 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aging of the immune system, also named immunosenescence, affects vaccine responses. However, the onset of age-related immunosenescence has been uncertain, in particularly with regard to vaccine responses. Here, we show that the formation of antibodies in response to vaccination against hepatitis B virus infection was significantly reduced for donors with a mean age of 61 y compared with a group with a mean age of 33 y. Booster vaccination sero-converted the elderly donors, but only at a reduced level, while a stronger response was found for the group of young donors. Agreeing with these findings, the hepatitis B surface antigen-specific proliferative responses by donor-derived T cells were reduced for the elder donors. Interestingly, the association between expression of the adhesion molecule CD62L (L-selectin) on naïve and central memory T cells and the formation of antigen-specific antibodies differed significantly between younger and elder donors. This finding corresponds well with the observation made previously that CD62L gene ablation in animals alters the formation of antigen-specific antibodies. We suggest that a complex interplay between the expression of CD62L and its ligands is a determinant in early-age immunosenescence affecting the response to HBV vaccination.
Introduction
Hepatitis B virus (HBV) infection is a major global health problem. A recent evaluation by The World Health Organization (WHO), estimated that 2 billion people worldwide are infected with the virus, including 350 million with a chronic infection. Chronic HBV infection is the leading cause of hepatocellular carcinoma (HCC) and a major contributing factor to liver cirrhosis.Citation1,Citation2 Prophylactic immunization is the main tool for controlling HBV infection. In most countries the vaccine is administered at birth as part of the childhood immunization program in compliance with the recommendations issued by the WHO. The benefit of universal newborn HBV vaccination is illustrated by elimination of acute symptomatic HBV as well as HCC among Alaska Native children.Citation1
The commercially available recombinant subunit vaccines are based on the highly immunogenic HBV surface antigen (HBsAg), which is part of the viral envelope.Citation3 Vaccine immunogenicity is evaluated by quantification of the humoral response. Follow-up studies have demonstrated that the HBV vaccine confers protection for more than 20 y when administered during childhood.Citation4 Protective immunological memory may even be life-long when adequate immunological priming is achieved.Citation5,Citation6 From these observations, a booster immunization is not recommended at present although this recommendation is still subject to debate.Citation7 A recent Cochrane review concluded that none of the existing randomized clinical trials have addressed booster immunization policies with regard to prevention of hepatitis B infection.Citation6 Induction of strong primary immune memory is indeed crucial for long-term protection against infection from exposure.Citation8 A Chinese study emphasized this point by reporting that almost 32% of weakly responding neonatal HBV vaccinees may be susceptible to HBV infection after childhood.Citation9 Chaves et al. observed additionally that only 70% of seronegative adolescents (10–15 y) immunized at birth developed an anamnestic antibody response following a booster dose.Citation10 The vaccine response is reduced later in life and has a limited effectiveness when administered to older adults comparable to the response detected in immunocompromised individuals.Citation11-Citation14 So far, the data from HBsAg vaccination studies suggest that the onset of reduced vaccine effectiveness is a distinctive feature of individuals older than 80 y. Impaired formation of antigen-specific antibodies in elderly individuals has also been reported for influenza vaccination.Citation15 Comparing the humoral response to influenza immunization in individuals below or above 58 y of age, a quantitative review found that age significantly influenced both seroconversion and seroprotection rates.Citation16
Immunosenescence encompasses changes of both humoral and cellular responses in healthy individuals. A decreased proportion of naïve (i.e., CD45RA+) CD4+ and CD8+ T cells, as well as an increased proportion of memory (i.e., CD45RO+) CD4+ and CD8+ T cells characterize the overall changes within T cell populations of elderly individuals.Citation17,Citation18 Thymic involution that begins early in lifeCitation19 contributes to this phenomenon and limits the generation of naïve T cells at ages as early as 40–50 y.Citation20 Changes within the CD4+ naïve and memory subsets have been shown to impair long-term CD4+ T cell responses to influenza vaccination.Citation21 One of the most consistent and well-established markers of immunological senescence is loss of the co-stimulatory molecule CD28 on antigen-experienced T cells.Citation22 This phenotypic change has been reported to influence the outcome of vaccination as well as the response to infection,Citation23 perhaps related to a reduced diversity of naïve T cells and altered cytokine environment in secondary lymphoid tissues.Citation24 Moreover an age-related decline in signal transduction through CD28 has been demonstrated and results in a decreased responsiveness of naïve T cells independent of antigen stimulation.Citation25 CD4+ T cells provide indispensable help to B cells during formation of germinal centers where somatic hypermutation, affinity maturation, and class switching modify the genes encoding immunoglobulins.Citation26,Citation27 Impaired T helper cell function may therefore be partly responsible for the reduced ability to produce antibodies in aged individuals. Indeed, a gradual decline in germinal center reactions (both in number and volume) has been correlated with reduced antibody responses in senescent mice.Citation25 On activated B cells, specific co-stimulatory molecules such as CD86 are critical for initiation of antibody responses. Murine studies show that diminished CD86 expression by aged B cells may be a contributing factor to reduced germinal center reaction,Citation25 and will most likely also be an important aspect in human immunosenescence.
The trafficking of T cells from the vasculature into secondary lymphoid organs and inflamed tissues is a complex process involving several adhesion molecules.Citation28,Citation29 Upon entry of T cells into lymph nodes, the initial cell-adhesive event involves interactions between carbohydrate ligands presented on high endothelial venules (HEV) and the constitutively expressed Ca2+-dependent lectin CD62L (also named L-selectin) on the surface of recirculating central memory and naïve T cells. The critical recognition determinants for CD62L are well-established.Citation30 As in the case of other selectins, CD62L recognizes sialyl 6-sulfo Lewis X albeit with low affinity. To obtain high affinity interactions sulfation of the ligand is required. CD62L ligands includes the sialomucins such as GlyCAM-1, CD34, and podocalyxin, which are part of the peripheral node adressin (PNAd) complex. Another ligand is shared with P-selectin (CD62P), namely P-selectin glycoprotein ligand-1 (PSGL-1).Citation30 Through the regulated exposure of such ligands CD62L mediates both primary capture of leukocytes by endothelial-cell-derived ligands as well as secondary capture by ligands expressed on leukocyte that have previously adhered to endothelium. In experimental animals, the ablation of CD62L altered the humoral response to antigens requiring T cell help for efficient formation of specific antibodies. This finding is consistent with alterations in the T cell distribution in lymph nodes as a consequence of the CD62L gene ablationCitation31 Other studies removing the synthesis of ligands for CD62L in mice also showed significant alterations in the cellular distribution in lymph nodes with a marked reduction in the frequency of B cells.Citation32 These effects also showed a time-course where aged mice had a disproportionate number of CD4+ T lymphocytes in circulation, suggesting that CD62L is of major importance in regulating the cellular distribution in this compartment as well.Citation33 Taken together, these data suggest that CD62L is a factor in regulating humoral immunity. In humans, evidence has been presented that the CD62L expression was enhanced nearly 2-fold on T lymphocytes from elderly people with a mean age of 90 y compared with a group of younger people with a mean age of 30 y.Citation34 While this finding was entirely observational, it clearly suggests that CD62L vary with age and that alterations in the CD62L expression level could affect the humoral immune response. It was hypothesized that the increased expression reflects a compensation of functional insufficiencies of the receptor in the aged immune system, but it remains unclear if CD62L functionality is changed as part of immunosenescence.
