-type immune response. Unfortunately, the Th2 systemic environment is reinforced by parasitic infections and tuberculosis affecting people living in developing areas.Citation24,Citation25 HIV-specific Th1 cells may play a major role in the contention of the virus and progression to AIDS. According to current thinking, the functional proficiency of these cells is mandatory to elicit an effective CTL response against the virusCitation26 and even for efficient access of CTL to mucosal sites.Citation27
Although the induction of T-cell-mediated responses is essential in therapeutic vaccination against the HIV/AIDS, the induction of antibodies should not be neglected since they might counteract the toxic effect of some viral antigens like Nef, Tat, Rev and gp120. It is possible that such HIV-derived antigens are the underlying cause of the progressive Th1 to Th2/Th0 cytokine-profile shift related to disease progression.Citation28,Citation29 For instance, it is known that high amounts of soluble Nef protein is in the sera of seropositive patients and this exogenous Nef subverts several important functions of the immune system.Citation30 Consequently, the induction of anti-Nef antibodies may help in the development of effective anti-HIV immune responses.
Another important challenge in therapeutic vaccination is viral variability. Although some authors have suggested that only stimulation of HIV-specific cellular response with autologous viral sequences (epitopes) will induce long-term effective viral controlCitation15 this thesis has not been yet demonstrated. Other studies in the pipeline might help to find out a definitive answer. In particular, the phase 1 clinical trial conducted by the University of Pittsburgh using autologous DC pulsed with conserved HIV-derived peptides and another one conducted by the Massachusetts General Hospital based on DC transfected with vectors encoding consensus HIV-1 Gag and Nef sequences will provide further clues.Citation31 If conserved viral sequences (epitopes) in such trials induce an effective cellular response and control over the viral load it would evidence that a personalized procedure is not necessary. Furthermore, a positive result will pave the way to an effective therapeutic vaccine of universal use.
What Outcome Might be Expected with an Effective Therapeutic Vaccination?
Although therapeutic vaccination using HIV-derived antigens might help to pump the viral reservoir out,Citation32 elimination of the virus is something beyond our expectations right now. A functional cure seems a rational and attainable objective.Citation4
Even a partially effective therapeutic vaccination might significantly decrease the expending on ARTs, the heterosexual transmission rate and it might allow ART vacations to counteract drug toxicities. It might also reduce viral diversity and hamper the appearance of resistance mutationsCitation33 which in turn might result in an enhancement of the efficacy of ART. Then, therapeutic vaccination might counteract the main factors contributing to virological failure for a given therapy: the appearance of resistance viruses and the poor adherence to the treatments (allowing some time off therapy).
Because the correlate of protection against the HIV-1 infection are still unknown, viral rebound dynamics following ART interruptions are still necessary as study endpoint to assess the impact of the immune response elicited after therapeutic vaccination.Citation34 Considering a possible impact on the viral set point several outcomes may be envisaged. If the viral load is reduced below 10,000 RNA copies/mL or 1.5 log under the relatively steady viral set point that has been established during the asymptomatic period then the risk of drop in CD4+ T cell and progression to AIDS is very low.Citation35 If the viral load is reduced below 1,700 RNA copies/mL heterosexual transmission probabilities decrease significantly,Citation36 and finally, a reduction below 1,000 RNA copies/mL accounts for almost no vertical transmission.Citation37
TERAVAC-HIV-1: A Case Study
