Abstract
This study was conducted to assess the impact of administration of two-dose rotavirus (RV) vaccine (RIX4414; GlaxoSmithKline Vaccines) among children aged less than 5 y in three states/territories of Australia. Aggregated and de-identified data on rotavirus gastroenteritis (RVGE) and all-cause gastroenteritis (AGE) from July 1998–June 2009 were obtained from the Australian Institute of Health and Welfare database. The baseline incidence (July 1998–June 2006) of RVGE hospitalizations before RV vaccine introduction in New South Wales (NSW), the Australian Capital Territory (ACT) and the Northern Territory (NT) were 33.75, 42.93 and 288.67 per 10 000 child-years, respectively among children aged 0–11 mo. Following RV vaccine introduction in NSW, the ACT and the NT, incidence of RVGE hospitalizations reduced to 13.06, 17.35 and 47.52 per 10 000 child-years, respectively, during July 2007–June 2008 and 3.87, 8.40 and 122.79 per 10,000 child-years, respectively, during July 2008–June 2009 among children aged 0–11 mo. Reductions in RVGE and AGE were also observed in all children below 5 y of age in NSW and the ACT. Overall reduction in hospitalizations due to RVGE and AGE was observed following RV vaccine introduction into the NIP in Australia.
Introduction
Globally, rotavirus (RV) is a leading cause of severe gastroenteritis (GE) among infants and young children resulting in substantial morbidity and mortality.Citation1 It is estimated that prior to the introduction of RV vaccines, RV was associated with nearly 453 000 deaths and over two million hospitalizations worldwide among children below 5 y of age.Citation1,Citation2 In Australia RV is estimated to account for nearly 10 000 hospitalizations annually prior to RV vaccine introduction, an estimated 50% of acute GE related hospitalizations being attributable to RV.Citation3
Two live attenuated oral RV vaccines: Rotarix™ (RIX4414) (GlaxoSmithKline Vaccines) and RotaTeq® (RV5) (Merck and Co., Inc.) are licensed in many countries worldwide.Citation4 Both these vaccines have shown to be safe and efficacious against rotavirus gastroenteritis (RVGE) in both developed and developing countries.Citation5-Citation12
RIX4414 and RV5 are available in Australia since 2006. Both were included in the government National Immunisation Program (NIP) on July 1, 2007, for all infants born May 1, 2007, onwards.Citation13,Citation14 Due to a high burden of RV disease in the Northern Territory (NT), RIX4414 was provided for all children born in the NT from August 1, 2006.Citation15
New South Wales (NSW), the NT, Tasmania and the Australian Capital Territory (ACT) selected RIX4414 for use in their state/territory immunization program; while Victoria, South Australia and Queensland (QLD) selected RV5. Western Australia selected RIX4414, subsequently changing to RV5 in May 2009.Citation13
Vaccination coverage is high in Australia. RV vaccination coverage at 12 mo of age was 91.0% and 88.0% in 2007 and 2008, respectively, in the ACT; 87.2% and 81.1% in 2007 and 2008, respectively, in the NT; 84.5% and 80.7%, respectively in 2007 and 2008, in QLD and 83.4% and 84.9% in 2007 and 2008, respectively, in NSW. The overall coverage of RV vaccines in 2007 and 2008 were 83.8% and 82.3%, respectively. RV vaccine coverage was lower than the overall coverage of other vaccines scheduled to be given at the same visits under the NIP, such as 7-valent pneumococcal conjugate vaccine (coverage of 90.9% and 91.1% in 2007 and 2008 respectively).Citation16,Citation17 The reason for this slightly lower coverage was presumed to be because of the strict upper age limit for RV vaccines.
The burden of RVGE was significantly higher in Indigenous children. In a study conducted by Newall et al., the average annual rate of hospitalizations for RVGE among Indigenous people of all ages between 2000 and 2002 was 107.7 per 100 000 population as compared with 21.9 per 100 000 population in the whole of Australia covered by the Australian Institute of Health and Welfare (AIHW) database.Citation18
In the Australian NIP, RIX4414 (2-dose vaccine) is scheduled to be administered at the 2- and 4-mo immunization visit; first dose between 6–14 weeks of age and second dose between 10–24 weeks of age, with at least four weeks interval between the doses.Citation19
The present study aimed to assess the impact of RV vaccination (RIX4414) on Australian children less than 5 y of age by comparing the incidence of hospitalizations for RVGE and all-cause GE (AGE) in the period prior to RV vaccine introduction (July 1998 to June 2006) with a period post vaccine introduction (July 2007 to June 2009) onto the NIP in NSW, ACT and the NT.