Here, we analyze the humoral and cell-mediated immune response to HBsAg vaccination in two groups of healthy adults, one comprising individuals with an age of 35 y or younger and the other 55 y of age or older carefully matched with regard to time of vaccination. The humoral response was related to the T cell proliferative response of individual donors and distribution of naïve, effector, and memory T cells. The formation of HBsAg-specific antibodies in the older vaccinees was less efficient compared with the younger group. In relation to earlier reports this finding suggests an earlier onset of immunosenescence with regard to forming a protective response to HBV vaccination. While the vaccine response in the younger vaccinees correlated with CD62L expression level this pattern was not found for the elderly vaccinees. This finding points to an altered interplay between CD62L and its ligands in the latter group of individuals.
Results
HBV vaccine-induced humoral immune response
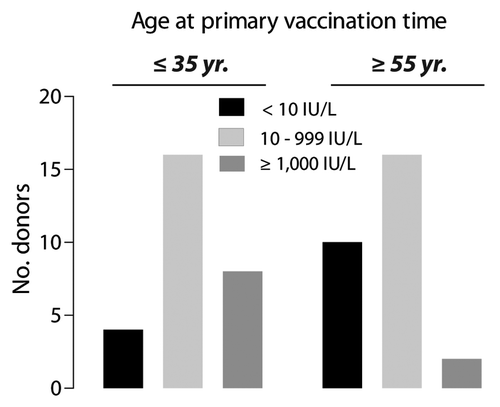
In the present study we analyzed the immune response among 56 donors () vaccinated with either the combined hepatitis A and B vaccine Twinrix® or the HBV subunit vaccine Engerix-B® (). Protective antibodies against HBV were found in 24 of the 28 (85.7%) vaccinees below 35 y and in 18 of the 28 (64.3%) vaccinees over 55 y. According to the assay report, levels of the antibody response stratified the donors into to non-responding (NR, anti-HBs ≤ 10 IU/L), intermediate (IR, 10 IUL/L < anti-HBs < 1,000 IU/L), and high responding (HR, anti-HBs ≥ 1000 IU/L) individuals. The distribution of the ≤ 35 y and ≥ 55 y subjects within the three antibody response categories showed a significant difference in the distribution of non-responding (anti-HBs < 10 IU/L) and high-responding (anti-HBs ≥ 1000 IU/L) individuals (p = 0.046). Inspection of the distributions attributed this difference to a higher number of HR individuals in subjects ≤ 35 y (). Sixteen young and 16 elderly individuals were identified as intermediate responders. Evaluation of the intermediate HBV-specific antibody titers (n = 32) revealed a statistically significant correlation between the in vivo antibody response and the in vitro T cell proliferation (r = 0.5174, p = 0.0029) (data not shown). This finding is consistent with previous reports concerning induction of antigen specific B and T cell responses.Citation35,Citation36
Table 1. Characteristics of the study population
Table 2. Characteristics of the specific vaccination regimes
Figure 1. Distribution of humoral responses to HBsAg in vacinees immunized when ≤ 35 y of age and ≥ 55 y of age. Antibody responses were categorized as non-response (NR) when titers were < 10 IU/L, intermediate response (IR) for titers 10–999 IU/L, or high response (HR) for titers ≥ 1000 IU/L. Application of a χ2-test demonstrated a significant difference (p = 0.046) between age at the time for the primary vaccination and the degree of antibody response.
A booster vaccination was offered to all individuals with a HBs-specific antibody response ≤ 20 IU/L. A typical anamnestic antibody response to revaccination was observed in all six young individuals, and in 10 out of 13 elderly individuals (). Three donors ≥ 55 y remained seronegative, and two of these were administered a second booster immunization. Following these two booster vaccinations both individuals seroconverted with an antibody response of 14 IU/L and 31 IU/L, respectively.
Table 3. Antibody response following primary and booster vaccination of vaccinees with low (≤ 20 IU/L) or no protective antibody response
The HBsAg-induced cellular immune response quantified by T cell proliferation
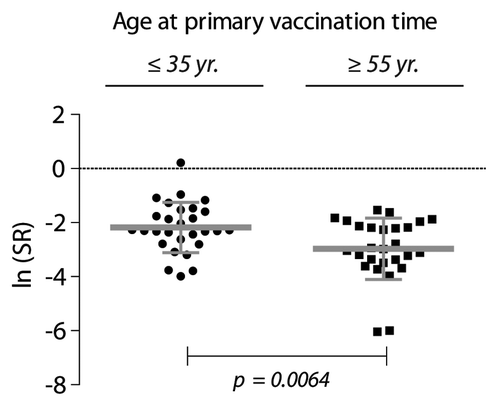
T cells from all subjects (≤ 35 y: n = 28, ≥ 55 y: n = 27) were proliferating in response to stimulation with antibodies to CD3 and CD28, and demonstrated full proliferative capacity (data not shown). As a means of robustly quantifying the antigen-induced proliferation, we used a Stimulation Ratio (SR) defined as the antigen-induced thymidine incorporation relative to the proliferative capacity of cultures activated with antibodies to CD3 and CD28. Since the cpm measurements were skewed to the right, SRs were transformed by the natural logarithm, according to Equation 1 defined in the Materials and Methods Section, to approximate a normal distribution as confirmed in QQ-plots (data not shown). In a simple comparison between the groups, the average SR for the young individuals was a factor 2.21 (95% CI: 1.26 – 3.86, p = 0.0064) larger than the elderly individuals ().
Figure 2. Proliferative recall response to HBsAg (10 µg/ml) tested by in vitro stimulation of PBMCs from HBV-vaccinated individuals. The donors were ≤ 35 y (n = 28), or ≥ 55 y (n = 27) when they received the primary immunization course. The stimulation ratio (SR) expresses the ln-transformed ratio of the antigen-specific response normalized to the polyclonal stimulation of T cell proliferation with antibodies to CD28 and CD3 according to Equation 1 (defined in the Materials and Methods). To compensate for right skewing of the data, the cpm values are shown as ln median ± SD.
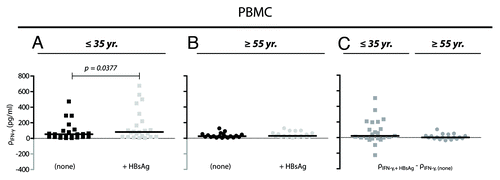
A quantitative sandwich ELISA assay was performed to measure the levels of IFN-γ protein in supernatants from PBMC cultures stimulated with HBsAg (10 μg/ml). Among the cells harvested from the young donors (), some cultures generated IFN-γ levels at a mass concentration higher than 200 pg/ml. The striking presence of cultures with a high response to HBsAg stimulation among young donors persisted even after subtraction of the IFN-γ production in the absence of HBsAg stimulation (). This was not found for any of the tested cultures from elderly donors (). Moreover, for the young donors stimulation with HBsAg increased significantly the IFN-γ production compared with unstimulated controls ().