TERAVAC-HIV-1 is a multiantigenic vaccine candidate comprising the recombinant protein CR3 (which stand for cellular response number 3), the surface (HBsAg) and the full-length nucleocapsid (HBcAg) antigens of Hepatitis B Virus (HBV). The CR3 antigen contains CTL and Th epitope-rich regions from the envelope glycoprotein gp120 and gp41, a portion of the reverse transcriptase, a fragment of p24 (Gag), one T helper epitope of Vpr and the central region of Nef. Previous studies showed that such virus-like particles (VLP) of the HBV provide a Th1 adjuvant effect on the CR3-specific immune-response.Citation38
According to our studies in mice, the multiantigenic formulation TERAVAC-HIV-1 induces anti-CR3 cellular responses after nasal and parenteral inoculations and best results are obtained in schedules with simultaneous parenteral and nasal co-administration.Citation38,Citation39 We have found a strong Th1 bias of the CR3-specific response, the induction of CD4+ and CD8+ T cells in mice’s spleen and IFN-γ-secreting cells in mesenteric lymph nodes. We have also found evidences that in an HIV-specific Th2 environment, subcutaneous and simultaneous nasal-subcutaneous administrations of TERAVAC-HIV-1 generate a Th1-cell-type response.Citation40
The induction of CR3 (HIV)-specific CD8+ cells might be explained because of the Th1 immune-deviation induced by the recombinant core particle on the immune response to codelivery antigens after parenteral and nasal inoculations and the non-covalent interaction of CR3 with the VLP,Citation38,Citation39 which enables the crosspriming after entry and processing of the recombinant protein inside antigen-presenting cells (APC) following the same routes as the HBV VLP (). It is noteworthy that both antigens can be taken up and processed by macrophages and DC, and its fragments efficiently presented via the class I and II antigen presentation pathways to naïve T helper and cytotoxic T lymphocytes.Citation41,Citation42 Of special interest is the fact that 5–10% of naïve B cells in the mice and humans natural repertoires bind to the core antigen.Citation43,Citation44 Furthermore, the recognition of this antigen by the B cell receptor induces the expression of costimulatory molecules rendering the B cells competent to prime cytotoxic T lymphocytes after efficient delivery to the class I pathway.Citation45 For the surface antigen something similar happens with memory B lymphocytes.Citation46 In this regard, this approach might circumvent the DC and macrophages dysfunction observed in chronic HIV infection mobilizing a significant number of core and surface antigen-specific B cells to prime and boost the HIV-specific cellular responses. Additionally, differential processing by an array of APC might allow diversification of the cellular responseCitation47 and might also contribute to the induction of relevant epitope-specific cytotoxic T cells.
Figure 1. Schematic diagram of the hypothetical events that might explain the induction of CR3(HIV)-specific CD4+ and CD8+ cellular response after IN and SC immunizations of TERAVAC-HIV-1 vaccine candidate. Explanations are in the text.
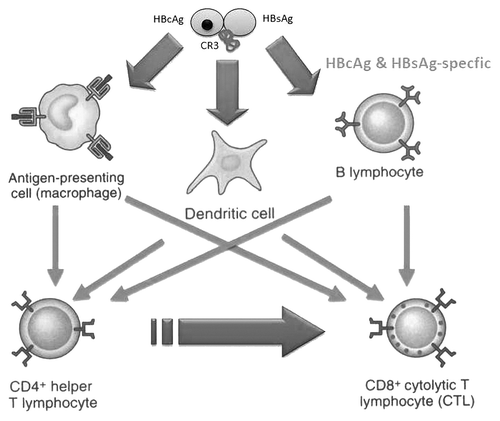
Although the CR3 protein was designed to induce HIV-specific cellular responses, it also contains some B-cell epitopes associated with several immune-effector mechanisms.Citation48 In this regard, the induction of anti-Nef antibodies have been found that may avoid the toxic effects of this viral antigen.Citation39 Finally, we also detected anti-HBsAg and anti-HBcAg cellular and humoral responses.Citation39 Consequently, the multiantigenic formulation might provide immunity to HBV as well, which would be of additional benefit considering the HIV–HBV coinfection rate reported worldwide.Citation49
Preclinical evaluation of this multiantigenic vaccine candidate was conducted up to the demonstration of the safety and immunogenicity of the formulation and a phase I therapeutic clinical trial is ongoing.
Conclusions
Progress in our understanding of the immunopathological events that occurs during chronic HIV infection, and the benefits and limitations of ART open a window of opportunities for therapeutic vaccination. This knowledge may lead to new therapeutic approaches to deal with chronic HIV-1 infection. Therapeutic vaccination is a possible alternative to the current antiretroviral chemotherapy. Even a partial effective vaccine might report some therapeutic, social, economic and epidemiologic benefits. Although the correlates of protection are still unknown, perhaps we are closer than ever to obtain a therapeutic vaccine against the HIV/AIDS.