Results
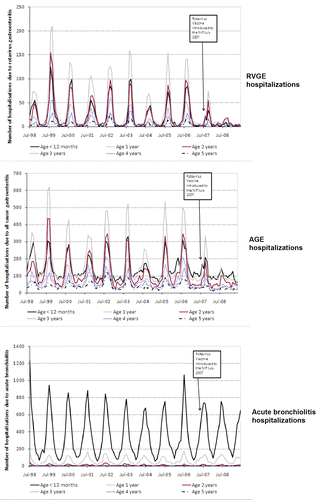
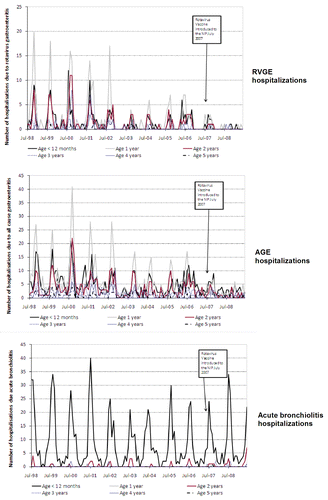
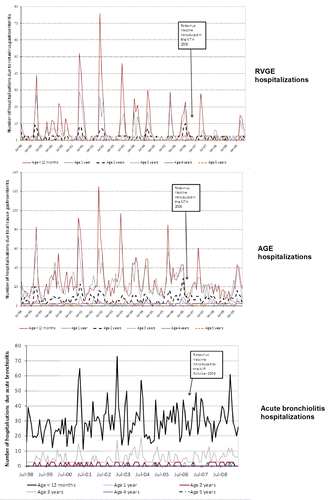
The number of hospitalizations due to RVGE and AGE reduced following the introduction of RIX4414 in each of the states/territories assessed; NSW, the ACT and the NT (; and ). A reduction in the incidence of RVGE- and AGE-associated hospitalizations was observed in all children below 5 y of age (which also included children who were ineligible for RV vaccination) in NSW and the ACT and in children below 3 y in the NT (; ; ).
Table 1. Incidence of RVGE, AGE and acute bronchiolitis hospitalizations among children below 5 y of age in NSW
Table 2. Incidence of RVGE, AGE and acute bronchiolitis hospitalizations among children below 5 y of age in ACT
Table 3. Incidence of RVGE, AGE and acute bronchiolitis hospitalizations among children below 5 y of age in NT
Impact on the incidence of RVGE and AGE hospitalizations, by region
Prior to the introduction of RV vaccination in NSW, the average baseline incidence of RVGE hospitalizations among children aged 0–11 mo was 33.75 [95% confidence intervals (CI): 32.39–35.15] per 10,000 child-years over the period July 1998–June 2006 (). Following introduction of RV vaccination, the incidence of RVGE hospitalizations reduced to 13.06 (95% CI: 10.81–15.64) per 10,000 child-years in the first year post introduction on the NIP (July 2007–June 2008) and further to 3.87 (95% CI: 2.71–5.35) per 10,000 child-years, in the second year post introduction on the NIP (July 2008–June 2009), respectively. The overall reduction in RVGE hospitalizations in NSW compared with baseline period (July 1998–June 2006) was 53.84% and 79.24% during July 2007–June 2008 and July 2008–June 2009, respectively, among all children aged less than 5 y.
In the ACT, the average baseline incidence of RVGE hospitalizations among children aged 0–11 mo prior to the introduction of RV vaccination was 42.93 (95% CI: 36.16–50.60) per 10,000 child-years. Following introduction of RV vaccination, the incidence of RVGE hospitalizations reduced to 17.35 (95% CI: 7.49–34.19) and 8.40 (95% CI: 2.29–21.50) per 10,000 child-years in July 2007–June 2008 and July 2008–June 2009, respectively (). Overall, 67.7% and 87.58% reduction in RVGE hospitalizations was observed during July 2007–June 2008 and July 2008–June 2009, respectively, as compared with baseline period (July 1998–June 2006) in all children less than 5 y.