Figure 3. IFN-γ production from non-stimulated and HBsAg-stimulated PBMCs. (A and B) The mass concentration of IFN-γ (ρ) was measured in supernatant from cultured cells 48 h-post stimulation with a final concentration of 10 μg/ml HBsAg (+HBsAg) or with no stimulation (none). Cells were derived from donors ≤ 35 y (A; n = 23) and ≥ 55 y (B; n = 19). Results for each of the donors are shown together with medians (black bars). The data were analyzed by Wilcoxon matched-pairs signed rank test. (C) Correction for IFN-γ baseline levels were performed by simple subtraction of the mass concentration of IFN-γ in supernatant from unstimulated cell cultures (ρIFN-γ, none) from the concentration obtained in HBsAg-stimulated cultures (ρIFN-γ, +HBsAg).
Analysis of T cell subsets by flow cytometry
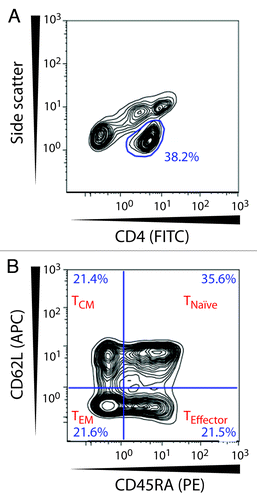
A flow cytometric analysis of cell surface markers was performed to dissect naïve and memory CD4+ T cell subsets. We used the markers CD45RA and CD62L to distinguish naïve (TNaïve), effector (TEffector), central memory (TCM), and effector memory (TEM) T cells. TNaïve are CD62L+CD45RA+, TEM lacks both of these receptors (CD62L-CD45RA-), while TEffector (CD62L-CD45+) and TCM (CD62L+CD45-) lack either.Citation37 The principles for gating these subsets are shown in .
Figure 4. Gating strategy for naive and memory CD4+ T lymphocytes. The CD4+ T cell population was selected in a side scatter vs. CD4-FITC dot plot. Within this subset CD4+ T cells were divided based on their expression of CD62L and CD45RA and were identified as naïve T cells (TNaïve; CD62L+CD45RA+), effector T cells (TEffector; CD62L-CD45RA+), central memory T cells (TCM; CD62L+CD45RA-), and effector memory T cells (TEM; CD62L-CD45-).
The lymph-node homing receptor CD62L is essential for the entry of TNaïve and TCM cells into the secondary lymphoid organsCitation38 where antigen-specific immune responses are initiated. To investigate if CD62L played a role in age-related changes in the immune response we focused on these subsets, while the TEM and TEffector subsets, which do not express CD62L, were not further investigated.
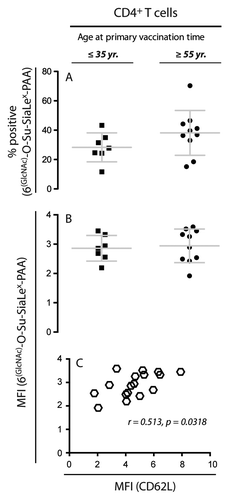
We examined the relation between serologic response and expression of CD62L among the vaccinees by stratifying the donors according to HBsAg-specific antibody titers into three groups, namely non-responders (NR) with titers < 10 IU/L, intermediate responders (IR) with titers 10–999 IU/L, and high-responders (HR) with titers ≥ 1000 IU/L (). Within the CD4+ T cells of the young individuals we detected a significant increase in the CD62L expression with an increasing antibody titer for both naïve (p = 0.0314) and central memory T cells (p = 0.0197). Correction for multiple comparisons (Bonferroni’s Multiple Comparison Test) defined a difference between individuals with no detectable antibodies and individuals with serum antibody titers ≥ 1000 IU/L (). The mean difference in CD62L expression was 1.98 (95% CI: 0.28–3.78), and 1.73 (95% CI: 0.30–3.25) for the TNaïve and TCM, respectively. By contrast, such differences were not found among the elderly donors (). To more directly address differences between the age groups, a two-way analysis of variance on both age groups was applied. The test for interaction between the age group and the HBsAg antibody response group gave p = 0.0359 for TCM and p = 0.1366 for TNaïve, indicating that the differences between the CD62L MFI in the three HBsAg groups were similar in the two age groups for TNaïve but not for TCM (). Interestingly, additional analysis revealed that CD62L expression level of donors ≥ 55 y with no detectable HBsAg-specific antibodies () was comparable to that of high-responding donors ≤ 35 y (). Based on this finding, we hypothesized that aging could have a possible functional impact on CD62L, i.e., an influence on the ability of the lymph node homing receptor to bind its saccharide ligand.
Figure 5. Association between CD62L expression levels and the humoral response to HBV vaccination. The donors were stratified into antibody response groups of non-responders (NR) (< 10 IU/L), intermediate responders (IR) (10–999 IU/L), and high responders (HR) (≥ 1000 IU/L). One-way ANOVA analyses for each age group were performed to test the relationship between CD62L expression and titers of HBsAg-specific antibodies. (A) The results are shown as a scatter plot with mean ± SD (a) for individuals ≤ 35 y [NR (n = 4), IR (n = 16), HR (n = 8)]. A statistically significant difference was found between the group mean CD62L MFI values for CD4+ TNaïve cells (p = 0.0314) and TCM cells (p = 0.0197). (B) Individuals ≥ 55 y [NR (n = 10), IR (n = 15), HR (n = 2)] showed no difference between the different response categories.
![Figure 5. Association between CD62L expression levels and the humoral response to HBV vaccination. The donors were stratified into antibody response groups of non-responders (NR) (< 10 IU/L), intermediate responders (IR) (10–999 IU/L), and high responders (HR) (≥ 1000 IU/L). One-way ANOVA analyses for each age group were performed to test the relationship between CD62L expression and titers of HBsAg-specific antibodies. (A) The results are shown as a scatter plot with mean ± SD (a) for individuals ≤ 35 y [NR (n = 4), IR (n = 16), HR (n = 8)]. A statistically significant difference was found between the group mean CD62L MFI values for CD4+ TNaïve cells (p = 0.0314) and TCM cells (p = 0.0197). (B) Individuals ≥ 55 y [NR (n = 10), IR (n = 15), HR (n = 2)] showed no difference between the different response categories.](/cms/asset/19af8c8a-a77a-46a0-9ebb-c55a81ef53fe/khvi_a_10924480_f0005.gif)
To investigate the function of CD62L, we performed a flow cytometric binding assay with a CD62L-specific polyvalent glycoprobe on the total CD4+ T cell population. shows a representative sequence for gating with antibody to CD4 and the glycoprobe. Initially, CD4+ T cells were identified according to their logarithmic side scatter and CD4-APC fluorescence in a contour plot. Subsequently, the expression of functional CD62L was gated based on FMO controls and the negative control probe devoid of the L-selectin binding structure. We found no difference in the percentage of CD4+ T cells that express functional CD62L molecules (). Likewise, the median expression level on single cells was unaffected (). Our assay was clearly able to distinguish the functional activity of CD62L on the cell surface since the expected correlation was found for the overall expression of CD62L, determined by monoclonal antibody binding, and ligand binding by this receptor as evaluated by the glycoprobe (Spearman r = 0.512; p = 0.032) ().