Disclosure of Potential Conflicts of Interest
The author have not conflicts of interest to disclose.
Acknowledgments
The author acknowledges the financial support received form the Center for Genetic Engineering and Biotechnology, Havana, Cuba.
References
- UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2012. Geneva, Switzerland, 2012.
- Guest I. Rape in Congo is not a myth – if anything, it is under-reported. The Guardian. http://www.guardian.co.uk/commentisfree/2012/nov/21/rape-congo-not-myth-under-reported, 2012.
- Zabarenko D. Countries must plan for climate refugees: report. Reuter. http://www.reuters.com/assets/print?aid=USTRE79Q6TS20111027, 2011.
- Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, et al, International AIDS Society Scientific Working Group on HIV Cure. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol 2012; 12:607 - 14; http://dx.doi.org/10.1038/nri3262; PMID: 22814509
- Deeks SG. Determinants of virological response to antiretroviral therapy: implications for long-term strategies. Clin Infect Dis 2000; 30:Suppl 2 S177 - 84; http://dx.doi.org/10.1086/313855; PMID: 10860903
- Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, et al. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol 2000; 74:11001 - 7; http://dx.doi.org/10.1128/JVI.74.23.11001-11007.2000; PMID: 11069995
- Kewenig S, Schneider T, Hohloch K, Lampe-Dreyer K, Ullrich R, Stolte N, et al. Rapid mucosal CD4(+) T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology 1999; 116:1115 - 23; http://dx.doi.org/10.1016/S0016-5085(99)70014-4; PMID: 10220503
- Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 1999; 284:816 - 9; http://dx.doi.org/10.1126/science.284.5415.816; PMID: 10221916
- Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med 2006; 12:289 - 95; http://dx.doi.org/10.1038/nm1380; PMID: 16520776
- Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, et al, RV254/SEARCH 010 Study Group. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7:e33948; http://dx.doi.org/10.1371/journal.pone.0033948; PMID: 22479485
- Lange CG, Lederman MM, Medvik K, Asaad R, Wild M, Kalayjian R, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS 2003; 17:2015 - 23; http://dx.doi.org/10.1097/00002030-200309260-00002; PMID: 14502004
- Price P, Keane NM, Lee S, Lim AF, McKinnon EJ, French MAA. A T2 cytokine environment may not limit T1 responses in human immunodeficiency virus patients with a favourable response to antiretroviral therapy. Immunology 2006; 119:74 - 82; http://dx.doi.org/10.1111/j.1365-2567.2006.02407.x; PMID: 16792698
- Gahery H, Daniel N, Charmeteau B, Ourth L, Jackson A, Andrieu M, et al. New CD4+ and CD8+ T cell responses induced in chronically HIV type-1-infected patients after immunizations with an HIV type 1 lipopeptide vaccine. AIDS Res Hum Retroviruses 2006; 22:684 - 94; http://dx.doi.org/10.1089/aid.2006.22.684; PMID: 16831093
- Girardi E, Sabin CA, Monforte AD. Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. J Acquir Immune Defic Syndr 2007; 46:Suppl 1 S3 - 8; http://dx.doi.org/10.1097/01.qai.0000286597.57066.2b; PMID: 17713423
- García F, Climent N, Guardo AC, Gil C, León A, Autran B, et al, DCV2/MANON07-ORVACS Study Group. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med 2013; 5:ra2; http://dx.doi.org/10.1126/scitranslmed.3004682; PMID: 23283367