In the NT, the baseline incidence of RVGE hospitalizations among children aged 0–11 mo prior to the introduction of RV vaccination was 288.67 (95% CI: 269.26–309.11) per 10,000 child-years (). Following the introduction of RV vaccination, the incidence of RVGE hospitalizations reduced to 47.52 (95% CI: 28.17–75.11) and 122.79 (95% CI: 90.22–163.28) per 10,000 child-years, during July 2007–June 2008 and July 2008–June 2009, respectively. The overall reduction in RVGE hospitalizations compared with baseline period (July 1998–June 2006) was 67.41% and 45% during July 2007–June 2008 and July 2008–June 2009, respectively, in the NT among all children aged less than 5 y.
Overall reductions in AGE hospitalizations were also observed in all children less than 5 y of age, including those ineligible of RV vaccination in NSW and the ACT ( and ).
Acute bronchiolitis hospitalizations, used as a non-vaccine preventable disease, did not show any trend to reduction in children aged below 5 y following RIX4414 vaccine introduction (; and ).
Discussion
The present study describes the impact of RV vaccine (RIX4414) introduction onto the NIP of three Australian States namely NSW, the ACT and the NT on RVGE and AGE hospitalizations.
The findings of the study indicate that the decrease in incidence of RVGE and AGE hospitalizations in young children is directly related to the introduction of RIX4414 onto the NIP. Importantly, from a public health perspective, reductions in RVGE and AGE-associated hospitalizations were not only observed among children aged less than 24 mo, who were eligible for RV vaccination during the study period, but also in older children in NSW and the ACT, who would not have received the vaccine, indicating a potential herd effect. This aligns with the findings of earlier studies including a single-center study in Sydney,Citation20 a multi-center study conducted in NSW, Victoria and QLDCitation21 and studies conducted in the USA,Citation22,Citation23 all of which reported a herd effect.
The public health benefits of RIX4414 in Australia, in terms of reduction in RVGE and AGE hospitalizations, are substantive in all of the regions studied. In NSW and the ACT immunization rates are high and the burden of RV disease is typical of that seen in a developed country setting. In the NT, the epidemiology of RV disease is different to that seen in the other regions of Australia. It has a significantly greater burden of RV disease with the highest rate of RV-associated hospitalizations and the longest median hospital stay being recorded. This has also been previously reported and attributed to several factors such as the high proportion of children of indigenous decent, where the lower health and nutritional status, remoteness of residence of the population, affect susceptibility, diagnosis and treatment of the disease, thereby increasing the disease burden.Citation24
Despite the high burden of RVGE, achieving high vaccine coverage rates among Indigenous children in the NT is highly challenging as many children live in remote communties.Citation25 Webby and coworkers found that in the NT the percentage of Indigenous children receiving RIX4414 in 2007 was 68.4% as compared with 77.8% in non-indigenous children. While in 2008, 69.0% of indigenous children received RIX4414 as compared with 84.5% of non-indigenous children.Citation25
Although environmental factors affected the RVGE and AGE hospitalizations in the NT, the introduction of RV vaccination reduced the incidence of RVGE and AGE hospitalizations considerably, as compared with the baseline period during both surveillance years (July 2007–June 2008 and July 2008–June 2009). The incidence of RVGE and AGE hospitalizations among vaccine-eligible children was slightly greater in second year of surveillance as compared with the first due to an outbreak of acute RVGE that occurred during February–April 2009 in the central region of the NT–a region where large cyclical outbreaks occur on a regular basis.Citation26 As the burden of RVGE in the NT is substantially higher than in NSW and the ACT, particularly in an outbreak year, the differential between the NT and the findings in NSW and the ACT may correspond to those observed in the efficacy trials in Africa,Citation7 AsiaCitation9 and Europe.Citation8 These trials indicated that the efficacy of RV vaccines was higher in lower RVGE disease environments. The challenges of vaccine coverage in the NT are likely to be a factor in the lower impact seen in this region. Due to the annual fluctuation in the incidence of RVGE in the NT, it may take several years to be confident before we understand the true impact of the vaccine in this population. In addition to RVGE, it would be of value to understand the role co-pathogens play in gastroenteritis in the NT where the incidence of disease caused by these pathogens is greater in Indigenous children as compared with children in NSW and the ACT.
Unlike in NSW and the ACT, no reduction in the incidence of RVGE hospitalizations in children too old to have been vaccinated was observed in the NT. This apparent absence of herd immunity maybe due to the high disease burden and early onset of RVGE in the NT resulting in almost all unvaccinated children being exposed to wild-type RV, early in their lives and developing a natural immunity, thereby masking the capacity of the vaccine to confer herd immunity in this specific population. During the outbreak in the NT, it was of note that a high burden of disease was seen in children at a very young age, indicating that early vaccination and early course completion maybe of value in this setting.