Figure 6. Gating strategy for identification of functional CD62L on CD4+ T lymphocytes. The CD4+ T cell population was selected based on side scatter and CD4-APC expression. Hereafter binding of the glycoprobe to functional CD62L molecules was distinguished by means of the fluorescent glycoprobe signal and forward scatter.
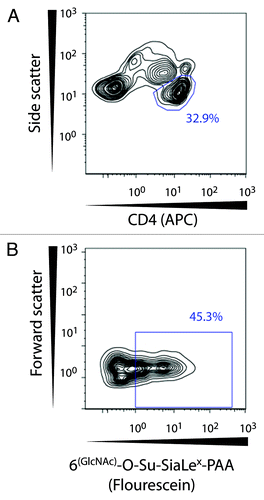
Figure 7. Flow cytometric analysis of glycoprobe-binding to CD62L+ T cells. To investigate if the expression of functional CD62L in NR donors ≥ 55 y of age could account for their inability to raise responses against HBsAg, the percentage of T cells binding the glycoprobe ligand for CD62L and the MFI for staining with the glycoprobe was compared for HR donors ≤ 35 y and NR donors ≥ 55 y. (A) Percentage of CD4+ T cells positive for staining with the glycoprobe analyzed in 17 subjects either ≤ 35 y (n = 7; anti-HBsAg titer ≥ 1000 IU/L) or ≥ 55 y (n = 10; anti-HBsAg titer < 10 IU/L) who had received the complete course of HBV vaccination. (B) Analysis of the MFI from staining with the glycoprobe after gating on CD4+ T cells. (C) Analysis of the correlation between the MFI for staining against CD62L with specific antibody and the MFI for the glycoprobe binding to CD4+ T cells.
Discussion
It is well known that induction of antibody responses to new antigens decline with age.Citation39 In this study we evaluated the immune response toward hepatitis B virus vaccination of young and elderly subjects one to six years following completion of their primary vaccination course. The present study reports on an age-related immunosenescence in people with an age of approximately 60 y and hence developing between the age of 35 and 55. Therefore, our study adds to a growing body of evidence that effects of immunosenescence have an onset earlier in life than previously thought.Citation40
The crucial events for obtaining protective immunity are the induction of antigen specific B- and T cell memory during initial vaccination. The data presented here demonstrate a higher proportion of elderly subjects with HBsAg antibody titers below the threshold for seroprotection as well a higher proportion of younger subjects with antibody responses above the range of the clinical assay. The loss of protective antibodies is not necessarily synonymous with loss of protection against infection. The long incubation time of the virus allow for a recall antibody response to be built to clear the infection, and thus avert acute diseaseCitation41 as well as chronic infection.Citation42 Judged by the presence of a strong anamnestic antibody response, specific immune memory against HBsAg was induced during primary vaccination for all individuals ≤ 35 y. Likewise, the existence of memory was confirmed among 77% of the elderly donors, although two individuals only responded weakly (14–42 IU/L). Administration of a second booster dose was needed to induce a serologic response in two of the three elderly donors. Indeed, memory function is impaired in old age seen by reduced expansion of B cells, impaired germinal center development and reduced IgG production.Citation43,Citation44 More recently, a vaccination study against tick-borne encephalitis demonstrated that the number of antigen-specific memory B cells generated during primary vaccination was approximately 3-fold lowered in older (60–80 y) than in young individuals (20–31 y).Citation45 In addition, the antibody response per memory B cell after revaccination was dramatically diminished within the group of old individuals.Citation45 Taken together these findings may explain the reduced antibody response in the group of elderly vaccinees reported here in our study.
With the close link between antigen recognition by the CD4+ T cells and differentiation of B cells into antibody secreting plasma cells and memory B cells, measurements on the humoral immune response may serve as a marker for the presence of antigen specific CD4+ T cells.Citation46 However, by use of proliferation assays and flow cytometric characterization of cellular phenotypes, it is possible to study the contribution of T cells to the production of HBsAg-specific antibodies more directly. Well-established cell surface markers include CD62L and CD45, which allow for the identification of naïve, effector, and memory T cellular subsets. Expression of CD45RA generally characterizes naïve T cells whereas CD45RO is expressed on memory T cells. The adhesion molecule CD62L is a lymph node homing receptor expressed on TNaïve and TCM cells, but not on TEM cells.Citation37 The molecule is essential for the initial adhesion to high endothelial venules prior to the entry into the lymph node. In humans, it has been reported that the CD62L expression decrease with age,Citation47-Citation49 but any functional correlate has, to our knowledge, not been reported previously. The leukocyte subset distribution of notably the CD4+ T cells in lymphoid tissue is obviously at the center of B cell differentiation and may influence both the formation of antibody-producing plasma cells as well as memory B cells. By stratifying the younger and older vaccinees according to their humoral response, we found that the differences between the CD62L MFI in the three HBsAg groups were similar in the two age groups for TNaïve but not for TCM cells. This finding consequently suggests that alterations in the CD62L expression on the TCM cell may regulate antibody production in young donors. The importance of TCM, rather than TNaïve, cells in this process is likely to be crucial in mounting efficient recall responses to HBsAg. By contrast, a similar phenomenon was not observed for the elderly vaccinees. Here, responding and non-responding vaccinees had high expression levels of CD62L, i.e. on par with the high-responding younger vaccinees.
Past reports on age-related deficiencies in the immune system were associated with the loss of components or altered regulation, one example being the age-related downregulation of Cd28 expression in elderly individuals.Citation40 Since CD62L expression was maintained at a high level among the non-responding elderly donors in our study, we analyzed the functional activity of the receptor. Previous studies have suggested that exogenous factors such as fatty acids may influence CD62L clustering in the membrane,Citation50 which alter the ability of the molecules to interact with ligands.Citation51 It is known that the fatty acid metabolism changes with age. As demonstrated for the CD28 family of adhesion molecules as well as for β2 integrins such receptor clustering may be a quantitative determinant in function.Citation52,Citation53 This way, an influence of the fatty acid metabolism on the function of CD62L could potentially contribute to some of the immunosenescence recorded in our study. The ability of fluorescein-conjugated 6(GlcNAc)-O-Su-SiaLex-PAA glycoprobe to stain the T cell subset of PBMCs from high-responding young donors and non-responding elderly donors was compared by flow cytometry. No significant difference between these groups was found in the CD62L ligand-binding capacity. This seems to exclude a functional impairment of the CD62L molecule itself or in other mechanism contributing to its ligand binding capacity, e.g., membrane fluidity. Therefore, our analyses suggest that the functionality of CD62L per se is not explaining age-related alteration in vaccine responses. With the current investigations on the “glycome,” it is now clear that the glycosylation of the macromolecules is a diverse and dynamic process, one prominent example being the wide diversity of sialic acid-modified carbohydrates.Citation54 While at present it is not well-studied how the glycome changes with age, some evidence exist to the point that the abundance in certain tissues of LewisX-modfied proteins may change with age, presumably, as suggested by Ogiso et al., altering cell-to-cell adhesion.Citation55 We suggest that the presentation of sialyl-LewisX ligand for CD62L may be subject to age-dependent quantitative and/or qualitative alterations as a consequence of changes in the processes that synthesize this complex carbohydrate structure. In theory, this would explain the high expression level of CD62L on the T cells from non-responding elderly donors as a compensatory response, which, however, fails to bring the function to a sufficient level for raising a response to the HBsAg on par with younger vaccinees. Genetic ablation in mice of CD62P (or P-selectin) apparently strengthens other mechanisms supporting cell adhesion through the binding of vascular cell adhesion molecule-1.Citation56 While our current set of analyses did not experimentally address the various contributions to CD62L functionality listed above, at the very least our data point to age-related changes in the availability of CD62L ligands as a possible influence on the vaccine response. It would be of interest to analyze the presentation of CD62L ligands in human lymphoid tissue, notably the high endothelial venules, with the aim of correlating changes in this critical tissue with the age of the donor.
