Abstract
The prevalence of seasonal allergic rhinitis in the western world is high and increasing. Besides considerably affecting physical and psychosocial aspects of patients’ lives, allergic rhinitis is often associated with allergic asthma and may aggravate this condition over time. Specific immunotherapy is currently the only approved therapy that can modify the underlying disease process and induce long-term tolerance to allergens. Pollinex Quattro is a subcutaneous four injections immunotherapy consisting of tyrosine-absorbed specific allergoids and enhanced with the adjuvant monophosphoryl lipid A (MPL®). MPL® induces a significant Th1-type immune response, characterized by an increase of allergen-specific IgG antibody levels and dampening of the IgE response during allergen exposure. Due to this dual action of stimulating the immune system, Pollinex Quattro is clinically effective after only four injections given pre-seasonally. A large clinical program has demonstrated efficacy and tolerability of Pollinex Quattro in children, adolescents and adults with grass and tree pollen allergy. A health economics study concluded that an immunotherapy with only 4 injections might be more cost-beneficial than other application forms of immunotherapy.
Introduction
Allergic rhinitis (AR), an inflammation of the nasal airways, affects about 30% of the Western industrialized population and the prevalence continues to increase.Citation1 The cause of AR is likely attributed to multiple factors, including an underlying genetic predisposition.Citation2 In addition, lack of exposure to certain environmental factors shaping the immune system might contribute to the increase in allergic diseases. AR is a type I allergic disease characterized by an immunoglobulin E (IgE)-mediated inflammation of the nasal mucosa. Untreated, it represents a major cause of morbidity that includes interference with usual daily activities and impaired quality of life. Furthermore it may progress over time as well in severity of symptoms possibly leading to more serious complications such as allergic asthma (AA) in about 20% of patients.Citation3 Whereas seasonal allergic rhinitis (SAR) is primarily related to a wide variety of grass, tree and weed pollen allergens, perennial AR is associated with dust mite, mold spore or animal dander allergens.
Current therapeutic options are 3-fold: avoidance of allergens, symptomatic medication and specific immunotherapy (SIT).Citation4 Antiallergic medication such as antihistamines, corticosteroids and β mimetics are effective in treating the symptoms of AR and AA, but do not alter the underlying disease. Subcutaneous immunotherapy (SCIT), i.e., allergy vaccination with gradually increasing quantities of specific allergen up to a top dose or maintenance dose, is currently the only treatment that can modify the underlying disease process and induce long-term tolerance. It is therefore recommended in patients with moderate to severe SAR].Citation1
The mechanism of allergy vaccination is not yet completely understood, but involves a shift of allergen-specific T cells from a more T-helper type 2 (Th2) to a more Th1-like phenotype and the induction of regulatory T cells (Treg).Citation5 The cytokine pattern associated with a Th2 cell response is an increase in interleukin (IL)-4, IL-5 and IL-13 leading to induction of IgE antibodies. A Th1 response involves elevated interferon-ɣ (IFNɣ), IL-12 and immunoglobulins IgG and IgA as well as downregulation of IgE. Although allergy vaccination has been used for a century, recent developments have resulted in safer, shorter and more effective regimens such as ultra short course immunotherapy.Citation6,Citation7 Pollinex Quattro is currently the only ultra short course subcutaneous immunotherapy. In contrast to conventional SCIT, which requires up to 90 injections over three to five years, ultra short course SCIT has been shown to be clinically effective against allergies in only four injections per cycle, administered in as little as 3 weeks.
Key Issues
Allergic rhinitis is a major health problem in industrialized countries, potentially causing allergic asthma and other serious complications.
Therapeutic options include avoidance of allergens, symptomatic medication and immunotherapy. However, immunotherapy is the only approved therapy, altering the disease course.
Specific immunotherapy, i.e., allergy vaccination, leads to a shift from a more T-helper type 2 (Th2) to a more Th1-like cell population.
Pollinex Quattro is a four injections immunotherapy consisting of tyrosine-absorbed specific allergoids enhanced with the adjuvant monophosphoryl lipid A (MPL®).
In contrast to conventional SCIT, an ultra short course SCIT with only four injections potentially leads to higher therapeutic adherence of the patient and enhanced safety (i.e., less adverse reactions; each allergy vaccine injection is inherently associated with a risk of adverse reactions).
Design of Specific Immunotherapy
The first generation of allergy vaccines was based on aqueous native allergens. These exhibited a certain efficacy but sometimes also significant systemic side effects.Citation8 Nowadays, aqueous native allergens have mostly been replaced by depot allergen extracts and chemically modified allergens, so called allergoids. Changes in the protein structure of allergens ( = allergoids) reduce their allergenic potential, i.e., minimizes IgE-reactivity, whereas the immunogenic potential, i.e., the ability to induce allergen-specific IgG antibodies, remains intact.Citation4 They are typically linked to a depot adjuvant such as aluminum hydroxide, calcium phosphate or l-tyrosine to enable slow release of the allergen.Citation9 By this, clinical efficacy as well as a product’s safety profile, especially with regard to the incidence of anaphylactic reactions, could be improved.
