Abstract
This manuscript presents data on the persistence of Hemagglutination Inhibition (HI) immune response against the A/California/7/2009 strain, six and 12 mo after adults received one dose (n = 138) or two doses (n = 102; 21 d apart) of a 3.75 µg Hemagglutinin antigen AS03-adjuvanted H1N1 2009 vaccine (NCT00968526). Two hundred forty subjects (18−60 y: 120; > 60 y: 120) were vaccinated. Immunogenicity end points were based on the European licensure criteria for pandemic influenza vaccines. Exploratory analyses assessed the cell-mediated immune response (CMI) up to Month 12 and the influence of previous influenza vaccination on persistence of immune response. At Month 6, the CHMP criteria were met in subjects aged 18−60 y who received one or two vaccine doses and in subjects aged > 60 y who received two vaccine doses. At Month 12, the CHMP criteria were met only in subjects aged 18−60 y who received two vaccine doses. Persistence of HI immune response against the vaccine strain was higher in subjects without prior influenza vaccination. Exploratory analyses showed that two doses of the H1N1 2009 vaccine induced persistence of H1N1-specific CD4+ T cells up to Month 6 and memory B cells up to Month 12. In conclusion, HI immune responses persisted up to 12 mo after vaccination with one-dose and two-dose regimens of the AS03-adjuvanted 3.75 µg HA H1N1 2009 pandemic influenza vaccine, although not all three CHMP guidance criteria for both groups were met at Month 6 and Month 12. The CD4+ T cell and B cell responses also persisted up to Month 12.
Keywords: :
Introduction
Based on previous experience with a split-virion H5N1 influenza vaccine [3.75 µg Hemagglutinin (HA)] adjuvanted with AS03 (an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion), an AS03-adjuvanted H1N1 2009 pandemic influenza vaccine (containing 3.75 µg HA) was developed. This vaccine was proven to be highly immunogenic (fulfilling the European and US regulatory guidance criteria for pandemic influenza vaccines) with a clinically acceptable safety profile in different populations.Citation1−Citation4
In a phase III, open-label study (NCT00968526), adults aged 18−60 y and > 60 y received either one or two doses (21 d apart) of this 3.75 µg HA AS03-adjuvanted H1N1 2009 vaccine. The European regulatory criteria were met and exceeded 21 d after the first vaccine dose; those who received the second dose mounted a stronger immune response 21 d later.Citation5 This manuscript presents the observations from an exploratory assessment of the cell-mediated immune (CMI) response in terms of frequencies of H1N1-specific T cells and H1N1-specific memory B cells before vaccination, 21 d after each dose (Days 21 and 42), at Month 6 and Month 12. Also presented here are the results of the protocol-planned assessment of the persistence of Hemagglutination Inhibition (HI) and microneutralization (MN) immune responses against the vaccine-homologous H1N1 A/California/7/2009 strain at Months 6 and 12 in these subjects categorized by history of prior vaccination for seasonal influenza (during the three previous seasons) and pre-vaccination serostatus.
Results
Study population
Of the 240 subjects aged 18−60 y (n = 120) and > 60 y (n = 120) who received one (n = 138) or two doses (n = 102) in the study, 236 and 237 subjects completed the study up to Month 6 and Month 12, respectively. The reasons for exclusion from the According-To-Protocol (ATP) cohort for persistence at Month 6 and Month 12 are presented in . The CMI subset consisted of 59 subjects.
Figure 1. Study design + CONSORT. Reasons for exclusions: *, essential serological data missing; **, essential serological data missing (1 subject), administration of vaccine forbidden in the protocol (1 subject); #, administration of vaccine forbidden in the protocol; ^ and ^^, essential serological data missing.