- Valor L, Navarro J, Carbone J, Rodríguez-Sáinz C, Gil J, López F, et al, STIR-2102 Team. Immunization with an HIV-1 immunogen induces CD4+ and CD8+ HIV-1-specific polyfunctional responses in patients with chronic HIV-1 infection receiving antiretroviral therapy. Vaccine 2008; 26:2738 - 45; http://dx.doi.org/10.1016/j.vaccine.2008.03.019; PMID: 18433946
- Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med 2004; 200:701 - 12; http://dx.doi.org/10.1084/jem.20041270; PMID: 15381726
- Vajdy M, Baudner B, Del Giudice G, O’Hagan D. A vaccination strategy to enhance mucosal and systemic antibody and T cell responses against influenza. Clin Immunol 2007; 123:166 - 75; http://dx.doi.org/10.1016/j.clim.2007.01.009; PMID: 17349825
- Gorse GJ, Otto EE, Powers DC, Chambers GW, Eickhoff CS, Newman FK. Induction of mucosal antibodies by live attenuated and inactivated influenza virus vaccines in the chronically ill elderly. J Infect Dis 1996; 173:285 - 90; http://dx.doi.org/10.1093/infdis/173.2.285; PMID: 8568287
- Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol 2008; 214:231 - 41; http://dx.doi.org/10.1002/path.2276; PMID: 18161758
- Costiniuk CT, Angel JB. Human immunodeficiency virus and the gastrointestinal immune system: does highly active antiretroviral therapy restore gut immunity?. Mucosal Immunol 2012; 5:596 - 604; http://dx.doi.org/10.1038/mi.2012.82; PMID: 22929559
- Fujihashi K, Kiyono H. Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol 2009; 30:334 - 43; http://dx.doi.org/10.1016/j.it.2009.04.004; PMID: 19540811
- Bergquist C, Johansson EL, Lagergård T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun 1997; 65:2676 - 84; PMID: 9199436
- Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY, et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol 2002; 32:1605 - 13; http://dx.doi.org/10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6; PMID: 12115643
- van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 2007; 212:475 - 90; http://dx.doi.org/10.1016/j.imbio.2007.03.009; PMID: 17544832
- Kaech SM, Ahmed R. Immunology. CD8 T cells remember with a little help. Science 2003; 300:263 - 5; http://dx.doi.org/10.1126/science.1084511; PMID: 12690179
- Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature 2009; 462:510 - 3; http://dx.doi.org/10.1038/nature08511; PMID: 19898495
- Collette Y, Dutartre H, Benziane A, Olive D. The role of HIV1 Nef in T-cell activation: Nef impairs induction of Th1 cytokines and interacts with the Src family tyrosine kinase Lck. Res Virol 1997; 148:52 - 8; http://dx.doi.org/10.1016/S0923-2516(97)81914-0; PMID: 9017835
- Blazevic V, Heino M, Lagerstedt A, Ranki A, Krohn KJ. Interleukin-10 gene expression induced by HIV-1 Tat and Rev in the cells of HIV-1 infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol 1996; 13:208 - 14; http://dx.doi.org/10.1097/00042560-199611010-00002; PMID: 8898665
- Quaranta MG, Mattioli B, Giordani L, Viora M. The immunoregulatory effects of HIV-1 Nef on dendritic cells and the pathogenesis of AIDS. FASEB J 2006; 20:2198 - 208; http://dx.doi.org/10.1096/fj.06-6260rev; PMID: 17077296
- Clayden P, Collins S, Daniels C, Geffen N, Harrington M, Jefferys R, et al. Pipeline Report In: Benzacar A, ed. New York: Treatment Action Group, 2012.