Although this study evaluated the impact of RIX4414 alone on RVGE and AGE hospitalizations, the results of the present surveillance are comparable with those observed earlier which assessed the impact of RIX4414 and RV5 in various Australian states/territories including NSW, Victoria, QLD, Western Australia and South Australia and the NT.Citation27,Citation28 Introduction of two RV vaccines, namely RIX4414 and RV5 considerably reduced the RV-associated hospitalizations.Citation27,Citation28
The strength of this study was the inclusion of a non-vaccine preventable disease, namely acute bronchiolitis, the use of which is not routine in studies that assess RV vaccine impact. The data on acute bronchiolitis addressed one of the confounding factors and was included to observe the trend in “non-vaccine preventable seasonal disease” following the introduction of RV vaccines. If a reduction in this disease was observed, it can be considered that other factors could have affected the decrease in the hospitalizations and not attributable to vaccination alone. Since no reduction in acute bronchiolitis hospitalizations was seen, we could deduce that any reduction in RVGE related hospitalization is most likely due to the vaccine itself. Furthermore, no unexpected changes in acute bronchiolitis hospitalizations were observed over the entire study period, it appears that the reduction in the incidence of infectious disease in the study population was not universal. These results consequently improve the confidence that the reductions in RVGE and AGE that were observed were related to the introduction of RV vaccines.
However, the results of the present study need to be interpreted with caution in light of several potential limitations. First, assigning a mean value, where hospitalization data was suppressed (i.e., where less than five hospitalizations in the month were recorded), could have affected the accuracy of the results observed in this study. However, sensitivity analyses suggest the effect of assigning a mean value would be small. Second, the precision of this study could have been affected by sensitivity of International Classification of Diseases (ICD) codes to reflect the burden of disease. Previous reports indicate that use of ICD codes for RVGE may lead to under-reporting and as a result, the true incidence of RVGE and the effect of RV vaccine might be higher than observed.Citation29,Citation30 In addition, ICD codes may not be uniformly applied in different locations, which could limit the value of any comparisons between states. Lack of information on the individual vaccination status of children enrolled in this study is another limitation that is acknowledged. The RVGE outbreak in the NT during the post-vaccination era, where increased number of RVGE and AGE hospitalizations occurred, might not be a true representative of the true impact of the RV vaccine in this region over time. It is also important to note that such outbreaks are more typically localized to central region of the NT. Moreover, in the central region of the NT, where gastrointestinal co-pathogens are not uncommon in Indigenous children, it is important that we better understand the causation of the GE.
In conclusion, this ecological study demonstrates substantial reductions in RVGE and AGE-associated hospitalizations and a decrease in incidence of RVGE and AGE hospitalizations in children in NSW, ACT and the NT following the introduction of RV vaccination onto the Australian NIP. A reduction in incidence of RVGE and AGE hospitalizations was also observed in children ineligible for vaccination in NSW and the ACT indicating a potential herd effect.
Long-term analysis of the impact of RV vaccination, particularly in the NT, is also warranted to further evaluate the public health impact of RV vaccination in Australia.
Materials and Methods
Study design and study population
This ecological study was conducted in the Australian states/territories that implemented RIX4414 namely NSW, ACT and the NT. The impact of RV vaccination on RVGE and AGE hospitalizations was assessed using data extracted from the AIHW database. The numerator consisted of data from the AIHW on children who received RIX4414 and reported RVGE, AGE and acute bronchiolitis, while the denominator consisted of data from the Australian Bureau of Statistics consisting of the total number of children screened. RVGE and AGE cases were coded according to the International Classification of Diseases, 10th Revision, Australian Modification (ICD-10-a.m.) codes.
Data on RVGE, AGE, and acute bronchiolitis-associated hospitalizations from July 1998 to June 2009 among children aged less than 5 y residing in NSW, the ACT and the NT was extracted from the AIHW databases.
Acute bronchiolitis was chosen as it was a common cause of hospitalization in young children, it was a non-vaccine preventable disease and it exhibited seasonal variation.
Since the data collected from the AIHW and ACIR databases were aggregated and de-identified (subject-level information was not accessed) in the present study, no ethics committee approval or informed consent was required.