Taken together, our study suggests a need for distinct guidelines concerning booster immunizations, including hepatitis B, and surveillance of immune status among people 55 y or older, especially if they received their primary vaccination as adults. The observation that donors with a mean age as low as 61 y show a significant decrease in their ability to raise a protective humoral response to HBV vaccination adds to the discussion on appropriate vaccine regimens for the elderly people and indicate that immunosenescence is seen at this age. The mechanistic aspect was investigated from our observation that CD62L expression correlates with vaccine response in younger donors. As discussed above, the experimental findings encourage the idea that such correlation reflects a physiological role of CD62L in the formation of the humoral response. However, a study on larger cohorts would clearly be needed to substantiate this finding. Furthermore, it is certainly possible that other factors than those directly related to the function of CD62L may play a role in shaping the immune response in elderly vs. young donors. In particular, the age-related changes in hormonal levels may also affect the immune response. Indeed, such changes may help to explain why no correlation between the CD62L expression and vaccine response was found among the elderly donors, in particular since both CD62L expression and functionality appears to be intact among the donors tested. We suggest that a complex interplay between ligand presentation and CD62L function may act to quench the correlation in elderly vaccinees between receptor expression and humoral responses to antigens requiring leukocyte homing to lymphoid tissue.
Materials and methods
Study population
Fifty-six healthy adults (52 males and 4 females), was included in the study. The participants were divided into two groups (A and B) based on the age of the vaccinees at the time of their primary vaccination. All individuals had completed a vaccination course of adult vaccine doses containing 20 μg recombinant HBsAg administered in three injections at 0, 1, and 6 mo. Individuals belonging to group A were 35 y or younger at the time of primary vaccination (n = 28, mean age 32.5, range 25–35), and individuals belonging to group B were 55 y or older (n = 28, mean age 61.2, range 55–65) (). The two groups were matched and stratified according to the number of years since completion of their primary vaccination. All vaccinees were without evidence of medical illness determined by their medical history and physical examination. Each subject was vaccinated with either the commercially available combined hepatitis A and B vaccine, (Twinrix®) or the HBV subunit vaccine (Engerix-B®) (GlaxoSmithKline Biologicals, Rixensart, Belgium). The vaccine administration is detailed in . Twinrix® contains the same amount of rHBsAg as Engerix-B®. One individual was tested positive for anti-HBV core antibodies (a marker for previous HBV infection) and was excluded from the study. The participants signed informed-consent forms and the protocol was approved by the Scientific Ethical Committee for Biomedical Research in the Central Denmark Region (Ref. No. M-20080033).
Quantification of the HBV vaccine humoral reponse
Quantitative determination of in vivo production of antibodies against HBsAg and HBcAg was performed on serum with the commercially available ARCHITECT® anti-HB ELISA assays (Abbott Laboratories, Chicago, Illinois, USA). Anti-HB titers were expressed in IU/l and the threshold for seroprotection was defined as an anti-HBsAg antibody concentration of at least 10 IU/l (CDC 1985, WHO 1992). The ARCHITECT® ELISA reader (Abbott) was used for the detection of anti-HBsAg and anti-HBc antibodies according to the guidelines provided by the manufacturer. The clinical assay had a detection range of 10–1000 IU/L. Anti-HB titers in the interval between 10 and 1000 IU/L were given as absolute titers, whereas titers above 1000 IU/L or below 10 IU/L were stated as ≥ 1000 IU/L and < 10 IU/L, respectively.
Preparation of peripheral mononuclear cells
Peripheral venous blood was collected for cellular assays in BD Vacutainer® Cell Preparation Tubes (CPTTM) with Sodium Heparin (BD, Franklin Lakes, NJ, USA) one, two, three, four or six years post HBV vaccination. Peripheral blood mononuclear cells (PBMCs) were prepared by density centrifugation according to the manufacturer. The isolated PBMCs were resuspended in freezing media containing 10% (v/v) DMSO and 12.5% (w/v) BSA, and initially placed at -80°C for 24–72 h before transfer into a liquid nitrogen tank.
Quantification of the HBV vaccine cellular response
An in vitro lymphocyte proliferation assay was used to measure the HBsAg-specific cellular immune response of the vaccinees. Unfractionated PBMCs were thawed in RPMI 1640 containing 10 mM HEPES and 0.292 μg/ml L-glutamine and resuspended in complete media (CM) consisting of AIM-V medium (Gibco Life technologies Europe) supplemented with 10 mM HEPES, 0.292 μg/ml L-glutamine, and 40 U/ml penicillin. The cells (2 × 105/well) were cultured for 7 d at 37°C in air with 95% relative humidity and 5% (v/v) CO2 in 96-well round-bottomed microtiter plates (NUNC, Roskilde, Denmark). Antigenic particles consisting of yeast-derived recombinant HBV envelope antigen (subtype adw, Diasorin, Saluggia, Italy) identical to the recombinant HBsAg vaccine was used as recall antigen at a final concentration of 10 μg/ml. The choice of this concentration was based on initial titration of the antigen where an antigen dose of 10 μg/ml was found to deliver optimal response. As a positive control for T cell activation and to establish a measure of the proliferative capacity, the PBMCs were stimulated with antibodies to CD3 (R&D Systems Europe, UK) and CD28 (BD PharMingen, Franklin Lakes, NJ, USA) precoated in the microtiter plates at a final concentration of 0.8 μg/ml in sterile PBS. This concentration was also based on initial dose-response experiments to assess the maximum proliferative response. Unstimulated control cultures were kept in culture medium without addition of antigen. All proliferation assays were performed in quadruplicates. Each microculture was pulsed with 1.0 μCi [3H]-thymidine (PerkinElmer Life Science, Boston, MA) 18 h before harvesting. The cultures were harvested onto glass-fiber filters (Wallac, Perkin Elmer Life Science, Turku, Finland) using an automated multichannel cell harvester (Tomtec™, Hamden, CT). Incorporation of [3H]-thymidine was measured in counts-per-minute (cpm) using a liquid scintillation and luminescence counter (1450 Microbeta Wallac Trilux, Perkin Elmer Life Science, Turku, Finland). Proliferation data for individual donors were expressed as median thymidine incorporation count for the four replica cultures treated with HBsAg () normalized to the median thymidine incorporation counts for the polyclonal T cell activation by means of CD3/CD28 stimulation (
). This stimulation ratio (SR) was transformed with the natural logarithm, i.e.,
Equation (1) 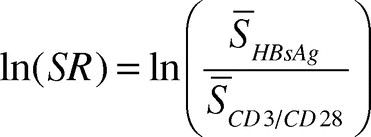
IFN-γ ELISA assay
PBMCs were thawed in PBS supplemented with 20% (v/v) heat-inactivated human AB serum (Life Technologies) and washed twice. The cells were cultured in CM (2 × 105/well) in 96-well round-bottomed microtiter plates (NUNC) and stimulated in duplica with 50 ng/ml PMA and 1 μg/ml ionomycin (Sigma-Aldrich), 10 μg/ml HBsAg (Aldevron), or AIM-V medium (Gibco) alone. The cultures were incubated for 48 h at 37°C in air with 95% relative humidity and 5% (v/v) CO2. The cells were thoroughly resuspended and samples were centrifuged at 1000 × g for 5 min before the supernatants were transferred to 96-well culture trays preblocked with 1 mg/ml HSA and stored at -20°C until required for cytokine analysis. IFN-γ concentrations were measured in cell supernatants using the human IFN-γ Ready-SET-Go! ELISA kit (eBioscience). The assay had a sensitivity of 4 pg/ml, and the range of 4–500 pg/ml; hence, measurements below the assay range was assigned the value 4 pg/ml and measurements above the assay range was assigned the value 500 pg/ml. Samples with replica variations > 50% was excluded based on a technical bias.