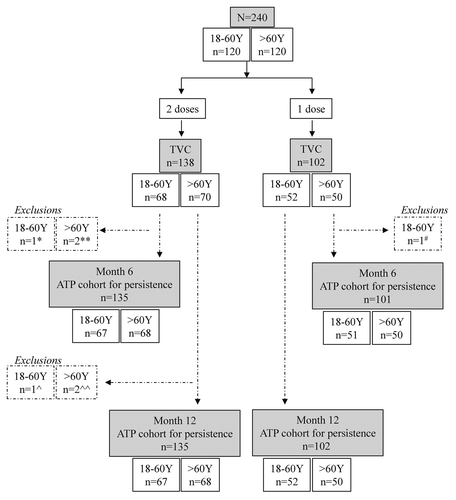
The mean ages of subjects in both study groups included in the ATP cohort for persistence at Month 12 was 39.5 y (standard deviation: 13.87 y; range: 19−60 y) in those aged 18−60 y and 69.1 y (standard deviation: 6.17 y; range: 61−85 y) in those aged > 60 y. The male to female ratios were 45.4%: 54.6% and 56.8%: 43.2% in the two study groups, respectively; all subjects were of Caucasian heritage.
Immunogenicity
HI immune response
At Month 6, all subjects (100.0%) aged 18−60 y who received one or two H1N1 2009 vaccine doses, all subjects (100.0%) aged > 60 y who received two vaccine doses and 92.0% of subjects aged > 60 y who received one vaccine dose, were seropositive for HI antibodies against the vaccine strain. The Committee for Medicinal Products for Human Use (CHMP) guidance criteria for seroconversion rate (SCR) and geometric mean fold rise (GMFR) for HI immune response against the H1N1 2009 strain were still met in both age groups irrespective of the number of vaccine doses received, indicating the persistence of HI immune response; the CHMP criterion for seroprotection rate (SPR) was met in subjects aged 18−60 y who received one or two vaccine doses and in subjects aged > 60 y who received two vaccine doses, but not in those aged > 60 y who received one vaccine dose (). Sub-group analyses showed that all three CHMP guidance criteria were met at Month 6 in subjects aged 71−80 y who received one or two vaccine doses and subjects aged 61−70 y and > 80 y who received two vaccine doses. Baseline serostatus appeared to influence the magnitude of the persisting HI immune response; the subjects in both 18−60 y and > 60 y age groups who were seropositive for HI antibodies against the vaccine strain at baseline retained a higher immune response than subjects who were seronegative at baseline irrespective of the number of doses received, as observed from the HI antibody geometric mean titers (GMTs) (data not shown). Exploratory analyses based on HI antibody GMTs showed that the magnitude of persistence of the HI immune response against the vaccine strain was higher in subjects without prior influenza vaccination. Serostatus at baseline, Days 21, 42,182 (Month 6) and 364 (Month 12) according to previous influenza vaccination history during the three previous seasons and age subgroup is shown in .
Table 1. Immune response in terms of hemagglutination inhibition antibodies against vaccine homologous A/California/7/2009 strain (CHMP criteria) (ATP cohort for persistence)
Table 2. Hemagglutination inhibition antibodies against vaccine homologous A/California/7/2009 strain pre-vaccination, at Day 21, Day 42, Months 6 and 12 by previous influenza vaccination history (ATP cohort for persistence)
At Month 12, the persistence of HI antibodies was evident in subjects aged 18−60 y and > 60 y irrespective of the number of vaccine doses received (seropositivity rate ranged between 82.0% and 100.0%). The CHMP guidance criteria for GMFR were met in both age groups after one or two vaccine doses; the CHMP criterion for SCR was met in all subjects except those aged > 60 y who received one vaccine dose; SPR was met only in subjects aged 18−60 y who received two vaccine doses (). Sub-group analyses showed that at Month 12 the CHMP guidance criteria for SCR and GMFR were met in subjects aged 61−70 y who received two vaccine doses and in subjects 71−80 y who received one or two vaccine doses (none met all three criteria), while all three CHMP guidance criteria were met in subjects aged > 80 y who received two vaccine doses. However, these observations should be made within the realms of the modest number of subjects in these age sub-groups. The magnitude of persistence of HI immune response against the vaccine strain at Month 12 was higher in subjects seropositive at baseline compared with those who were seronegative at baseline. Exploratory analyses based on HI antibody GMTs showed that the immune response to the vaccine strain appeared to be hindered by previous influenza vaccination (although less pronounced in subjects aged > 60 y) ().
It is to be noted that for the HI assay the samples from the time points Day 0, Month 6 and Month 12 were tested separately. Due to potential assay variability, a comparative interpretation of the HI responses at Month 6 and Month 12 with earlier time points should be done with caution.