Concurrent with the challenge of development of safer allergens was the development of specific adjuvants. Similar challenges were faced in the development of novel vaccines.Citation10 Aluminum salts were the first adjuvants to be used in vaccines and in specific immunotherapy about 80 y ago, and are still by far the most widely used adjuvants in vaccines and SIT.Citation8,Citation10 However, sensitivity to aluminum and/or partially bothersome itching and/or persistent granuloma at the injection site may develop in rare cases.Citation9,Citation11 A non-toxic alternative to aluminum is the physiological amino acid l-tyrosine.Citation12 Naturally occurring in the blood, l-tyrosine has a high adsorptive potential for proteins and is only weakly soluble at neutral pH-value.Citation13 In addition, immunological data indicate that l-tyrosine may support Th1-mediated immune responses by inducing the production of IgG while inhibiting IgE secretion.
Identification of Toll-like receptors (TLRs) and their association with the innate and adaptive immune system have led to the development of immunomodulating adjuvants that specifically support the Th1-shift associated with tolerance induction.Citation6,Citation10 These receptors specifically bind to structurally conserved molecules derived from different pathogens.Citation14 The activation of TLRs generally induces a strong, unspecific immune response, alerting cells of the innate immune system to fight against a pathogen immediately after entering the body. The activation of the innate immune system via TLRs also seems to be a prerequisite for inducing a specific immune response and thus essential in treatment with SIT.Citation15
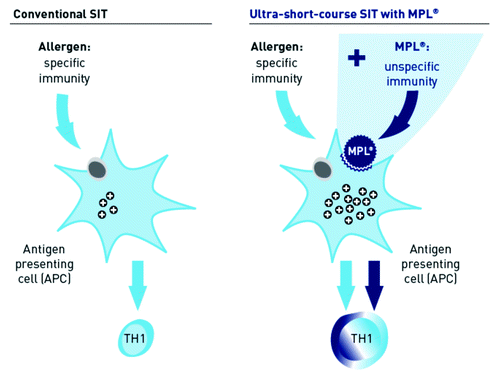
In the last decade, a variety of TLR agonists have been identified and tested as immunomodulators.Citation10 One such TLR agonist is the adjuvant monophosphoryl lipid A (MPL®), a detoxified derivative of the lipopolysaccharide cell wall component from Salmonella minnesota R595.Citation10,Citation14 MPL® acts via binding to Toll-like receptor 4 on antigen presenting cells (APCs). MPL® induces the secretion of gamma interferon (IFNɣ), IL-10 and IL-12 in APCs and in turn promotes the Th1 pathway of the unspecific immune response.Citation6 MPL® in combination with a specific antigen causes APCs to activate the immune system specifically in response to exposure to the corresponding allergen, and as such intensifies immunogenicity to these allergens ().Citation14,Citation15 Pollinex Quattro is an ultra short course, broad-spectrum allergy vaccine for the treatment of seasonal allergy. It contains glutaraldehyde-modified allergoids, linked to l-tyrosine as well as the adjuvant MPL®.Citation16 Through the dual action of MPL® in combination with highly tolerable allergoids on APCs and the resulting activation of both specific and unspecific immune responses, Pollinex Quattro can effectively induce tolerance to specific allergens within only four injections per year.
Pollinex Quattro
Depending on patient needs, one or more allergens (e.g., 13 grasses for grass allergic patients) are extracted, diafiltered using nominal molecular weight to cut off membranes at 5kDa and chemically treated with glutaraldehyde to form a series of allergoids. The allergoids are formed from natural allergens solubilised from one extract or a mix of extracts. For each Pollinex Quattro product, total protein and total allergenic activity have been specified and the major allergens have been identified (see example for 13 grass products in the ). The reproducibility of allergoid formulation is measured qualitatively by High Performance Liquid Chromatography – Size Exclusion Chromatography (HPLC-SEC) from which the chromatogram shows a characteristic shift based upon molecular weight increase associated with allergoid formation and quantitatively by measuring the change in primary amino groups present, which represents the degree of reduction in allergenicity.
Table 1. Thirteen grass product
The strength of the product is declared in Standardized Units (SU). The dosage levels of Pollinex Quattro with seasonal allergens are generally 300SU, 800SU and 2000 SU, administered in weekly 1.0 ml injections, followed by a further top dose injection of 2000 SU one week later to complete the 4 injection treatment course. These levels have been determined safe and effective via clinical experience as described in the respective sections below.
