Abstract
Invasive pneumococcal disease continues to be important problem for older adults. Pneumococcal polysaccharide vaccine (PPV23) has a clinical effectiveness of 43–81%, and following primary vaccination and revaccination, antibody responses last 5–10 y. Hyporesponsiveness to a second dose of vaccine has not been shown to be a significant problem. The use of pneumococcal conjugate vaccines (initially PCV7; more recently PCV13) has led to a dramatic fall in the incidence of conjugate vaccine-type invasive pneumococcal disease in children. Because PCVs are immunogenic in older adults, the question has arisen as to whether to also use PCVs in this age group. However, PCV vaccination of children has also reduced the incidence of conjugate vaccine-serotype disease in older adults, and so wherever PCVs are used in children, there is no epidemiological reason to vaccinate older adults with PCV. The cost-effectiveness of PPV for older adults has changed wherever PCVs have been used for children, and this needs to be periodically re-evaluated.
Early mortality in pneumococcal bacteremia
Historical studies have shown the importance of early intervention in improving survival in patients with pneumococcal bacteremia and pneumococcal pneumonia (non-bacteremic as well as bacteremic cases) ().Citation1 This insight provided the basis for developing the first pneumococcal polysaccharide vaccine. The clinical efficacy of an experimental 13-valent pneumococcal polysaccharide vaccine was shown in a clinical trial among South African gold miners. In this trial, vaccine efficacy in preventing pneumococcal bacteremia was 82.3% (Table 1).Citation2 The vaccine was also efficacious in preventing all cases of pneumococcal pneumonia and in preventing more than half of all cases of radiographically-confirmed pneumonia, regardless of microbial cause.Citation3
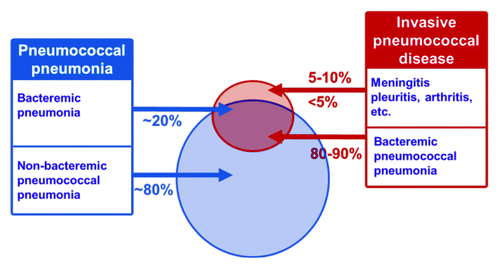
Figure 1. Diagrammatic representation of the overlap between pneumococcal pneumonia and invasive pneumococcal disease.
A number of case-control studies have been conducted to assess the effectiveness of pneumococcal polysaccharide vaccine in preventing invasive pneumococcal disease in older adults. Vaccination effectiveness has ranged from 43–81%. A recent report has reviewed the long-term antibody responses to 23-valent pneumococcal polysaccharide vaccine (PPV23) in older adults and the impact of vaccination on serotype-specific incidence of invasive pneumococcal disease.Citation4 The reviewers concluded that PPV23 was effective, particularly in healthy individuals less than 75 y of age, but protection waned after 5 y. There was no discernible impact of PPV23 on population incidence of IPD. Based on vaccine efficacy estimates, PPV23 was still considered a cost-effective intervention for the low-risk elderly.
Long-term protection
Antibody persistence following PPV23 vaccination was examined in a large study of ambulatory adults ≥ 50 y of age.Citation5 Study subjects were PPV23 naïve and had no previous history of invasive pneumococcal disease. Immunoglobulin G (IgG) and functional antibody levels were measured in 444 primary vaccination subjects and in 564 revaccination subjects. In the primary vaccination group, IgG antibodies were evaluated in 308 subjects at 5 y and in 72 subjects at 10 y. In the revaccination group (median time since primary vaccination = 3.9 y), IgG and functional antibody levels 3–5 y after primary vaccination and revaccination were similar. Ten years after revaccination, mean IgG concentrations still exceeded vaccine-naïve levels.Citation5 Although the results indicated that increased antibody levels persisted for many years, there are still no data on long-term clinical protection afforded by primary vaccination or revaccination with PPV23.
Revaccination and hyporesponsiveness?
It has been hypothesized that revaccination of older adults with PPV23 is associated with hyporesponsiveness.Citation6 Earlier studies of combined schedules of PPV23 and pneumococcal conjugate vaccine (PCV) vaccination showed lower antibody responses one month after the second dose in subjects previously vaccinated with PPV23. These findings suggested that PPV23 vaccination caused hyporesponsiveness following subsequent vaccination with PPV23 and PCV, and called into question the value of PPV23 revaccination. To test this hypothesis, PPV23 revaccination was evaluated in adults aged 55–74 y who had been vaccinated with 1 to 4 doses of PPV23.Citation7 Antibody responses were measured 30 d after vaccination. Repeat PPV23 vaccination of older adults was found to be safe and immunogenic, and there was no evidence of hyporesponsiveness. Other studies in infants have shown that nasopharyngeal carriage of S. pneumoniae shortly before pneumococcal conjugate vaccine (PCV) 7 causes serotype-specific hyporesponsiveness in infants.Citation8 Clearly, some degree of hyporesponsiveness may occur in some individuals following both repeated doses of PPV23 and recent natural exposure to S. pneumonia. Nonetheless, there is no evidence that these findings are clinically important.