MN immune response
At Month 6, persistence of MN response was shown in all participants, regardless of age-group and numbers of vaccine dose received, with SPRs ranging from 88.9% to 100.0%. Exploratory analyses based on GMTs showed that the persistence of MN antibodies at Month 6 was higher in subjects seropositive at baseline compared with those seronegative at baseline (data not shown). Also, subjects aged 18–60 y with a history of previous influenza vaccination displayed lower GMTs compared with subjects without previous influenza vaccination. This was not observed in subjects aged > 60 y. In this age group MN GMTs were higher in subjects with a history of influenza vaccination during the previous three seasons than in subjects without a history of previous seasonal influenza vaccination ().
Table 3. Neutralizing antibodies against Flu A/Neth/602/09 H1N1 strain pre-vaccination, at Day 21, Day 42 and Month 6 by previous influenza vaccination history (ATP cohort for persistence)
CMI response
CD4+ T cell response: pre-existing H1N1-specific CD4+ T cells were observed both in subjects aged 18−60 y and > 60 y, which appeared to be quite variable across different subjects ().
Figure 2. (A) H1N1-split-specific CD4+ T cell frequencies pre-vaccination (PRE), at Day 21 (D21) and 42 (D42), at Month 6 (M6) and Month 12 (M12) in subjects aged 18−60 y and > 60 y following one or two vaccine doses (ATP cohort for persistence). (B) H1N1-split-specific CD4+ T cell frequencies pre-vaccination (PRE), at Day 21 (D21) and 42 (D42), at Month 6 (M6) and Month 12 (M12) in subjects aged 18−60 y and > 60 y following one or two vaccine doses by previous influenza vaccination history (ATP cohort for persistence). N, without influenza vaccination during previous three seasons; F, with previous influenza vaccination.
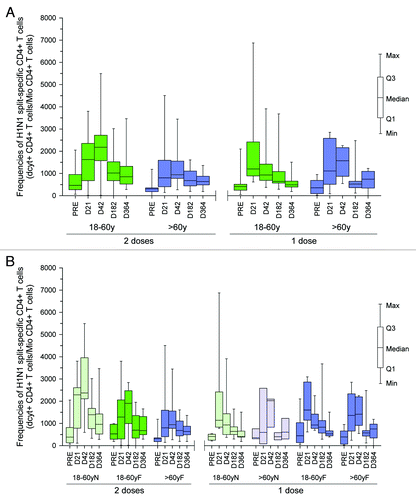
The vaccine-induced immune response was quite variable between subjects. In subjects who received two vaccine doses, the H1N1 2009 vaccine induced strong H1N1-specific immune responses, with still a trend for higher CD4+ T cell frequencies at Month 12 compared with the pre-vaccination frequencies. The data indicated that the second vaccine dose further boosted the CD4+ T cell H1N1-specific immune response induced by the first vaccine dose in subjects aged 18−60 y; however, the same boosting effect was not as evident in those aged > 60 y (). No obvious difference had been observed in terms of vaccine induced CD4+ T cells in subjects with or without seasonal influenza vaccination history (). As all subjects aged > 60 y had seasonal influenza vaccination history, no parallel comparisons could be drawn.
In subjects who received a single vaccine dose, a frequencies of specific CD4+ T cell at Month 12 waned up to the pre-vaccination frequency level. In the one dose vaccine, a delay in the CD4+ T cell peak was observed in subjects aged > 60 y. No particular difference in persistence of CD4+ T cells was observed between the two age groups. Further, exploratory analyses did not show a clear impact of previous seasonal influenza vaccination on persistence of CD4+ T cell frequencies ().
B cell response: Pre-existing H1N1-specific B cells were observed in subjects aged 18−60 y and > 60 y, which appeared to be quite variable across different subjects ().