Immunological Changes after Pollinex Quattro
Immunological changes associated with the use of Pollinex Quattro in patients with SAR indicate an effective modification of allergic response in favor of a more Th1-like response. Treatment with Pollinex Quattro stimulated an increase in allergen-specific IgG, suppressed the seasonal rise in allergen-specific IgE, and induced CD4+, CD25+ and Fox P3+ regulatory T cells.Citation17-Citation20 In addition, specific immunotherapy significantly reduced skin-prick sensitivity reactions.Citation17,Citation18,Citation20,Citation21
In vitro, Pollinex Quattro induces a shift from a more Th2-type to a Th1-type immune reaction. Such immune responses are characterized by an increase of the anti-allergic cytokines IL-10, IL-12, TGF-β and IFNɣ.Citation22,Citation23 In contrast, Puggioni et al. reported no increase in IL-4 and IL-5, both typical Th2-type cytokines.Citation23
This was shown by an in vitro study using human mononuclear cells from patients with grass pollen allergy. Incubation of the cells with grass pollen allergen alone induced the production of IL-5 but only a low IFNɣ secretion in some T cell cultures. Addition of MPL® to allergen-stimulated cell cultures led to a significant increase in IFNɣ production (p < 0.001) and a decrease in IL-5 secretion.
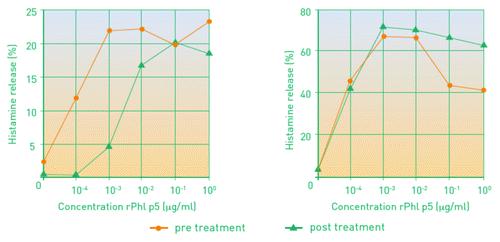
Mothes et al. analyzed the immunoglobulin responses and allergen-dependent basophil histamine release in 20 patients treated with Pollinex Quattro or placebo.Citation19 This was a subgroup analysis of a multicenter, double-blind, placebo-controlled phase 3 study in patients with grass pollen allergy.Citation17
Corresponding to significant clinical improvement, there was a strong induction of allergen-specific IgG1 and IgG4 antibodies in actively treated patients only.Citation19 Compared with placebo-treated patients, specific IgE levels were lower in patients who received a specific immunotherapy. Allergen-dependent basophil histamine release was inhibited in patients after short course immunotherapy but not before SIT or in the placebo group ().
Figure 2. Basophil histamin-release in patients treated with four injections of Pollinex Quattro (left) or placebo (right) before and after treatment.Citation19
Further immunological data were obtained from another double-blind, placebo-controlled phase 3 study conducted on patients with tree pollen allergy.Citation24 Patients were randomized to either four injections of Pollinex Quattro or placebo. In a subgroup analysis on 14 patients, Interferon-ɣ production increased significantly in the active treatment group upon re-exposure to allergen in comparison to patients in the placebo-group (p < 0.001). Contrarily, concentrations of IL-4-and IL-5 increased significantly in the placebo group, but not in the SIT group (p < 0.01).
The effects of specific immunotherapy on the activation and function of B cells were analyzed in 12 patients with seasonal allergic rhinoconjunctivitis.Citation25 Patients received either Pollinex Quattro (n = 8) or placebo (n = 4). The expression of CD23, CD54 and HLA-DR-II on peripheral B cells decreased during immunotherapy, an indication of reduced B-cell activation.
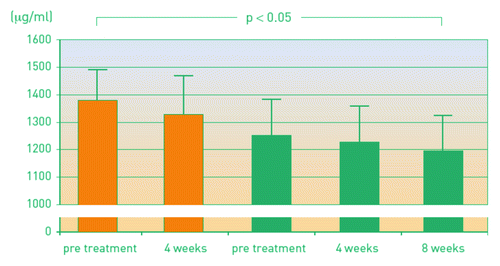
Tolerance induction was examined in a prospective study with 34 children/ adolescents (6 to 17 y of age) with grass pollen allergy.Citation20 Pollinex Quattro was administered over two years with four injections per course/year. After the first year, treatment generated only a modest increase of allergen-specific IgG and IgG4. However, after the second year this increase was pronounced (p < 0.0001), whereas specific IgE levels decrease slightly (not significant). In addition, a significant increase of CD4+CD25+FoxP3+ regulatory T cells could be demonstrated after the second course (p < 0.01 for comparison of baseline vs. end of second cycle). Treg cells are considered as important regulators of a balanced immune response due to suppressing or lowering proliferation of cytokine production of effector T cells after antigen exposure.Citation6 In a subset of nine patients, inhibiting activity of allergen-specific antibodies was further investigated by measuring leukotriene levels in the supernatant.Citation20 During immunotherapy there was a continuous decrease in leukotrienes (p < 0.05 comparing week 4 after the 2nd cycle vs. baseline) ().
Figure 3. Leukotriene levels as a surrogate parameter of inhibiting antibodies before and after immunotherapy.Citation20
Therapeutic Efficacy of Pollinex Quattro in Clinical Trials
The clinical efficacy of Pollinex Quattro for SIT has been evaluated in three phase 3 randomized, double-blind placebo-controlled trials in adult patients with SAR,Citation17,Citation18,Citation26 one open study in children and adolescentsCitation21 and one large post-marketing surveillance studyCitation27-Citation29 (summarized in ).
Table 2. Overview of phase 3 clinical studies and post-marketing trials with Pollinex Quattro: study design and key results
Two randomized placebo-controlled studies examined the safety and efficacy of Pollinex Quattro in the treatment of seasonal AR/AA caused by grass pollenCitation17 or tree pollen.Citation18 In both trials, patients received four pre-seasonal injections of Pollinex Quattro (300, 800, 2000 and 2000 SU/mL) or placebo (l-tyrosine).