PCV7 vs. PPV23
The immunogenicity of PCV7 vs. PPV23 was compared in adults 50–80 y of age.Citation9 IgG antibody responses to both vaccines were similar, suggesting that either vaccine could be administered to these individuals, However, epidemiological studies in the US and other countries have shown dramatic reductions in the incidence of invasive pneumococcal disease (IPD) in older adults after the introduction of PCV7 vaccination of children.Citation10 IPD rates caused by serotype 19A and other non-PCV7 types have increased, but have remained low relative to decreases in PCV7-type IPD. When changes in the incidence of IPD were examined for older adults in England and Wales during the period 1998/99 to 2009/10, it was found that while the overall incidence of all serotype- and PPV23-serotype IPD did not change, the incidence of PCV7-serotype IPD declined after PCV7 was introduced for children. The incidence of IPD due to serotypes unique to PPV23 (not in PCV7) increased, as did the incidence for non-vaccine type IPD.Citation4,Citation11,Citation12
Economic considerations
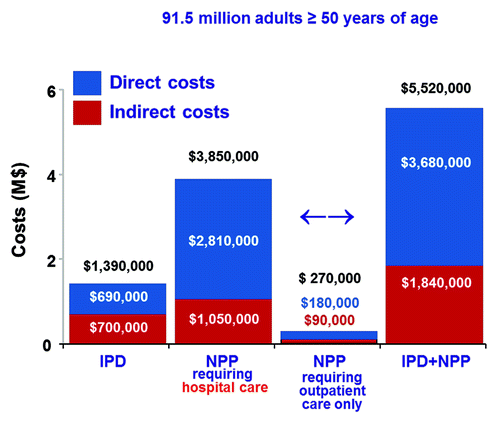
Data from the US have shown that the direct and indirect costs of pneumococcal diseases among older adults are substantial (), despite increased coverage with PPV23 and indirect benefits afforded by PCV7 vaccination of young children.Citation13
PPV23
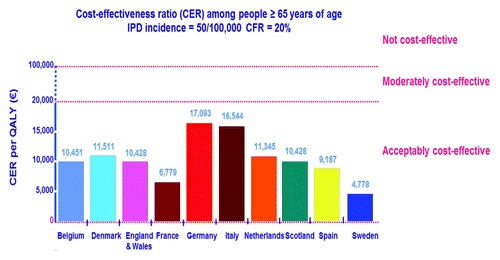
Several years ago, before the widespread introduction of PCV7 vaccination for children, the cost-effectiveness of PPV23 vaccination to prevent invasive pneumococcal disease was evaluated in 10 Western European countries (PPV23 was given concomitantly with influenza vaccine).Citation14 PPV23 was found to be acceptably cost-effective in all 10 countries studied ().
Figure 3. Data showing the cost-effectiveness of PPV23 vaccination to prevent invasive pneumococcal disease alone in 10 Western European Countries.
An earlier study reported that even a low level of clinical effectiveness against non-bacteremic pneumococcal pneumonia dramatically improved the overall cost-effectiveness of PPV.Citation15 While encouraging, these studies may not be relevant in countries that have introduced widespread vaccination of children with pneumococcal conjugate vaccines.
PCV13
Immunogenicity studies of PCVs in older adults have found no convincing advantage of PCVs over PPV23. Because PCV7 vaccination of children has led to a decrease in PCV7 serotype IPD in older adults, PCV13 vaccination of children should result in a similar decrease in PCV13 serotype IPD in older individuals. As a result, there will be little PCV13 serotype pneumococcal disease in this age group that could be prevented by vaccination with PCV13. It follows that PCV13 vaccination of older adults will not be cost-effective in preventing either IPD or non-bacteremic pneumococcal pneumonia. However, because of the overall decline in the incidence of PCV13 serotype disease following the introduction of PCV13 vaccination of children, the cost-effectiveness of PPV23 vaccination in older adults will inevitably decrease.Citation16
Conclusions
IPD and pneumococcal pneumonia remain important health problems for older adults. PPV23 is clinically effective and cost-effective in reducing IPD and community-acquired pneumonia hospitalizations and deaths. Antibody responses to PPV23 vaccination and revaccination last up to 10 y, although direct evidence of long-term clinical protection afforded by PPV23 vaccination has never been obtained. PCV vaccination of children leads to lower incidence of PCV serotype IPD in older adults. Many have suggested that older adults should be vaccinated with PCV13 instead of PPV23. However, immunogenicity studies show no convincing advantage of PCVs over PPV23 in older adults.Citation17 Moreover, soon after the introduction of PCV13 vaccination for children, the incidence of PCV13 serotype disease in older adults will become so low that there will be no epidemiological reason to vaccinate them with PCV13.Citation18
In future years, health officials should continue their surveillance of IPD in all age groups following the introduction of PCV13 vaccination of children. They should also evaluate the changing cost-effectiveness of PPV23 in older adults. Ultimately, better control of pneumococcal disease in populations will probably require a universal, protein-based vaccine for both children and adults. Better management of individual patients with severe pneumococcal infections may eventually be achieved by treating them with immunomodulatory agents (e.g., statins).Citation19
Disclosure of Potential Conflicts of Interest
DSF discloses no conflicts of interest.