Flow cytometric identification of CD4+ T cell subsets
Flow cytometry was performed to define naïve (CD45RA+CD62L+) central memory (TCM: CD45RA-CD62L+), and effector memory (TEM: CD45RA-CD62L-) subsets of CD4+ T cells. Cryopreserved PBMCs were thawed in a 37 °C water bath and washed in PBS containing 0.5% (w/v) bovine serum albumin (BSA) and 20% (v/v) fetal calf serum (FCS). Subsequently, the cells were washed and resuspended in PBS with 0.5% (w/v) BSA and 0.09% (w/v) NaN3. Expression of cell surface markers was determined by staining the cells with mouse monoclonal antibodies (mAb) specific for: anti-CD4 fluorescein isothiocyanate (FITC)-conjugated (clone MT310 [IgG1] DAKO, Glostrup, Denmark), anti-CD45RA phycoerythrin (PE)-conjugated (clone 4KB5 [IgG1κ] DAKO), and anti-CD62L allopycocyanin (APC)-conjugated (clone DREG-56 [IgG1κ], BD PharMingen, Franklin Lakes, NJ, USA. The following isotype controls were used at the same protein concentration as the test antibody: mouse IgG1 FITC (clone DAK-G01, DAKO), mouse IgG1 PE (clone DAK-G01, DAKO), mouse IgG1 APC (clone MOPC-21, BD PharMingen) and mouse IgG1 PC7 (clone 679.1Mc7, Beckman Coulter). All samples were stained for 20 min at room temperature in the dark. Afterwards the cells were washed twice in PBS containing 0.5% (w/v) BSA and 0.09% (w/v) NaN3 and fixed in PBS (pH 7.4) with 0.9% (v/v) formaldehyde. Data acquisition was performed using an FC500 flow cytometer (Beckman Coulter), collecting 50,000 gated lymphocytes. FlowJo software (TreeStar Inc., Ashland, OR, USA) was used for the subsequent data analysis. To verify the compensation matrix, single-stained samples and “fluorescence-minus-one”Citation57 (FMO) samples were used. Results were expressed as median fluorescence intensity (MFI). For naïve and memory CD4+ subpopulations the gates were placed based on both FMO controls and analysis of contour plots of the CD4+ T cell population.
Assay for the CD62L ligand-binding capacity
Unfractionated PBMCs were thawed in a 37°C water bath and washed twice in PBS containing 20% (v/v) FCS. The cells were resuspended in binding buffer containing 10 mM Hepes, 150 mM NaCl, 5 mM KCl, 1mM MgCl2, 1.8 mM CaCl2, and 0.2% (w/v) BSA. A flexible polyacrylamide (PAA)-based polyvalent glycoprobe was employed for evaluation of CD62L ligand-binding capacity. The glycoprobe was directly conjugated with fluorescein (6(GlcNAc)-O-Su-SiaLex-PAA-fluorescein) and was used in a final concentration of 5 μg/ml. As control for unspecific binding, the 6-Su-3′SLN-PAA-fluorescein glycoprobe, devoid of the CD62L binding motif, was used at the same concentration. In addition, cell surface marker analysis was conducted using mouse monoclonal antibodies to CD3 (conjugated with PE; clone UCHT1 [IgG1], DAKO), CD4 (APC; clone MT310 [IgG1] BD PharMingen), and CD8 (PC7; clone SFCI21Thy2D3 [IgG1], Beckman Coulter). Isotypes were applied as described above and used as controls at the same protein concentration as the test antibody. The samples were stained and incubated in the dark at 4°C for 40 min. Next, the cells were washed once, resuspended in Binding buffer and data were acquired within 24 h using an FC500 flow cytometer (Beckman Coulter), and collecting 10,000 events within the lymphocyte gate. FlowJo software (TreeStar Inc., Ashland, OR, USA) was used for the subsequent data analysis. To verify the compensation matrix, single-stained and FMO samples were used as described above. Results were expressed as MFI.
Statistical analyses
For comparison of only two groups, an unpaired two-tailed t-test was performed. When comparing more than two groups, either a one-way or two-way analysis of variance (ANOVA), followed by Bonferroni’s Multiple Comparison Test was used. Wilcoxon matched-pairs signed rank test was performed with donor number as the repeated factor, and afterwards a Mann Whitney test was used for non-parametric comparison of the two groups. Correlations between two variables were analyzed for significance by Spearman’s rank order correlation. Distribution of donors within different antibody response categories was analyzed by a χ2-test. Differences were considered to be significant at the 5% level. These analyses were made using GraphPad Prism 5.0c for Mac OS X. QQ-plots and two-way analysis of variance were made using SAS® Analytical Software.
| Abbreviations: | ||
| APC | = | allopycocyanin |
| ANOVA | = | analysis of variance |
| BSA | = | bovine serum albumin |
| CM | = | complete medium |
| cpm | = | counts per minute |
| FITC | = | fluorescein isothiocyanate |
| FMO | = | fluorescence-minus-one |
| HCC | = | hepatocellular carcinoma |
| HBsAg | = | HBV surface antigen |
| HBV | = | Hepatitis B virus |
| HEV | = | high endothelial venules |
| HR | = | high responders |
| IR | = | intermediate responders |
| IU | = | international units |
| MFI | = | median fluorescence intensity |
| PE | = | phycoerythrin |
| PNAd | = | peripheral node adressin |
| NR | = | non-responders |
| PSGL-1 | = | P-selectin glycoprotein ligand-1 |
| SR | = | stimulation ratio |
| TCM | = | central memory T cell |
| TEM | = | effector memory T cell |
| TNaïve | = | naïve T cell |
| WHO | = | World Health Organization |
| ρ | = | mass concentration |
Acknowledgments
The employees of Danfoss, Nordborg, Denmark, who donated blood for the serological and T cell studies, are gratefully acknowledged. We thank Drs. Anné Møller-Larsen and Per Höllsberg for advice and cand.scient. Eva Kläning for comments on a draft version of the manuscript. We wish to thank Bettina W. Grumsen for excellent technical assistance. The Manufacturer Mads Clausen’s Foundation and the Karen Elise Jensen Foundation kindly supported the study. Dr. Nicolai Bovin was supported by the Program in Molecular and Cell Biology, RAS.