Figure 3. (A) split H1N1-specific memory B cell frequencies at Month 6 and Month 12 in subjects aged 18−60 y and > 60 y following one or two vaccine doses (ATP cohort for persistence). (B) recHA specific memory B cell frequencies at Month 6 and Month 12 in subjects aged 18−60 y and > 60 y following one or two vaccine doses (ATP cohort for persistence)
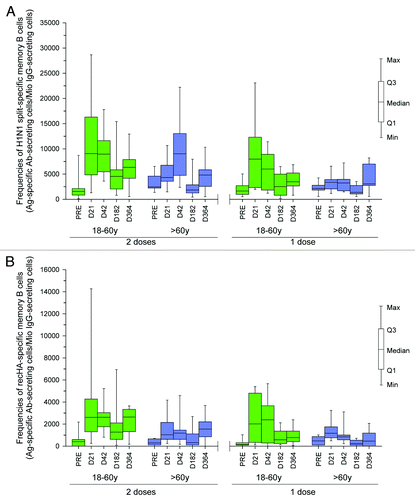
Overall, the H1N1-specific memory B cells persisted at comparatively higher frequencies at Month 12 compared with those prior to vaccination, in subjects who received either one or two vaccine doses. No additive effect of the second vaccine dose was apparent in subjects aged 18−60 y, as it did not appear to induce H1N1-specific memory B cells. However, the second vaccine dose did boost the frequency of H1N1-specific memory B cells in subjects aged > 60 y, which was low after the first vaccine dose. A substantial proportion of the H1N1-split-specific memory B cells were specific to recHA antigen (). Exploratory analyses showed no clear impact of previous seasonal influenza vaccination on persistence of memory B cells frequencies for neither the one-dose or two-dose schedule ().
Figure 4. (A) split H1N1-specific memory B cell frequencies at Month 6 and Month 12 in subjects aged 18−60 y and > 60 y following one or two vaccine doses by previous influenza vaccination history (ATP cohort for persistence). (B) recHA specific memory B cell frequencies at Month 6 and Month 12 in subjects aged 18−60 y and > 60 y following one or two vaccine doses by previous influenza vaccination history (ATP cohort for persistence). N, without influenza vaccination during previous three seasons; F, with previous influenza vaccination
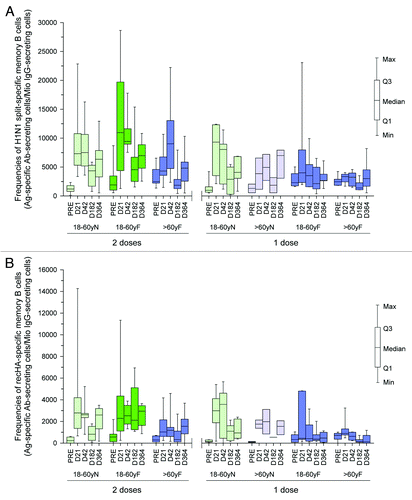
These CMI results should be interpreted within the limitations of unbalanced and low sample size in some of the subgroups.
Safety and reactogenicity
Overall, 29 serious adverse events (SAEs) were reported in 16 subjects (10 subjects received two vaccine doses and 6 subjects received one vaccine dose) during the entire study period. None of the SAEs were considered by the investigators to be vaccination-related. Two subjects were withdrawn from the study due to SAEs—a female subject aged 68 y was diagnosed with a breast cancer 116 d after the second vaccine dose and a male subject aged 70 y suffered from multiple fractures, pneumothorax and subdural hematoma following an accident 180 d after the second vaccine dose. Both subjects recovered by the end of the study. No fatalities were reported. No potential immune mediated diseases (pIMDs) were recorded during the study period.
Discussion
This manuscript completes the data presented previously from a study assessing the immune response induced by one or two doses of an AS03-adjuvanted H1N1 2009 pandemic influenza vaccine in young and elderly adults.Citation5 Further to the strong HI antibody immune responses observed 21 d after the second vaccine dose, this is one of the first studies to present data on the long-term persistence of humoral immune response in terms of HI and MN antibody levels and CMI in terms of CD4+ T cell and memory B cell frequencies up to Month 12.
Even at Month 6, all three CHMP guidance criteria for HI immune response against the H1N1 2009 strain were met in all subjects but for those aged > 60 y who received one vaccine dose. This is contrary to observations in a previous study which reported that all subjects aged 18−60 y and > 60 y who received one or two doses of the AS03-adjuvanted 3.75µg H1N1 2009 vaccine continued to meet the CHMP criteria at Month 6.Citation6 Two other studies in adults reported that the immune response induced by two doses of the 3.75 µg HA AS03-adjuvanted H1N1 2009 vaccine persists up to six months after vaccination.Citation7,Citation8 At Month 12, all three CHMP guidance criteria were met only in subjects aged 18−60 y who received two vaccine doses; none of the subjects aged > 60 y met all three CHMP criteria irrespective of the number of vaccine doses they received. This long-term persistence data supplements already available data that the AS03-adjuvanted H1N1 2009 pandemic influenza vaccine (antigen content: 3.75 or 1.9 µg HA) induces HI antibody responses that meet all CHMP guidance criteria for pandemic influenza vaccines, 21 d after one or two doses (administered 21 d apart) in different age groups.Citation1-Citation4,Citation9 The HI antibody results in this study should be interpreted in the light of assay variability inherent for HI assays.