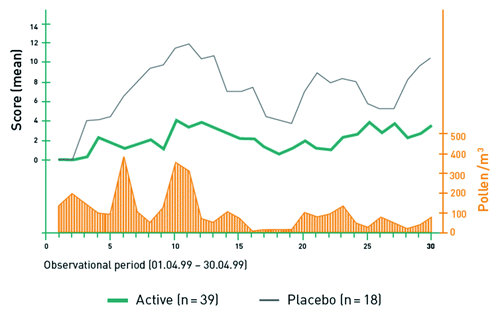
These studies showed significantly lower mean combined symptom/medication scores for patients treated with grass pollen (0.65 vs. 0.83; p = 0.013) and tree pollen (2.2 vs. 7.2; p = 0.028) allergoids (). Titrated skin prick testing in both trials revealed a significant reduction of skin sensitivity after immunization (grass pollen, p = 0.03; tree pollen, p < 0.01) and a significant decrease of mean wheal area in the active treatment group compared with placebo (grass pollen, p = 0.04; tree pollen, p = 0.03). In patients with grass pollen allergy mean scores for ocular symptoms (0.82 vs. 1.12, p = 0.003), nasal symptoms (1.21 vs. 1.46, p = 0.016) and combined nasal, ocular and pulmonary symptoms (0.75 vs. 0.95, p = 0.003) were significantly lower in patients treated with ultra short course immunotherapy. In patients with tree pollen allergy, use of symptomatic medication decreased significantly (p = 0.019).
Figure 4. Combined Symptom/medication Score in the randomized, placebo-controlled phase 3 study in patients with tree pollen allergy (n = 58).Citation18
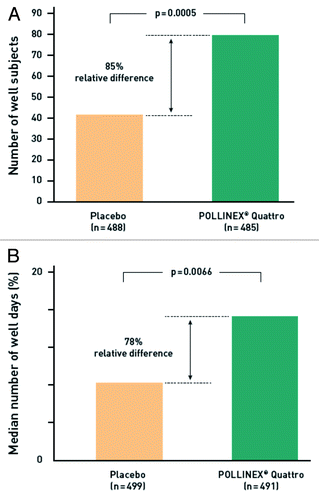
A third randomized controlled study compared the symptom/medication score of patients given four injections of Pollinex Quattro (in an optimized formulation) to those receiving placebo.Citation26 This multicenter trial enrolled 1,028 patients. Before entering the study, patients had an average history of over 20 y of SAR with moderate to severe symptoms. Patients were randomized to either four pre-seasonal injections of Pollinex Quattro (300, 800, 2000 and 2000 SU/0.5 mL) or placebo (l-tyrosine). Patients in the active group had a 13.6% reduction of the combined symptom/medication score during the four peak pollen weeks of the season (p = 0.0038) in comparison to placebo. In the prospectively defined patient population of those who fully recorded key outcomes (n = 343), there was a 24.3% improvement with Pollinex Quattro over placebo in the same efficacy endpoint (p = 0.0031). In addition, actively treated patients experienced significantly more “well days” and fewer “bad days” than subjects in the placebo group. The proportion of well subjects in the Pollinex group was 85% higher compared with the control group (). Significant benefits of treatment over placebo were observed in patients with a higher burden of disease (31.0%; p = 0.0001) und in those with long-standing rhinoconjunctivitis for up to 35 y (up to 31.4%; p = 0.0059).
Figure 5. Proportion of well subjects; i. e. CSMS ≤ 2 during the peak pollen season (a; n = 973) and median number of well days; i.e., patients with a score ≤ 2 and no anti-allergic medication (b; n = 990) in the randomized, placebo-controlled phase 3 study.Citation26
The data of this study were re-analyzed retrospectively to investigate in greater depth the clinical treatment effects dependent on local pollen exposure at study sites during the trial.Citation30 Potential benefits of treatment depend on the severity and duration of symptoms, which, in turn, depend on local pollen exposure. In the above mentioned trials as in other trials on specific immunotherapy, allergen exposure was assessed by local pollen counts. However, if study participants are located at a substantial distance from pollen count stations, the associated pollen counts may misrepresent the actual exposure received. In the re-analysis, the four weeks at each study center in which placebo-treated subjects had the highest combined symptom/medication scores (CSMS) were identified. The difference in CSMS between active and placebo groups was compared during the four peak placebo score weeks (PlSW) and the four peak pollen count weeks (PoCW). The benefit of active treatment vs. placebo in the PlSW analysis (18.5%) was greater than in the PoCW analysis (13.6%), with increased statistical significance (p = 0.0001 vs. 0.0038, respectively). Similarly, improved power of discrimination was observed when analyzing benefits in subgroups of patients with pollen-induced cough or asthma (16% vs. 13.4%; p = 0.04 vs. 0.08), with a high disease burden (35% vs. 31%; p < 0.0001 for both comparisons), and for patients enrolled in Europe (27.3 vs. 22%; p = 0.03 vs. 0.08).