Conflicts of interest
LVB and EF are employees of Danfoss A/S, Nordborg, Denmark. In the past, LVB has received a honorarium for 3 × 2 h of lectures, paid by GlaxoSmithKline.
References
- McMahon BJ, Bulkow LR, Singleton RJ, Williams J, Snowball M, Homan C, et al. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology 2011; 54:801 - 7; http://dx.doi.org/10.1002/hep.24442; PMID: 21618565
- Tan YJ. Hepatitis B virus infection and the risk of hepatocellular carcinoma. World J Gastroenterol 2011; 17:4853 - 7; http://dx.doi.org/10.3748/wjg.v17.i44.4853; PMID: 22171125
- Zuckerman AJ. Novel hepatitis B vaccines. J Infect 1986; 13:Suppl A 61 - 71; http://dx.doi.org/10.1016/S0163-4453(86)92713-1; PMID: 2427591
- Huang LM, Lu CY, Chen DS. Hepatitis B virus infection, its sequelae, and prevention by vaccination. Curr Opin Immunol 2011; 23:237 - 43; http://dx.doi.org/10.1016/j.coi.2010.12.013; PMID: 21257300
- Centers for Disease C. Hepatitis B virus.. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep 1991; 40:RR-13 1 - 25; PMID: 1835756
- Poorolajal J, Mahmoodi M, Haghdoost A, Majdzadeh R, Nasseri-Moghaddam S, Ghalichi L, et al. Booster dose vaccination for preventing hepatitis B. Cochrane database of systematic reviews (Online) 2010; (11):CD008256.
- Han K, Shao X, Zheng H, Wu C, Zhu J, Zheng X, et al. Revaccination of non- and low- responders after a standard three dose hepatitis B vaccine schedule. Hum Vaccin Immunother 2012; 8:1845 - 49; PMID: 22906933
- Banatvala J, Van Damme P, Oehen S. Lifelong protection against hepatitis B: the role of vaccine immunogenicity in immune memory. Vaccine 2000; 19:877 - 85; http://dx.doi.org/10.1016/S0264-410X(00)00224-3; PMID: 11115711
- Zhu CL, Liu P, Chen T, Ni Z, Lu LL, Huang F, et al. Presence of immune memory and immunity to hepatitis B virus in adults after neonatal hepatitis B vaccination. Vaccine 2011; 29:7835 - 41; http://dx.doi.org/10.1016/j.vaccine.2011.07.098; PMID: 21816197
- Chaves SS, Fischer G, Groeger J, Patel PR, Thompson ND, Teshale EH, et al. Persistence of long-term immunity to hepatitis B among adolescents immunized at birth. Vaccine 2012; 30:1644 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.12.106; PMID: 22245310
- Jadoul M, Goubau P. Is anti-hepatitis B virus (HBV) immunization successful in elderly hemodialysis (HD) patients?. Clin Nephrol 2002; 58:301 - 4; PMID: 12400846
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol 2004; 5:133 - 9; http://dx.doi.org/10.1038/ni1033; PMID: 14749784
- Tohme RA, Awosika-Olumo D, Nielsen C, Khuwaja S, Scott J, Xing J, et al. Evaluation of hepatitis B vaccine immunogenicity among older adults during an outbreak response in assisted living facilities. Vaccine 2011; 29:9316 - 20; http://dx.doi.org/10.1016/j.vaccine.2011.10.011; PMID: 22015390
- Williams RE, Sena AC, Moorman AC, Moore ZS, Sharapov UM, Drobenuic J, et al. Hepatitis B vaccination of susceptible elderly residents of long term care facilities during a hepatitis B outbreak. Vaccine 2012; 30:3147 - 50; http://dx.doi.org/10.1016/j.vaccine.2012.02.078; PMID: 22421557
- Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis 2000; 31:578 - 85; http://dx.doi.org/10.1086/313947; PMID: 10987724
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159 - 69; http://dx.doi.org/10.1016/j.vaccine.2005.08.105; PMID: 16213065
- Dock JN, Effros RB. Role of CD8 T Cell Replicative Senescence in Human Aging and in HIV-mediated Immunosenescence. Aging Dis 2011; 2:382 - 97; PMID: 22308228
- Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol 2009; 30:306 - 12; http://dx.doi.org/10.1016/j.it.2009.03.013; PMID: 19540809
- Jamieson BD, Douek DC, Killian S, Hultin LE, Scripture-Adams DD, Giorgi JV, et al. Generation of functional thymocytes in the human adult. Immunity 1999; 10:569 - 75; http://dx.doi.org/10.1016/S1074-7613(00)80056-4; PMID: 10367902
- Hakim FT, Gress RE. Immunosenescence: immune deficits in the elderly and therapeutic strategies to enhance immune competence. Expert Rev Clin Immunol 2005; 1:443 - 58; http://dx.doi.org/10.1586/1744666X.1.3.443; PMID: 20476994
- Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol 2004; 173:673 - 81; PMID: 15210831
- Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology 2011; 134:17 - 32; http://dx.doi.org/10.1111/j.1365-2567.2011.03470.x; PMID: 21711350
- Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev 2005; 205:147 - 57; http://dx.doi.org/10.1111/j.0105-2896.2005.00259.x; PMID: 15882351
- Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol 2009; 30:325 - 33; http://dx.doi.org/10.1016/j.it.2009.05.004; PMID: 19541535
- Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997; 89:587 - 96; http://dx.doi.org/10.1016/S0092-8674(00)80240-8; PMID: 9160750
- Jacobson EB, Caporale LH, Thorbecke GJ. Effect of thymus cell injections on germinal center formation in lymphoid tissues of nude (thymusless) mice. Cell Immunol 1974; 13:416 - 30; http://dx.doi.org/10.1016/0008-8749(74)90261-5; PMID: 4217657
- Maizels N, Bothwell A. The T-cell-independent immune response to the hapten NP uses a large repertoire of heavy chain genes. Cell 1985; 43:715 - 20; http://dx.doi.org/10.1016/0092-8674(85)90244-2; PMID: 2416469
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol 2005; 6:1182 - 90; http://dx.doi.org/10.1038/ni1275; PMID: 16369557
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994; 76:301 - 14; http://dx.doi.org/10.1016/0092-8674(94)90337-9; PMID: 7507411
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol 2004; 22:129 - 56; http://dx.doi.org/10.1146/annurev.immunol.21.090501.080131; PMID: 15032576
