Abstract
The 2009 influenza A(H1N1) pandemic strain was for the first time included in the 2010–2011 seasonal trivalent influenza vaccine (TIV). We conducted a double-blind, randomized trial in Chinese population to assess the immunogenicity and safety of the 2010–2011 TIV manufactured by GlaxoSmithKline and compared it with the counterpart vaccines manufactured by Sanofi Pasteur and Sinovac Biotech. Healthy toddlers (6–36 mo), children (6–12 y) and older adults (≥60 y) with 300 participants in each age group were enrolled to randomly receive two doses (toddlers, 28 d apart) or one dose (children and older adults). The immunogenicity was assessed by hemagglutination-inhibition (HI) assay. The solicited injection-site and systemic adverse events (AEs) were collected within 7 d after vaccination. All the three TIVs were well-tolerated with 15.1% of participants reporting AEs, most of which were mild. No serious AEs and unusual AEs were reported. Fever and pain were the most common systemic and injection-site AEs, respectively. The three TIVs showed good immunogenicity. The seroprotection rates against both H1N1 and H3N2 strains were more than 87% in toddlers after two doses and more than 95% in children and more than 86% in older adults after one dose. The seroprotection rates against B strain were 68–71% in toddlers after two doses, 70–74% in children and 69–72% in older adults after one dose. In conclusion, the three 2010–2011 TIVs had good immunogenicity and safety in Chinese toddlers, children and older adults and were generally comparable in immunogenicity and reactogenicity.
Introduction
Influenza is one of the most common acute respiratory tract diseases that causes substantial disease burden in population at high risk. Seasonal trivalent influenza vaccine (TIV) consisting of influenza A(H1N1), A(H3N2) and B viruses has been used for more than three decades, and annual vaccination is considered to be the most effective way to reduce the disease burden.Citation1,Citation2 However, influenza viruses undergo continuous changes in their surface antigens, resulting in the immunity induced by the current influenza vaccine not necessarily protective against the viruses circulating in the future. Hence, new influenza vaccines must be designed annually to match the circulating viruses, which are expected to cause the next epidemic.Citation3
In April 2009, an emerging influenza A(H1N1) virus had caused a pandemic global as declared by the World Health Organization (WHO) on June 11, 2009.Citation4 Many monovalent 2009 influenza A(H1N1) vaccines had been developed, which through mass immunization, had shown effectiveness in combating this pandemicCitation5 and contributed to the termination of the pandemic after August 2010.Citation6 In the post-pandemic period, WHO expected the 2009 influenza A(H1N1) virus to take on the behavior of a seasonal influenza virus and continue to circulate for some years. Therefore, a trivalent influenza vaccine containing 2009 A(H1N1) pandemic strain was recommended by WHO for the 2010–2011 influenza season.Citation7
The monovalent 2009 influenza A(H1N1) vaccines had been well-evaluated in Chinese population.Citation8,Citation9 However, the 2010–2011 TIV containing 2009 influenza A(H1N1) antigen had not been assessed in Chinese population. Furthermore, since the emerging 2009 influenza A(H1N1) virus had a unique combination of genes that had not previously been identified in human or swine populations,Citation10 the inclusion of a genetically and antigenically novel strain into the traditional TIV for the first time warranted more study.
In this study, we aimed to assess the immunogenicity and safety of the 2010–2011 TIV manufactured by GlaxoSmithKline and meanwhile compared it with other two 2010–2011 TIVs manufactured by Sanofi Pasteur and Sinovac Biotech in Chinese toddlers, school-aged children and older adults. To the best of our knowledge, this was the first study comparing three licensed 2010–2011 TIVs head-to-head.