An open multicenter study including 90 children and adolescents (6–17 y) with grass or tree pollen allergy showed comparable results with previous studies in adults.Citation21 Symptom scores, use of anti-allergic medication and reactivity to skin prick testing decreased significantly compared with previous pollen seasons (p < 0.01 for each).
A large post-marketing surveillance study confirmed the clinical efficacy and safety of ultra short course immunotherapy in the real-life setting.Citation27-Citation29 This trial enrolled 3,114 patients (2.692 adults and 422 children) with tree and/or grass pollen-induced AR and/or AA. Subjects were given Pollinex Quattro for three consecutive years according to the recommended immunization scheme. After three years, improvement of symptoms was observed in 93% of patients and consumption of medication decreased in 75.3% of cases (). Efficacy increased with treatment time and was also found in patients older than 50 y.
Figure 6. Use of symptomatic medication during therapy in 324 patient completing 3 y of immunization.Citation28
Quality of life was evaluated in the large phase 3 study described above and one open extension study in adults with SAR.Citation26,Citation31,Citation32 In the phase 3 study, patients treated with Pollinex Quattro vs. placebo experienced a statistically significant improvement in quality of life during the pollen season (p = 0.015). The multicenter extension study enrolled 132 patients with SAR with or without asthma. Patients answered rhinitis and asthma quality of life questionnaires (RQLQ and AQLQ) at baseline before allergy vaccination, during and after the pollen season in each of the three years of immunization, and at follow-up one year after treatment. There were statistically significant improvements in the global RQLQ and AQLQ after each of the three therapeutic years vs. the baseline scores (2009 vs. 2006; p < 0.0001). After an additional year of follow-up further improvements in RQLQ and AQLQ were observed: RQLQ from 38.3% to 41.1% (2010 vs 2006; p < 0.0001) and AQLQ from 45.5% to 48.5% (2010 vs 2006; p < 0.0001).
To date, different specific immunotherapy regimens have not been tested in a prospective, randomized head-to-head-study. However, conventional subcutaneous immunotherapy has been compared with ultra short course immunotherapy (Pollinex Quattro) in a multicenter observational trial.Citation33 A total of 209 subjects with tree or grass pollen rhinoconjuctivitis with/without asthma were enrolled into the study. Immunotherapies were given according to the approved dosing schemes. The study demonstrated non-inferiority of the ultra short course SCIT to conventional SCIT with regard to tree pollen induced rhinitis, conjunctivitis and respiratory symptoms as well as grass pollen induced rhinitis and conjunctivitis.
Health Economics of Pollinex Quattro
A German health economics study using a Markov model evaluated the direct costs of hypothetical pediatric cohorts suffering from SAR.Citation34 These cases were followed over three years with and without an allergen-specific immunotherapy in the following application forms: long-term subcutaneous immunotherapy (SCIT), short course SCIT, ultra short course SCIT (i.e., Pollinex Quattro) and sublingual immunotherapy (SLIT). In addition, several long-term scenarios were prepared analyzing the effectiveness, adherence and prevention of SAR-complications over 15 y. Average costs after three years of therapy were estimated at up to 4,269 €, 2,584 €, 2,523 € and 2,080 € for SLIT, long-term SCIT, short course SCIT and adjuvanted ultra short course SCIT with four injections, respectively. Direct costs of the four injections SCIT were higher in the beginning, but after week 20, 23 and 27 costs of short course SCIT (including maintenance injections), SLIT and long-term SCIT, respectively, exceeded the costs of ultra short course SCIT. Furthermore, results from long-term analyses support the advantage of short course and ultra short course SCIT with regard to patient adherence and sustained clinical effects in comparison to long-term-SCIT, SLIT or no SCIT. Hence, comparison of single drug unit prices does not allow conclusions to be drawn regarding the cost-effectiveness of different application forms.
Tolerability of Pollinex Quattro
Clinical experience with Pollinex Quattro is broad, with more than 5,000 subjects treated in clinical and post-marketing studies and approximately 220,000 patients who have received the drug up to now. In addition, MPL® is an adjuvant in the approved vaccines Fendrix® (hepatitis B vaccine) and Cervarix® (human papilloma virus vaccine) with safety data gathered in controlled trials including more than 37,000 subjects.Citation35
In general, Pollinex Quattro has been shown to be very well tolerated. In phase 3 clinical trials local reactions occurred more frequently after administration of Pollinex Quattro than after placebo ().Citation17,Citation18,Citation26 The majority of these were redness and swelling of > 5 cm in diameter, pain and/or itching at the injection site. None of these reactions required medical intervention beyond cooling and resolved after a few days. There was no significant difference in the incidence of systemic reactions between the active and placebo groups. Systemic reactions, mainly rhinitis and conjunctivitis, were generally mild to moderate. No serious or severe systemic reactions, including anaphylactic reactions, occurred either in the placebo-controlled studies or the open-label study in children and adolescents.Citation17,Citation18,Citation21,Citation26 In the large phase 3 study described previously, subject withdrawals due to adverse events were low (2.5% in the Pollinex Quattro arm vs. 0.4% in the placebo arm).Citation26 Furthermore, compliance rates were higher than previously recorded in any other comparable allergy vaccine study with 95.3% of patients receiving the planned number of injections.