Although not within the primary scope of investigation in this study, exploratory analyses indicated that the magnitude of persistence of the HI immune response against the vaccine strain was higher in subjects without prior influenza vaccination. These observations are in agreement with those from a previous study with a similar formulation of the study vaccine Citation4,Citation5,Citation10 and others using the AS03-adjuvanted 3.75 µg HA H5N1 vaccine.Citation11,Citation12 Although some researchers have invoked the infection block hypothesis to explain this inability to mount an optimal cross-reactive immune response,Citation13 the opinion on this topic has been varied, with some studies even reporting contrasting observations.Citation14-Citation16
The AS03-adjuvanted H1N1 2009 vaccine administered in one or two dose schedule induced a MN response that persisted for six months after the first vaccine dose in all age groups. Similar long-term persistence data have been previously reported in studies enrolling Japanese adults randomized to receive oneCitation17 or two dosesCitation18 of a study vaccine with a similar formulation. Exploratory analyses showed that the persistence of MN immune response was higher in subjects aged 18–60 y without previous influenza vaccination and in subjects aged > 60 y with a history of influenza vaccination during the previous three seasons. Note that these results must be interpreted with caution due to small number of subjects analyzed in each sub-group.
The data from this study showed that the AS03-adjuvanted H1N1 2009 vaccine administered following a one- or two-dose schedule induced CD4+ T cell and B cell responses in both age groups, irrespective of whether the subject had previously received seasonal influenza vaccination. The CMI response persisted up to 12 mo after the first vaccine dose and a systematic trend for higher CD4+ T cell and B cell frequencies compared with the pre-vaccination time point was observed. The trend for higher responses in the 18–60 y age group was not observed consistently at all time points (pre-vaccination, Day 21, Day 42, Month 6 and Month 12) or between subjects who received either one or two vaccine doses. These observations may be attributed to the intrinsic variability of the CD4+ T cell and B cell responses and the low sample size of the study. It may not be appropriate at this stage to correlate this observation to immunosenescence, although it has been reported previously that lower pre-existing T cell immunity leads to lower vaccine-induced responses post-primary vaccination as well as upon boosting, in the elderly as compared with in adults.Citation19 No clear trends could be identified to draw inferences on the impact of previous seasonal influenza vaccination on CMI.
In conclusion, although not all three CHMP guidance criteria were met at the long-term follow-up time points at Month 6 and Month 12, HI immune responses persisted up to 12 mo after vaccination with the one-dose and the two-dose regimens of the AS03-adjuvanted 3.75 µg HA H1N1 2009 pandemic influenza vaccine, in subjects aged 18−60 y and > 60 y. Despite the modest number of subjects in the sub-groups and the exploratory nature of the assessment of the CMI response, a persistent CD4 and B cell response was observed up to Month6 and Month 12. This aspect warrants further investigations to better understand the interplay between the magnitude of CMI response and persistence of protection against the H1N1 2009 virus. The long-term persistence data presented in this manuscript is expected to add to the existing literature on the well established immunogenicity profile of this vaccine.
Materials and Methods
Study design and subjects
In the study, 240 subjects aged ≥ 18 y received one dose of the 3.75 µg HA AS03-adjuvanted H1N1 2009 vaccine; at Day 21 subjects were randomized into two study groups (allocation ratio- 1:1) to either receive another vaccine dose or no vaccination, to facilitate assessments as per the study objective.Citation5 The 12-mo long-term follow-up of this study was completed on September 23, 2010.
Study vaccine
The H1N1 2009 pandemic influenza vaccine was a monovalent, inactivated, split-virion antigen with AS03A (Pandemrix™, GlaxoSmithKline Vaccines)Citation20 as described previously.5 AS03A is an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion (squalene 10.69 mg, DL- α-tocopherol 11.86 mg and polysorbate 804.86 mg).