Results
Study population
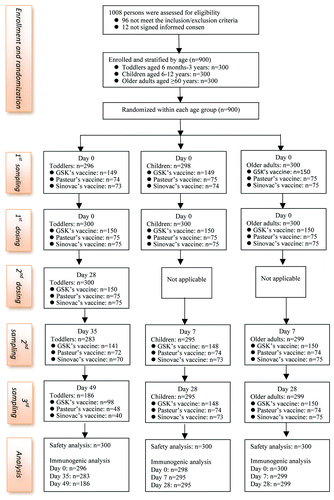
All the 900 participants received the first dose, completed the safety observation and were included in total vaccinated cohort for safety analysis. All the 300 toddlers received the second dose and were included for safety analysis of the second dose. Before vaccination, blood samples were collected from 894 participants due to incompliance with the sampling by 6 participants or their guardians. After vaccination, 877 and 780 participants donated the second samples (day 35 for toddlers, day 7 for children and older adults) and third samples (day 49 for toddlers, day 28 for children and older adults), respectively. Immunogenic analysis included those participants for whom HI titers against the three strains were available (). The demographic details of the participants are summarized in . All the participants were Chinese ethnically. No statistical differences were found among the three arms within each age group with respect to the age and sex ratio (p = 0.223–0.960).
Table 1. Demographic characteristic of the enrolled participants
Safety
All the three TIVs were well tolerated without immediate systemic allergic reactions or serious adverse events. The adverse events are shown in . Overall, a total of 136 participants (15.1%) reported adverse event with mild and moderate being 12.8% and 2.3%, respectively. No severe adverse events were reported. The proportions of participants having adverse events among the three vaccine arms were 15.3%, 15.1% and 14.7% with no significant difference (p = 0.974). In toddlers, the second dose did not increase the frequency of adverse events (6.3% vs. 4.3%, p = 0.271). Across the three age groups, pain (5.9%) was the most common injection-site adverse events followed by swelling (1.3%), and fever (6.1%) was the most common systemic adverse events followed by fatigue (1.7%). Other solicited adverse events were less common. All the adverse events were resolved by the end of the study. During the study, three unsolicited adverse events were reported but not causally related to vaccination.
Table 2. Participants with adverse events (total vaccinated cohort)
Immunogenicity
The baseline immunity is shown in . Across the three age groups, the seroprotection rates against H1N1, H3N2 and B were 33–37%, 37–45% and 5–8%, respectively, and the baseline GMTs against H1N1, H3N2 and B varied from 17.5–20.9, 14.5–24.3 and 4.5–5.5, respectively. The pre-existing immunity against B was significant lower than those against H1N1 and H3N2. Within each age group, there was no statistical difference among the three vaccine arms with respect to seroprotection rates and GMTs against all the three vaccine strains (p = 0.245–0.992).
Table 3. Baseline immunity in toddlers, children and older adults
All the 300 toddler participants received two doses on day 0 and 28. Seven days after the second dose (day 35), the seroconversion rates and seroprotection rates were at least 24.3% and 42.9%, respectively. Although the highest seroconversion and seroprotection rates were induced by GSK’s TIV, statistical difference among the three arms was shown only in seroconversion rate against H1N1 (p = 0.032). Multiple comparison did not find statistical differences between any two arms (p = 0.025–0.802, α’ = α/m = 0.05/3 = 0.017 according to Bonferroni adjustment). Twenty-one days after the second dose (day 49), the antibody level increased further. The three TIVs showed similar immunogenicity with respect to GMT, serocoversion rate and seroprotection rate. The seroconversion rates were more than 40% except for those against B in GSK’s TIV and Pasteur’s TIV arms. The seroprotection rates were more than 70% except for that against B in Sinovac’s TIV arm ().
Table 4. Immune response in toddlers
The children and older adults received one dose. Seven days after dosing, obvious immune response was observed in both age groups especially against H1N1 and H3N2 (). In children, the seroprotection rates against H1N1 and H3N2 were all more than 70%. In older adults, the seroprotection rates against H1N1 and H3N2 were all more than 60%, and the seroconversion rates against the two strains were all more than 30%. Twenty-eight days after vaccination, the antibody level increased further. In children, except for against B in Sinovac’s TIV arm, the seroconversion rates were all more than 40% and seroprotection rates were all more than 70%. In older adults, the seroconversion rates were all more than 30% and seroprotection rates were all more than 60%. Although statistical differences in GMT (p = 0.031) and seroconversion rate (p = 0.019) among the three arms were observed in children against H1N1 seven days after vaccination, no statistical differences among the three arms were observed in children and older adults against H1N1, H3N2 and B strains 28 d after vaccination with respect to GMT, seroconversion rate and seroprotection rate. Multiple comparison only found statistical higher sercoconversion rate elicited by GSK’s TIV than Pasteur’s TIV (48.7% vs. 31.1%, p = 0.013, α′ = α/m = 0.05/3 = 0.017).