Table 3. Summary of local adverse events in clinical trials during the treatment period
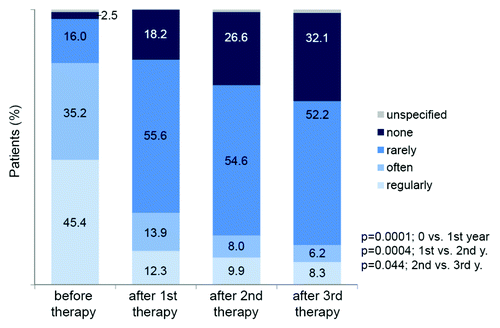
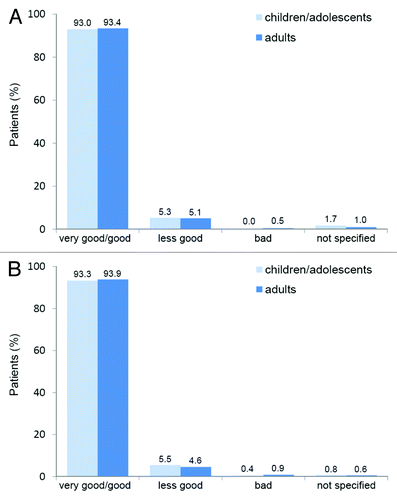
The large post-marketing surveillance study confirmed the tolerability of Pollinex Quattro in clinical practice. Local reactions occurred at a frequency of 8.1%, 2.2% and 2.7% during the first, second and third year, respectively.Citation28 Only few systemic reactions were reported, most commonly mild rhinitis. No anaphylactic reactions or serious adverse events were reported.Citation28,Citation29 Physician and patient assessment of tolerability and acceptance was „very good“ or „good“ in 93% of the population (). There was no difference in tolerability and acceptance between adults and children/adolescents.Citation28
Figure 7. Therapy tolerability (a) and acceptance (b) in adults (n = 2,692) and children/adolescents (n = 422).Citation28
Dosage and Administration
Formulations of Pollinex Quattro contain MPL® 50 µg/mL, l-tyrosine 2% w/v and specific glutaraldehyde-modified allergens.Citation36 This ultra short course immunotherapy is indicated for children, adolescents and adults with seasonal allergic rhinitis, conjunctivitis and/or asthma caused by grass, weed or tree pollen. Vaccination may be performed at any time before the associated pollen season each year. The vaccine should be administered for at least three consecutive years, since SIT has consistently demonstrated persistent long-term effects after 3 y of treatment.Citation37 The recommended vaccination scheme consists of four 1.0 mL injections of the following increasing strengths: 300, 800 and 2000 SU/mL followed by a fourth full-strength dose of 2000 SU/mL.Citation36 According to the manufacturer, dose-escalation should be performed only in the event of good tolerability of the last injection and adherence to injection intervals. In highly sensitized patients, dosage and dose escalation should be adapted according to the SmPC and at the physician’s discretion. Intervals between the first three injections should be between 7 and 14 d, and the fourth injection should be administered 1 to 4 weeks after the third injection. Should the allowed injection interval be exceeded by more than 7 d, the dose-escalation scheme should be restarted.
Future Perspective
To further improve tolerability, low-volume applications of Pollinex Quattro using an injection volume of 0.5 mL instead of 1.0 mL are currently in development.Citation26
More recently alternative modes of delivery of immunotherapy have been explored, such as sublingual immunotherapy (i.e., drops or tablets placed under the tongue). This application form might be particularly beneficial for children and patients with fear of injections. However, as with conventional immunotherapy, SLIT has a lengthy administration course. Short course MPL®-adjuvanted SLIT was recently evaluated in a double-blind, placebo-controlled phase 1/2 study.Citation38 The results suggest a favorable safety profile and efficacy of MPL®-adjuvanted SLIT.
Furthermore, a new allergy vaccine containing the adjuvant MPL® is currently in clinical development for the treatment of perennial allergy caused by house dust mites.Citation39 To date, preclinical data have shown no toxicological findings of significance at levels much higher to those proposed for clinical use.