Immunogenicity assessments
In the long-term follow-up phase, serum samples were collected six months and 12 mo after the first vaccine dose (Month 6 and Month 12) to be tested at GlaxoSmithKline Vaccines central laboratory using a validated in-house HI assay (cut-off: ≥ 1:10) as previously described.Citation21 Immunological assessments for HI immune response against the H1N1 A/California/07/2009 strain were based on the CHMP guidance criteriaCitation22 - GMT, SPR (proportion of subjects with HI antibody titer > 1:40), SCR (proportion of subjects with either a pre-vaccination HI antibody titer < 1:10 and post-vaccination titer > 1:40 or a pre-vaccination HI antibody titer > 1:10 and at least a 4-fold increase in post-vaccination HI antibody titers) and GMFR (ratio of post-vaccination vs. pre-vaccination HI antibody titers).
The MN immune response was evaluated in a subset of 22 participants, stratified by age groups (18–60 y and > 60 y age groups), history of seasonal influenza vaccination during previous three seasons, pre-vaccination serostatus and study group. To evaluate the persistence of this immune response, serum samples were collected six months after the first vaccine dose (Month 6) and tested at Viroclinics laboratories (Rotterdam, Nederland) using the A/Netherlands/602/9H1N1v strain which is antigenically similar to the A/California/7/09 vaccine strain as previously described.Citation23 The cut-off value used for MN antibody titers was 1:8.Citation17 Neutralizing antibody testing at Day 364 was cancelled.
As part of the exploratory assessments, the CMI response was evaluated in a subset of 59 subjects (and balanced between the 18–60 y and > 60 y age groups and the two study groups) at the Center for Vaccinology, Ghent University and Hospital (CEVAC). The CD4+ and CD8+ T cell frequencies were measured using Intracellular Cytokine Assay (ICA). To identify the memory B cells specific to the split H1N1 antigen as well as those specific to HA, the frequencies of memory B cell specific to split H1N1 and recombinant recHA proteinCitation24 (recHA protein A/Texas/5/2009) were measured at GlaxoSmithKline Vaccines by the Human Cellular Immunology team using an enzyme-linked immunosorbent spot assay, as described previously.Citation25
Safety and reactogenicity assessments
All pIMDs and SAEs occurring during the entire study period were recorded.
Statistical analyses
The end points for assessment of HI immune response were calculated with 95% confidence interval (CI) in terms of HI antibody GMT, SPR, SCR at Months 6 and 12. Also, GMTs and SPRs at Days 21, 42 and 182 (Month 6) were calculated with 95%CI. An exploratory end point was the frequency of influenza-specific CD4+ T cells and influenza-specific memory B cells before vaccination, at Days 21, 42 and Months 6 and 12. The analyses of immunogenicity were performed on the ATP cohort for persistence at Month 6 and at Month 12, and the analyses of safety were performed on the Total Vaccinated Cohort (TVC). The ATP cohort for persistence included all subjects who met the eligibility criteria, complied with the protocol-defined procedures during the entire study period and for whom the immunogenicity data was available at the two long-term time points—Months 6 and 12. The TVC included all vaccinated subjects.
| Abbreviations: | ||
| ATP | = | according-to-protocol |
| CHMP | = | Committee for Medicinal Products for Human Use |
| CEVAC | = | Center for Vaccinology, Ghent University and Hospital |
| CI | = | confidence interval |
| CMI | = | cell-mediated immune response |
| GMFR | = | geometric mean fold rise |
| GMT | = | geometric mean titer |
| HA | = | hemagglutinin |
| HI | = | hemagglutination inhibition |
| ICA | = | intracellular cytokine assay |
| pIMD | = | potential immune-mediated disease |
| SAE | = | serious adverse event |
| SCR | = | seroconversion rate |
| SPR | = | seroprotection rate |
| TVC | = | total vaccinated cohort |
Financial Disclosure
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis (ClinicalTrials.gov Identifier: NCT01001169). GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data and the corresponding author had final responsibility to submit for publication.