This study was conducted as per the guidelines of Good Epidemiological Practices and as per the principles of the Declaration of Helsinki and all applicable subject privacy requirements.
Statistical analyses
The period before the introduction of RV vaccines in Australia from July 1998 to June 2006 was considered as baseline/pre-vaccine introduction period, while the post vaccine introduction periods were from July 2007 to June 2008 and July 2008 to June 2009.
Incidence of RVGE, AGE and acute bronchiolitis hospitalizations were calculated with their respective 95% CI. The method of calculating 95% CIs was using the relation between Poisson distribution and chi square distribution.Citation31
The overall reduction was calculated by computing the difference between the average baseline value (pre-vaccination time points) and the post-vaccination time- points (July 2007 to June 2008 and July 2008 to June 2009) among all children (0–59 mo) with RVGE/ AGE hospitalizations, dividing by the average baseline value and expressed as percentage.
Sensitivity analyses
In the source data, when the total number of monthly hospitalizations for RVGE was very low, i.e., one to four (inclusive) admissions/month, the number of hospitalizations for that month were suppressed (i.e., no data provided) by the AIHW in order to protect patient privacy.
The suppressed data was coded as two cases/month in the analyses (the mean of one to four was 2.5; the minimum rounding off of 2.5 was two).
Trademark statement
Rotarix is a registered trademark of the GlaxoSmithKline group of companies. Rotateq is a trademark of Merck and Co., Inc.
| Abbreviations: | ||
| ACT | = | Australian Capital Territory |
| ACIR | = | Australian Childhood Immunisation Register |
| AGE | = | all-cause gastroenteritis |
| AIHW | = | Australian Institute of Health and Welfare |
| CI | = | confidence intervals |
| GE | = | gastroenteritis |
| ICD | = | International Classification of Diseases |
| NIP | = | National Immunisation Program |
| NSW | = | New South Wales |
| NT | = | Northern Territory |
| QLD | = | Queensland |
| RV | = | rotavirus |
| RVGE | = | rotavirus gastroenteritis |
Acknowledgments
This study (eTrack: 114910) was sponsored by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA also funded all costs associated with the development of the manuscript. ClinicalTrials.gov Identifier: NCT01236066
The authors would like to thank the Departments of Health of Northern Territory, New South Wales and Australian Capital Territory for kindly providing access to the data used in this study. The authors are grateful to Deloitte Access Economics Pty Ltd for the data request and analysis. The authors also thank Harshith Bhat for medical writing and Lakshmi Hariharan for editorial assistance and coordination in the development of the manuscript (both employed by the GlaxoSmithKline group of companies).
Disclosure of Potential Conflicts of Interest
CC, SPN, EL, and GR are employed by the GlaxoSmithKline group of companies. AP, MG, and YL were employed by the GlaxoSmithKline group of companies at the time of study. MG has received money for travel related to the presentation of the results from this study. SPN and EL hold shares of GlaxoSmithKline.
References
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9:565 - 72; http://dx.doi.org/10.3201/eid0905.020562; PMID: 12737740
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136 - 41; http://dx.doi.org/10.1016/S1473-3099(11)70253-5; PMID: 22030330
- Galati JC, Harsley S, Richmond P, Carlin JB. The burden of rotavirus-related illness among young children on the Australian health care system. Aust N Z J Public Health 2006; 30:416 - 21; http://dx.doi.org/10.1111/j.1467-842X.2006.tb00456.x; PMID: 17073221
- WHO. Wkly Epidemiol Rec 2007; 82:285-96. Accessed November 21, 2012 from: http://www.who.int/wer.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al, Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23 - 33; http://dx.doi.org/10.1056/NEJMoa052664; PMID: 16394299
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al, Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11 - 22; http://dx.doi.org/10.1056/NEJMoa052434; PMID: 16394298
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289 - 98; http://dx.doi.org/10.1056/NEJMoa0904797; PMID: 20107214
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757 - 63; http://dx.doi.org/10.1016/S0140-6736(07)61744-9; PMID: 18037080
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 2009; 27:5936 - 41; http://dx.doi.org/10.1016/j.vaccine.2009.07.098; PMID: 19679216
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615 - 23; http://dx.doi.org/10.1016/S0140-6736(10)60755-6; PMID: 20692031
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606 - 14; http://dx.doi.org/10.1016/S0140-6736(10)60889-6; PMID: 20692030
- Vesikari T, Karvonen A, Ferrante SA, Ciarlet M. Efficacy of the pentavalent rotavirus vaccine, RotaTeq®, in Finnish infants up to 3 years of age: the Finnish Extension Study. Eur J Pediatr 2010; 169:1379 - 86; http://dx.doi.org/10.1007/s00431-010-1242-3; PMID: 20559656
- Kirkwood CD, Boniface K, Bishop RF, Barnes GL. Australian Rotavirus Surveillance Program: annual report, 2009/2010. Commun Dis Intell Q Rep 2010; 34:427 - 34; PMID: 21413527
- Rotavirus vaccines for Australian children. 2009. Accessed November 21, 2012 from: http://www.ncirs.edu.au/immunisation/fact-sheets/rotavirus-fact-sheet.pdf.