Conclusion
Conventional allergen immunotherapy consists of up to 90 injections over three to five years. These long dosing regimens can represent a significant treatment and economic burden for allergy sufferers, not to mention health insurers and employers, potentially resulting in poor adherence to therapy. Pollinex Quattro, an ultra short course allergy vaccine consisting of four injections of allergoids adsorbed to l-tyrosine and MPL® adjuvant, offers distinct clinical advantages over conventional immunotherapies. Allergoids have reduced allergenicity and are thereby less likely to cause anaphylactic reactions or other systemic reactions. MPL® improves both the specific and unspecific immune responses, thus enabling efficacy similar to conventional immunotherapies but in a much shorter treatment course. In summary, through enhanced adherence to treatment, reduced direct and indirect costs, and minimized disruption to daily activities and occupations, Pollinex Quattro is an effective and efficient alternative to conventional allergy vaccinations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al, World Health Organization, GA(2)LEN, AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008; 63:Suppl 86 8 - 160; http://dx.doi.org/10.1111/j.1398-9995.2007.01620.x; PMID: 18331513
- Patel P, Salapatek AM. Pollinex Quattro: a novel and well-tolerated, ultra short-course allergy vaccine. Expert Rev Vaccines 2006; 5:617 - 29; http://dx.doi.org/10.1586/14760584.5.5.617; PMID: 17181436
- Corren J. The connection between allergic rhinitis and bronchial asthma. Curr Opin Pulm Med 2007; 13:13 - 8; http://dx.doi.org/10.1097/MCP.0b013e328010d0db; PMID: 17133119
- van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR, et al, European Academy of Allergology and Clinical Immunology. Consensus statement on the treatment of allergic rhinitis. Allergy 2000; 55:116 - 34; http://dx.doi.org/10.1034/j.1398-9995.2000.00526.x; PMID: 10726726
- Wheeler AW, Woroniecki SR. Allergy vaccines--new approaches to an old concept. Expert Opin Biol Ther 2004; 4:1473 - 81; http://dx.doi.org/10.1517/14712598.4.9.1473; PMID: 15335314
- Rolland JM, Gardner LM, O’Hehir RE. Allergen-related approaches to immunotherapy. Pharmacol Ther 2009; 121:273 - 84; http://dx.doi.org/10.1016/j.pharmthera.2008.11.007; PMID: 19111571
- Gawchik SM, Saccar CL. Pollinex Quattro Tree: allergy vaccine. Expert Opin Biol Ther 2009; 9:377 - 82; http://dx.doi.org/10.1517/14712590802699596; PMID: 19216626
- Rose MA. Das Adjuvans Monophosphoryl-Lipid A (MPL) - Vom Endotoxin zur effizienten SIT. Derm 2/2012.
- Pfaar O, Klimek L. Einsatz von Adjuvanzien bei der allergen-spezifischen Immuntherapie. Allergo J 2011; 20:60 - 70
- Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines 2007; 6:723 - 39; http://dx.doi.org/10.1586/14760584.6.5.723; PMID: 17931153
- Brehler R, Kleine-Tebbe J. Extraktvarianten und Adjuvanzien zur spezifischen Immuntherapie. Allergo J 2008; 17:224 - 7
- Wheeler AW, Moran DM, Robins BE, Driscoll A. l-Tyrosine as an immunological adjuvant. Int Arch Allergy Appl Immunol 1982; 69:113 - 9; http://dx.doi.org/10.1159/000233157; PMID: 7107028
- Baldrick P, Richardson D, Wheeler AW. Review of L-tyrosine confirming its safe human use as an adjuvant. J Appl Toxicol 2002; 22:333 - 44; http://dx.doi.org/10.1002/jat.869; PMID: 12355563
- Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, et al. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther 2004; 4:1129 - 38; http://dx.doi.org/10.1517/14712598.4.7.1129; PMID: 15268679
- Akira S. Innate immunity and adjuvants. Philos Trans R Soc Lond B Biol Sci 2011; 366:2748 - 55; http://dx.doi.org/10.1098/rstb.2011.0106; PMID: 21893536
- McCormack PL, Wagstaff AJ. Ultra-short-course seasonal allergy vaccine (Pollinex Quattro). Drugs 2006; 66:931 - 8; http://dx.doi.org/10.2165/00003495-200666070-00004; PMID: 16740007
- Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy 2001; 56:498 - 505; http://dx.doi.org/10.1034/j.1398-9995.2001.056006498.x; PMID: 11421893
- Drachenberg KJ, Heinzkill M, Urban E. Kurzzeit-Immuntherapie mit Baumpollen-Allergoiden und dem Adjuvans Monophosphoryl Lipid-A. Allergologie 2002; 25:466 - 74
- Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy 2003; 33:1198 - 208; http://dx.doi.org/10.1046/j.1365-2222.2003.01699.x; PMID: 12956739
- Rosewich M, Schulze J, Eickmeier O, Telles T, Rose MA, Schubert R, et al. Tolerance induction after specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A in children. Clin Exp Immunol 2010; 160:403 - 10; http://dx.doi.org/10.1111/j.1365-2249.2010.04106.x; PMID: 20345983