Conflict of Interest
Dr Froukje Kafeja has no conflict of interest to declare. Prof Pierre Van Damme acts as Chief and Principal Investigator for vaccine trials conducted on behalf of the University of Antwerp, for which the University obtains research grants from several vaccine manufacturers. Drs. EH, FR, PG, PM and VB are employees of GlaxoSmithKline group of companies. Drs. EH, FR, PG and PM report ownership of stock options.
Acknowledgments
All authors participated in the implementation of the study including substantial contributions to conception and design, the gathering of the data, or analysis and interpretation of the data. All authors were involved in the drafting of the article or revising it critically for important intellectual content, and final approval of the manuscript.
We are grateful to the New York Medical College, New York for providing the vaccine virus reassortant and to the National Institute for Biological Standards and Control (NIBSC, UK) and Therapeutic Goods Administration (TGA) from the Australian Government for providing the reference standards. The authors are indebted to the participating study volunteers, clinicians, nurses and laboratory technicians at the study sites as well as to the sponsor’s project staff for their support and contributions throughout the study. We are grateful to all teams of GlaxoSmithKline Vaccines for their contribution to this study, especially Annaêlle Delhaege and Karolien Peeters for clinical study management and site monitoring, and Caroline Hervé from the clinical, serological and cellular laboratory teams, Dorothy Slavin (Clinical Safety Representative) and Edith Lepine for project management. Authors would like to thank Dr James Stevens, CDC for providing recHA protein for the memory B cell assay. Finally the authors thank Dr Karl Walravens for critical review of the manuscript, Avishek Pal (GlaxoSmithKline Vaccines) and Adriana Rusu (XPE Pharma and Science) for providing medical writing services and Dr. Sophie Tambour and Dr Santosh Mysore (XPE Pharma and Science, on behalf of GlaxoSmithKline Vaccines) for editorial assistance and manuscript coordination.
Trademark Statement
Pandemrix is a trade mark of the GlaxoSmithKline group of companies.
References
- Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine 2010; 28:1740 - 5; http://dx.doi.org/10.1016/j.vaccine.2009.12.014; PMID: 20034605
- Carmona A, Omeñaca F, Tejedor JC, Merino JM, Vaman T, Dieussaert I, et al. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6-35 months. Vaccine 2010; 28:5837 - 44; http://dx.doi.org/10.1016/j.vaccine.2010.06.065; PMID: 20600478
- Ikematsu H, Nagai H, Kawashima M, Kawakami Y, Tenjinbaru K, Maeda A, et al. Immunogenicity and safety of a novel AS03(A)-adjuvanted H1N1 2009 pandemic influenza vaccine in adults in Japan. Hum Vaccin 2010; 6:888 - 93; http://dx.doi.org/10.4161/hv.6.11.12851; PMID: 20980795
- Roman F, Clément F, Dewé W, Walravens K, Maes C, Willekens J, et al. Effect on cellular and humoral immune responses of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol 2011; 18:835 - 43; http://dx.doi.org/10.1128/CVI.00480-10; PMID: 21450978
- Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis 2010; 51:668 - 77; http://dx.doi.org/10.1086/655830; PMID: 20687838
- Ferguson M, Risi G, Davis M, Sheldon E, Baron M, Li P, et al. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. J Infect Dis 2012; 205:733 - 44; http://dx.doi.org/10.1093/infdis/jir641; PMID: 22315336
- Nicholson KG, Abrams KR, Batham S, Clark TW, Hoschler K, Lim WS, et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis 2011; 11:91 - 101; http://dx.doi.org/10.1016/S1473-3099(10)70296-6; PMID: 21168369
- Madhun AS, Akselsen PE, Sjursen H, Pedersen G, Svindland S, Nøstbakken JK, et al. An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. Vaccine 2010; 29:266 - 73; http://dx.doi.org/10.1016/j.vaccine.2010.10.038; PMID: 21034828
- Saitoh A, Tamura S, Nagai A, Tsuchida N, Sako M, Maekawa T, et al. Clinical evaluation of an AS03-adjuvanted pandemic influenza H1N1 2009 vaccine in children (Preliminary report). [Japanese.] J Jap Pediatr Soc. 2011; 115:578 - 84