- Barnes GL, Bishop RF. Rotavirus vaccine--time to act. Med J Aust 2006; 185:352 - 3; PMID: 17014400
- Hull BP, Deeks S, Menzies RI, McIntyre P. Immunisation coverage annual report, 2007. Commun Dis Intell Q Rep 2009; 33:170 - 87; PMID: 19877535
- Hull BP, Mahajan D, Dey A, Menzies RI, McIntyre PB. Immunisation coverage annual report, 2008. Commun Dis Intell Q Rep 2010; 34:241 - 58; PMID: 21090180
- Newall AT, MacIntyre R, Wang H, Hull B, Macartney K. Burden of severe rotavirus disease in Australia. J Paediatr Child Health 2006; 42:521 - 7; http://dx.doi.org/10.1111/j.1440-1754.2006.00915.x; PMID: 16925538
- The Australian Technical Advisory Group on Immunisation (ATAGI) and the National Health and Medical Research Council. (NHMRC) (2008) Australian Immunisation Handbook, 9th edition, 2008:265-73. Accessed November 21, 2012 from: http://immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook-rotavirus.
- Macartney KK, Porwal M, Dalton D, Cripps T, Maldigri T, Isaacs D, et al. Decline in rotavirus hospitalisations following introduction of Australia’s national rotavirus immunisation programme. J Paediatr Child Health 2011; 47:266 - 70; http://dx.doi.org/10.1111/j.1440-1754.2010.01953.x; PMID: 21244557
- Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr Infect Dis J 2011; 30:Suppl S25 - 9; http://dx.doi.org/10.1097/INF.0b013e3181fefdee; PMID: 21183837
- Payne DC, Szilagyi PG, Staat MA, Edwards KM, Gentsch JR, Weinberg GA, et al. Secular variation in United States rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program. Pediatr Infect Dis J 2009; 28:948 - 53; http://dx.doi.org/10.1097/INF.0b013e3181a6ad6e; PMID: 19859013
- Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis 2010; 201:1617 - 24; http://dx.doi.org/10.1086/652403; PMID: 20402596
- Schultz R. Rotavirus gastroenteritis in the Northern Territory, 1995-2004. Med J Aust 2006; 185:354 - 6; PMID: 17014401
- Webby R, Nagy C, Krause V, Darwin CDC. Childhood immunisation coverage and timeliness in the NT. The Northern Territory Disease Control Bulletin 2009; 16:12 - 5
- Cook H, Krause V. A review of G2P[4] rotavirus outbreaks in Central Australia and how the introduction of Rotarix® has affected the epidemiology. The Northern Territory Disease Control Bulletin 2010; 17:14 - 21
- Lambert SB, Faux CE, Hall L, Birrell FA, Peterson KV, Selvey CE, et al. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med J Aust 2009; 191:157 - 60; PMID: 19645646
- Dey A, Wang H, Menzies R, Macartney K. Changes in hospitalisations for acute gastroenteritis in Australia after the national rotavirus vaccination program. Med J Aust 2012; 197:453 - 7; http://dx.doi.org/10.5694/mja12.10062; PMID: 23072242
- Riordan FA, Quigley T. Estimating hospital admissions due to rotavirus gastroenteritis from hospital episode statistics. J Infect 2004; 49:13 - 6; http://dx.doi.org/10.1016/j.jinf.2004.02.006; PMID: 15194242
- Hsu VP, Staat MA, Roberts N, Thieman C, Bernstein DI, Bresee J, et al. Use of active surveillance to validate international classification of diseases code estimates of rotavirus hospitalizations in children. Pediatrics 2005; 115:78 - 82; PMID: 15629984
- Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol 1990; 131:373 - 5; PMID: 2296988