- Drachenberg KJ, Heinzkill M, Urban E, Woroniecki SR. Efficacy and tolerability of short-term specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A (MPL) for children and adolescents. Allergol Immunopathol (Madr) 2003; 31:270 - 7; http://dx.doi.org/10.1016/S0301-0546(03)79195-2; PMID: 14572416
- Martin M, Michalek SM, Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun 2003; 71:2498 - 507; http://dx.doi.org/10.1128/IAI.71.5.2498-2507.2003; PMID: 12704121
- Puggioni F, Durham SR, Francis JN. Monophosphoryl lipid A (MPL) promotes allergen-induced immune deviation in favour of Th1 responses. Allergy 2005; 60:678 - 84; http://dx.doi.org/10.1111/j.1398-9995.2005.00762.x; PMID: 15813815
- Stuck BA, Schneide-Gene S, Schäfer D, et al. Short-term preseasonal immunotherapy with birch pollen allergoid plus monophosphoryllipid A (MPL). Allergy Clin Immunol Int 2004; 16:60 - 4; http://dx.doi.org/10.1027/0838-1925.16.2.60
- Roever AC, Reimann S, Zuberbier T, Worm M. Immunophenotypic characterization of peripheral B cells. During short-term immunotherapy with tree pollen allergoid and the immunoadjuvant monophosphoryl lipid A. J Invest Allergol. Clin Immunol 2002; 12:227 - 223
- DuBuske LM, Frew AJ, Horak F, Keith PK, Corrigan CJ, Aberer W, et al. Ultrashort-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc 2011; 32:239 - 47; http://dx.doi.org/10.2500/aap.2011.32.3453; PMID: 21535913
- Drachenberg KJ, Proell S, Urban E, Woroniecki SR. Short-term immunotherapy using an allergy vaccine adjuvanted with monophosphoryl lipid A: a post-marketing surveillance study. Int Rev Allergol Clin Immunol 2002; 8:219 - 23
- Zielen S, Metz D, Sommer E, Scherf HP. Short-term immunotherapy with allergoids and the adjuvant monophosphoryl lipid A. Allergologie 2007; 30:Suppl 1 S1 - 8
- Rosewich M, Schulze J, Fischer von Weikersthal-Drachenberg KJ, Zielen S. Ultra-short course immunotherapy in children and adolescents during a 3-yrs post-marketing surveillance study. Pediatr Allergy Immunol 2010; 21:e185 - 9; http://dx.doi.org/10.1111/j.1399-3038.2009.00953.x; PMID: 20003062
- Frew AJ, DuBuske L, Keith PK, Corrigan CJ, Aberer W, Fischer von Weikersthal-Drachenberg KJ. Assessment of specific immunotherapy efficacy using a novel placebo score-based method. Ann Allergy Asthma Immunol 2012; 109:342 - 7, e1; http://dx.doi.org/10.1016/j.anai.2012.08.013; PMID: 23062390
- Rabe U, Fiedler G, Piller M. 4-Jahresdaten zur Verbesserung der Lebensqualität bei Patienten mit Baumpollenallergie nach subkutaner Kurzzeit-Immuntherapie (SCIT) mit Allergoiden und dem Adjuvans Monophosphoryl-Lipid A (MPL®); 6. Deutscher Allergiekongress Wiesbaden 2011, Poster-Nr. 11.
- Rabe U, Fiedler G, Piller M. 4-Jahresdaten zur Verbesserung der Lebensqualität bei Patienten mit Graspollenallergie nach subkutaner Kurzzeit-Immuntherapie (SCIT) mit Allergoiden und dem Adjuvans Monophosphoryl-Lipid A (MPL®); 6. Deutscher Allergiekongress Wiesbaden 2011, Poster-Nr. 12.
- Stollewerk D, Reiber R, Greving J, et al. Comparison of efficacy of conventional subcutaneous immunotherapy (SCIT) and ultra-short SCIT containing Monophosphoryl Lipid A (MPL): results of a multicentre observational study after two years of treatment. 29th Congress of the European Academy of Allergy and Clinical Immunology London, 2010; Poster #670.
- Claes C, Mittendorf T, Graf von der Schulenburg JM. Gesundheitsökonomische Modellierung der allergenspezifischen Immuntherapie bei saisonaler allergischer Rhinitis aus Sicht der Kostenträger. Allergo J 2009; 18:60 - 70
- Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008; 26:6630 - 8; http://dx.doi.org/10.1016/j.vaccine.2008.09.049; PMID: 18845199
- Pollinex Quattro. Summary of Product Characteristics. Bencard Allergie GmbH. June 2011.
- Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med 1999; 341:468 - 75; http://dx.doi.org/10.1056/NEJM199908123410702; PMID: 10441602
- Pfaar O, Barth C, Jaschke C, Hörmann K, Klimek L. Sublingual allergen-specific immunotherapy adjuvanted with monophosphoryl lipid A: a phase I/IIa study. Int Arch Allergy Immunol 2011; 154:336 - 44; http://dx.doi.org/10.1159/000321826; PMID: 20975285
- Baldrick P, Richardson D, Wheeler AW. Safety evaluation of a glutaraldehyde modified tyrosine adsorbed housedust mite extract containing monophosphoryl lipid A (MPL) adjuvant: a new allergy vaccine for dust mite allergy. Vaccine 2001; 20:737 - 43; http://dx.doi.org/10.1016/S0264-410X(01)00413-3; PMID: 11738737