- Saitoh A, Nagai A, Tenjinbaru K, Li P, Vaughn DW, Roman F, et al. Safety and persistence of immunological response 6 months after intramuscular vaccination with an AS03-adjuvanted H1N1 2009 influenza vaccine: an open-label, randomized trial in Japanese children aged 6 months to 17 years. Hum Vaccin Immunother 2012; 8:749 - 58; http://dx.doi.org/10.4161/hv.19684; PMID: 22495117
- Leroux-Roels I, Roman F, Forgus S, Maes C, De Boever F, Dramé M, et al. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine 2010; 28:849 - 57; http://dx.doi.org/10.1016/j.vaccine.2009.10.017; PMID: 19835828
- Nolan T, Richmond PC, Formica NT, Höschler K, Skeljo MV, Stoney T, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine 2008; 26:6383 - 91; http://dx.doi.org/10.1016/j.vaccine.2008.08.046; PMID: 18801398
- Cowling BJ, Ng S, Ma ES, Cheng CK, Wai W, Fang VJ, et al. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis 2010; 51:1370 - 9; http://dx.doi.org/10.1086/657311; PMID: 21067351
- National Institute of Allergy and Infectious Diseases (NIAID). Early Results: NIAID Trial Supports Co-Administration of 2009 H1N1 Influenza Vaccine and Seasonal Influenza Vaccine Bulletin 2009; October 09, 2009. Available: http://www.niaid.nih.gov/news/newsreleases/2009/pages/h1n1plusseasonalvax.aspx. Accessed: February 20, 2012.
- Regner S, Peeters M, Schmitt B, Vaman T, Devaster JM, Rombo L. Immunogenicity, safety and reactogenicity of the AS03A-adjuvanted influenza A (H1N1) 2009 vaccine following sequential or co-administration with a licensed seasonal trivalent vaccine in adults aged ≥61 years. Accepted to be presented at the XIII International Symposium on Respiratory Viral Infections 2011, 13−16 March 2011, Rome, Italy.
- Vajo Z, Tamas F, Sinka L, Jankovics I. Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009-10 influenza season: a multicentre, randomised controlled trial. Lancet 2010; 375:49 - 55; http://dx.doi.org/10.1016/S0140-6736(09)62039-0; PMID: 20018367
- Ikematsu H, Tenjinbaru K, Li P, Madan A, Vaughn D. Evaluation of immune response following one dose of an AS03A-adjuvanted H1N1 2009 pandemic influenza vaccine in Japanese adults 65 years of age or older. Hum Vaccin Immunother 2012; 8:1119 - 25; http://dx.doi.org/10.4161/hv.21081; PMID: 22854661
- Ikematsu H, Nagai H, Kawashima M, Kawakami Y, Tenjinbaru K, Li P, et al. Characterization and long-term persistence of immune response following two doses of an AS03A-adjuvanted H1N1 influenza vaccine in healthy Japanese adults. Hum Vaccin Immunother 2012; 8:260 - 6; http://dx.doi.org/10.4161/hv.18469; PMID: 22426369
- Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines 2008; 7:467 - 79; http://dx.doi.org/10.1586/14760584.7.4.467; PMID: 18444893
- GlaxoSmithKline (GSK) Biologicals. Pandemrix™ suspension and emulsion for emulsion for injection. Summary of product characteristics. March 02, 2010.
- Hehme NW, Künzel W, Petschke F, Gisela T, Carmen R, Christian Van H, et al. Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin Drug Investig 2002; 22:751 - 69; http://dx.doi.org/10.2165/00044011-200222110-00004
- European Committee for Proprietary Medicinal Products (CHMP). Guideline on influenza vaccine prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context (EMEA/CHMP/VWP/263499/2006). European Agency for the Evaluation of Medicinal Products, January 24, 2007.
- Leroux-Roels I, Borkowski A, Vanwolleghem T, Dramé M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580 - 9; http://dx.doi.org/10.1016/S0140-6736(07)61297-5; PMID: 17707753
- Yang H, Carney P, Stevens J. Structure and Receptor binding properties of a pandemic H1N1 virus hemagglutinin. PLoS Curr 2010; 2:RRN1152; http://dx.doi.org/10.1371/currents.RRN1152; PMID: 20352039
- Moris P, van der Most R, Leroux-Roels I, Clement F, Dramé M, Hanon E, et al. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol 2011; 31:443 - 54; http://dx.doi.org/10.1007/s10875-010-9490-6; PMID: 21174144