- Shete A, Thakar M, Singh DP, Gangakhedkar R, Gaikwad A, Pawar J, et al. Short communication: HIV antigen-specific reactivation of HIV infection from cellular reservoirs: implications in the settings of therapeutic vaccinations. AIDS Res Hum Retroviruses 2012; 28:835 - 43; http://dx.doi.org/10.1089/aid.2010.0363; PMID: 21936714
- Hoffmann D, Seebach J, Cosma A, Goebel FD, Strimmer K, Schätzl HM, et al. Therapeutic vaccination reduces HIV sequence variability. FASEB J 2008; 22:437 - 44; http://dx.doi.org/10.1096/fj.06-7975com; PMID: 17932027
- Kutzler MA, Jacobson JM. Treatment interruption as a tool to measure changes in immunologic response to HIV-1. Curr Opin HIV AIDS 2008; 3:131 - 5; http://dx.doi.org/10.1097/COH.0b013e3282f54cde; PMID: 19372954
- Ledergerber B, Lundgren JD, Walker AS, Sabin C, Justice A, Reiss P, et al, PLATO Collaboration. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet 2004; 364:51 - 62; http://dx.doi.org/10.1016/S0140-6736(04)16589-6; PMID: 15234856
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, et al, Rakai Project Team. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 2001; 357:1149 - 53; http://dx.doi.org/10.1016/S0140-6736(00)04331-2; PMID: 11323041
- Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, et al, Women and Infants Transmission Study Group. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med 1999; 341:394 - 402; http://dx.doi.org/10.1056/NEJM199908053410602; PMID: 10432324
- Iglesias E, Thompson R, Carrazana Y, Lobaina Y, García D, Sánchez J, et al. Coinoculation with hepatitis B surface and core antigen promotes a Th1 immune response to a multiepitopic protein of HIV-1. Immunol Cell Biol 2006; 84:174 - 83; http://dx.doi.org/10.1111/j.1440-1711.2005.01408.x; PMID: 16519735
- Iglesias E, García D, Carrazana Y, Aguilar JC, Sánchez A, Gorobaya L, et al. Anti-HIV-1 and anti-HBV immune responses in mice after parenteral and nasal co-administration of a multiantigenic formulation. Curr HIV Res 2008; 6:452 - 60; http://dx.doi.org/10.2174/157016208785861186; PMID: 18855656
- García-Díaz D, Rodríguez I, Santisteban Y, Márquez G, Terrero Y, Brown E, et al. Th2-Th1 shift with the multiantigenic formulation TERAVAC-HIV-1 in Balb/c mice. Immunol Lett 2013; 149:77 - 84; http://dx.doi.org/10.1016/j.imlet.2012.11.007; PMID: 23183092
- Schirmbeck R, Böhm W, Melber K, Reimann J. Processing of exogenous heat-aggregated (denatured) and particulate (native) hepatitis B surface antigen for class I-restricted epitope presentation. J Immunol 1995; 155:4676 - 84; PMID: 7594467
- Milich DR, Chen M, Schödel F, Peterson DL, Jones JE, Hughes JL. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci U S A 1997; 94:14648 - 53; http://dx.doi.org/10.1073/pnas.94.26.14648; PMID: 9405667
- Cao T, Lazdina U, Desombere I, Vanlandschoot P, Milich DR, Sällberg M, et al. Hepatitis B virus core antigen binds and activates naive human B cells in vivo: studies with a human PBL-NOD/SCID mouse model. J Virol 2001; 75:6359 - 66; http://dx.doi.org/10.1128/JVI.75.14.6359-6366.2001; PMID: 11413302
- Lazdina U, Cao T, Steinbergs J, Alheim M, Pumpens P, Peterson DL, et al. Molecular basis for the interaction of the hepatitis B virus core antigen with the surface immunoglobulin receptor on naive B cells. J Virol 2001; 75:6367 - 74; http://dx.doi.org/10.1128/JVI.75.14.6367-6374.2001; PMID: 11413303
- Lazdina U, Alheim M, Nyström J, Hultgren C, Borisova G, Sominskaya I, et al. Priming of cytotoxic T cell responses to exogenous hepatitis B virus core antigen is B cell dependent. J Gen Virol 2003; 84:139 - 46; http://dx.doi.org/10.1099/vir.0.18678-0; PMID: 12533710
- Barnaba V, Franco A, Alberti A, Benvenuto R, Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature 1990; 345:258 - 60; http://dx.doi.org/10.1038/345258a0; PMID: 2110296
- Steers NJ, Currier JR, Kijak GH, di Targiani RC, Saxena A, Marovich MA, et al. Cell type-specific proteasomal processing of HIV-1 Gag-p24 results in an altered epitope repertoire. J Virol 2011; 85:1541 - 53; http://dx.doi.org/10.1128/JVI.01790-10; PMID: 21106750
- Iglesias E, Ruiz M, Carrazana Y, Cruz LJ, Aguilar A, Jimenez V, et al. Chimeric Proteins Containing HIV-1 T Cell Epitopes: Expression in E. Coli, Purification and Induction of Antibodies in Mice. J Biochem Mol Biol Biophys 2001; 5:109 - 22
- Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res 2010; 85:303 - 15; http://dx.doi.org/10.1016/j.antiviral.2009.10.021; PMID: 19887087