- Lowe JB. Glycosylation in the control of selectin counter-receptor structure and function. Immunol Rev 2002; 186:19 - 36; http://dx.doi.org/10.1034/j.1600-065X.2002.18603.x; PMID: 12234359
- Harp JR, Onami TM. Naïve T cells re-distribute to the lungs of selectin ligand deficient mice. PLoS One 2010; 5:e10973; http://dx.doi.org/10.1371/journal.pone.0010973; PMID: 20532047
- Steeber DA, Green NE, Sato S, Tedder TF. Lyphocyte migration in L-selectin-deficient mice. Altered subset migration and aging of the immune system. J Immunol 1996; 157:1096 - 106; PMID: 8757614
- De Martinis M, Modesti M, Profeta VF, Tullio M, Loreto MF, Ginaldi L, et al. CD50 and CD62L adhesion receptor expression on naive (CD45RA+) and memory (CD45RO+) T lymphocytes in the elderly. Pathobiology 2000; 68:245 - 50; http://dx.doi.org/10.1159/000055933; PMID: 11493756
- Chedid MG, Deulofeut H, Yunis DE, Lara-Marquez ML, Salazar M, Deulofeut R, et al. Defect in Th1-like cells of nonresponders to hepatitis B vaccine. Hum Immunol 1997; 58:42 - 51; http://dx.doi.org/10.1016/S0198-8859(97)00209-7; PMID: 9438208
- Gujar SA, Michalak TI. Primary occult hepadnavirus infection induces virus-specific T-cell and aberrant cytokine responses in the absence of antiviral antibody reactivity in the Woodchuck model of hepatitis B virus infection. J Virol 2009; 83:3861 - 76; http://dx.doi.org/10.1128/JVI.02521-08; PMID: 19193791
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708 - 12; http://dx.doi.org/10.1038/44385; PMID: 10537110
- Kawashima H, Fukuda M. Sulfated glycans control lymphocyte homing. Ann N Y Acad Sci 2012; 1253:112 - 21; http://dx.doi.org/10.1111/j.1749-6632.2011.06356.x; PMID: 22288521
- LeMaoult J, Szabo P, Weksler ME. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol Rev 1997; 160:115 - 26; http://dx.doi.org/10.1111/j.1600-065X.1997.tb01032.x; PMID: 9476670
- Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol 2005; 17:468 - 75; http://dx.doi.org/10.1016/j.coi.2005.07.020; PMID: 16098723
- Wu Q, Zhuang GH, Wang XL, Wang LR, Li N, Zhang M. Antibody levels and immune memory 23 years after primary plasma-derived hepatitis B vaccination: results of a randomized placebo-controlled trial cohort from China where endemicity is high. Vaccine 2011; 29:2302 - 7; http://dx.doi.org/10.1016/j.vaccine.2011.01.025; PMID: 21277403
- Chen CY, Hsu HY, Liu CC, Chang MH, Ni YH. Stable seroepidemiology of hepatitis B after universal immunization in Taiwan: A 3-year study of national surveillance of primary school students. Vaccine 2010; 28:5605 - 8; http://dx.doi.org/10.1016/j.vaccine.2010.06.029; PMID: 20598405
- Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A 2003; 100:15053 - 8; http://dx.doi.org/10.1073/pnas.2433717100; PMID: 14657384
- Weksler ME, Szabo P. The effect of age on the B-cell repertoire. J Clin Immunol 2000; 20:240 - 9; http://dx.doi.org/10.1023/A:1006659401385; PMID: 10939711
- Aberle JH, Stiasny K, Kundi M, Heinz FX. Mechanistic insights into the impairment of memory B cells and antibody production in the elderly. Age 2013; 35:371 - 81; PMID: 22282053
- Goodell V, dela Rosa C, Slota M, MacLeod B, Disis ML. Sensitivity and specificity of tritiated thymidine incorporation and ELISPOT assays in identifying antigen specific T cell immune responses. BMC Immunol 2007; 8:21; http://dx.doi.org/10.1186/1471-2172-8-21; PMID: 17850666
- Cossarizza A, Ortolani C, Paganelli R, Barbieri D, Monti D, Sansoni P, et al. CD45 isoforms expression on CD4+ and CD8+ T cells throughout life, from newborns to centenarians: implications for T cell memory. Mech Ageing Dev 1996; 86:173 - 95; http://dx.doi.org/10.1016/0047-6374(95)01691-0; PMID: 8733112
- Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naïve T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology 2005; 114:37 - 43; http://dx.doi.org/10.1111/j.1365-2567.2004.02006.x; PMID: 15606793
- Pawelec G, Barnett Y, Forsey R, Frasca D, Globerson A, McLeod J, et al. T cells and aging, January 2002 update. Front Biosci 2002; 7:d1056 - 183; PMID: 11991846
- Leid JG, Jutila MA. Impact of polyunsaturated fatty acids on cytoskeletal linkage of L-selectin. Cell Immunol 2004; 228:91 - 8; http://dx.doi.org/10.1016/j.cellimm.2004.04.004; PMID: 15219460
- Mattila PE, Green CE, Schaff U, Simon SI, Walcheck B. Cytoskeletal interactions regulate inducible L-selectin clustering. Am J Physiol Cell Physiol 2005; 289:C323 - 32; http://dx.doi.org/10.1152/ajpcell.00603.2004; PMID: 15788481
- Bakker GJ, Eich C, Torreno-Pina JA, Diez-Ahedo R, Perez-Samper G, van Zanten TS, et al. Lateral mobility of individual integrin nanoclusters orchestrates the onset for leukocyte adhesion. Proc Natl Acad Sci U S A 2012; 109:4869 - 74; http://dx.doi.org/10.1073/pnas.1116425109; PMID: 22411821
- Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, et al. The interaction properties of costimulatory molecules revisited. Immunity 2002; 17:201 - 10; http://dx.doi.org/10.1016/S1074-7613(02)00362-X; PMID: 12196291
- Cohen M, Varki A. The sialome--far more than the sum of its parts. OMICS 2010; 14:455 - 64; http://dx.doi.org/10.1089/omi.2009.0148; PMID: 20726801
- Ogiso M, Irie A, Kubo H, Hoshi M, Komoto M. Senile cataract-related accumulation of Lewis(x) glycolipid in human lens. J Biol Chem 1992; 267:6467 - 70; PMID: 1348055
- Gironella M, Mollà M, Salas A, Soriano A, Sans M, Closa D, et al. The role of P-selectin in experimental colitis as determined by antibody immunoblockade and genetically deficient mice. J Leukoc Biol 2002; 72:56 - 64; PMID: 12101263
- Tung JW, Parks DR, Moore WA, Herzenberg LA, Herzenberg LA. New approaches to fluorescence compensation and visualization of FACS data. Clin Immunol 2004; 110:277 - 83; http://dx.doi.org/10.1016/j.clim.2003.11.016; PMID: 15047205