References
- Austrian R, Gold J. Pneumococcal bacteremia with special reference to bacteremic pneumococcal pneumonia. Ann Intern Med 1964; 60:759 - 76; http://dx.doi.org/10.7326/0003-4819-60-5-759; PMID: 14156606
- Austrian R, Douglas RM, Schiffman G, Coetzee AM, Koornhof HJ, Hayden-Smith S, et al. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians 1976; 89:184 - 94; PMID: 14433
- Fedson DS. Pneumococcal vaccination for older adults: the first 20 years. Drugs Aging 1999; 15:Suppl 1 21 - 30; http://dx.doi.org/10.2165/00002512-199915001-00003; PMID: 10690792
- Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 2012; 30:6802 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.09.019; PMID: 23000122
- Musher DM, Manoff SB, McFetridge RD, Liss CL, Marchese RD, Raab J, et al. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Hum Vaccin 2011; 7:919 - 28; http://dx.doi.org/10.4161/hv.7.9.15996; PMID: 21860256
- O’Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue?. Lancet Infect Dis 2007; 7:597 - 606; http://dx.doi.org/10.1016/S1473-3099(07)70210-4; PMID: 17714673
- Hammitt LL, Bulkow LR, Singleton RJ, Nuorti JP, Hummel KB, Miernyk KM, et al. Repeat revaccination with 23-valent pneumococcal polysaccharide vaccine among adults aged 55-74 years living in Alaska: no evidence of hyporesponsiveness. Vaccine 2011; 29:2287 - 95; http://dx.doi.org/10.1016/j.vaccine.2011.01.029; PMID: 21255685
- Dagan R, Givon-Lavi N, Greenberg D, Fritzell B, Siegrist CA. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis 2010; 201:1570 - 9; http://dx.doi.org/10.1086/652006; PMID: 20384496
- Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, et al. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50-80 years. Clin Infect Dis 2009; 49:1318 - 25; http://dx.doi.org/10.1086/606046; PMID: 19814624
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al, Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32 - 41; http://dx.doi.org/10.1086/648593; PMID: 19947881
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11:760 - 8; http://dx.doi.org/10.1016/S1473-3099(11)70090-1; PMID: 21621466
- Flasche S, Slack M, Miller E. Long term trends introduce a potential bias when evaluating the impact of the pneumococcal conjugate vaccination programme in England and Wales. Euro Surveill 2011; 16:19868; PMID: 21616047
- Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine 2010; 28:4955 - 60; http://dx.doi.org/10.1016/j.vaccine.2010.05.030; PMID: 20576535
- Evers SM, Ament AJ, Colombo GL, Konradsen HB, Reinert RR, Sauerland D, et al. Cost-effectiveness of pneumococcal vaccination for prevention of invasive pneumococcal disease in the elderly: an update for 10 Western European countries. Eur J Clin Microbiol Infect Dis 2007; 26:531 - 40; http://dx.doi.org/10.1007/s10096-007-0327-z; PMID: 17570001
- Ament A, Fedson DS, Christie P. Pneumococcal vaccination and pneumonia: even a low level of clinical effectiveness is highly cost-effective. Clin Infect Dis 2001; 33:2078 - 9; http://dx.doi.org/10.1086/324356; PMID: 11700581
- Rozenbaum MH, van Hoek AJ, Fleming D, Trotter CL, Miller E, Edmunds WJ. Vaccination of risk groups in England using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ 2012; 345:e6879; http://dx.doi.org/10.1136/bmj.e6879; PMID: 23103369
- Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence?. Clin Infect Dis 2011; 52:633 - 40; http://dx.doi.org/10.1093/cid/ciq207; PMID: 21292668
- Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults?. Clin Infect Dis 2012; 55:265 - 7; http://dx.doi.org/10.1093/cid/cis364; PMID: 22495544
- Doshi SM, Kulkarni PA, Liao JM, Rueda AM, Musher DM. The impact of statin and macrolide use on early survival in patients with pneumococcal pneumonia. Am J Med Sci 2013; 345:173 - 7; http://dx.doi.org/10.1097/MAJ.0b013e3182639c26; PMID: 23111390