Table 5. Immune response in children and older adults
Discussion
The 2009 influenza A(H1N1) pandemic strain was different from previously circulating influenza A(H1N1) strains in annual epidemics with respect to genetic and antigenic characteristics. How would the inclusion of the genetically and antigenically novel strain impact on the safety and immunogenicity of 2010–2011 TIV is worthy of more study. In this study, we assessed the immunogenicity and safety of the 2010–2011 TIV manufactured by GSK and compared with the counterpart vaccines manufactured by Pasteur and Sinovac head-to-head in various age groups. Our study showed that the three TIVs had good safety profile in Chinese toddlers, school-aged children and older adults. During the study, 15.1% of participants reported adverse events, most of which were mild. No severe adverse,events, SAE and unusual adverse events were reported. This finding in safety profile was consistent with previous studies conducted in ethnically different populations.Citation11,Citation12 The inclusion of 2009 influenza A (H1N1) pandemic strain in 2010–2011 seasonal vaccine did not change the safety characteristics as seen with previous seasonal influenza vaccine.
Before vaccination, a significant proportion of the participants had seroprotective titer against H1N1 (33–37%) and H3N2 (37–45%). The level of pre-existing immunity was not completely unexpected taking into account previous vaccination with monovalent 2009 influenza A(H1N1) vaccine and/or 2009–2010 TIV or previous infection with the relevant strains. This may also account for the results that the toddlers, children and older adults had similar pre-existing seroprotective rates against H1N1. In addition, the pre-existing seroprotective rate in this study was similar to that found in a previous study where seroprotective rates of 27% against H1N1 and 54% against H3N2 were observed in population aged ≥61 y,Citation12 and lower than that found in another study where seroprotective rates of 54–74% against H1N1 and 73–80% against H3N2 were observed in population aged 3 to 17 y.Citation13 Our study indicated that the three 2010–2011TIVs manufactured by different companies were able to elicit robust immune response to H1N1 and H3N2 even though a significant part of the participants had pre-existing immunity against the two strains.
A rapid immune response elicited 7–8 d after TIV vaccination was reported previously.Citation14-Citation16 By adding a blood sampling point 7 d after completion of vaccination, we also intended to investigate whether a rapid immune response could be elicited. Our results showed that a rapid immune response to H1N1 and H3N2 strains was indeed induced 7 d after completion of vaccination with seroprotection rates of more than 74% in toddlers and children and more than 66% in older adults. However, a rapid immune response to B strain seemed not to be induced 7 d after completion of vaccination. Many studies with seasonal TIVs adopted two-dose schedule given at day 0 and 21 for toddlers. In our study, the toddlers received two injections at day 0 and 28, which was in line with the licensed product labels of the three vaccines in China. The timing for the second post-vaccination samples in toddlers, i.e., 21 d after the second dose, followed the EU CHMP recommendation. In children and older adults, the second post-vaccination samples were collected 28 d after vaccination that followed the practice in most vaccine trials.
For the yearly evaluation of licensed seasonal influenza vaccines, the EU CHMP released criteria for adults aged from 18 to 60 y and older adults aged more than 60 y.Citation17 This study showed that 7 d after receiving one of the three TIVs the immunity in older adults met EU CHMP criteria against H1N1 and H3N2 but not against B. Twenty-eight days after one dose, the immunity in older adults elicited by all the three TIVs against all the three strains met the criteria. Because of no EU CHMP criteria for population under 18 y old, we temporarily adopted EU CHMP criteria for adults to assess the serological performance in toddlers and children. In toddlers, the antibody against H1N1 and H3N2 but not B met EU CHMP criteria 7 d after the second dose, and met the criteria against all the three strains 21 d after the second dose, regardless of the vaccines used. In children, all the three TIVs induced immune response against H1N1 and H3N2 satisfying the criteria 7 d after one dose, and against all the three strains satisfying the criteria 28 d after one dose. It seemed that B antigen was less immunogenic than H1N1 and H3N2 antigens regardless of the manufacturers and age groups, which was also observed in other trials.Citation11,Citation12
Twenty-one days after the second dose (day 49), 114 (38%) toddlers who were still available for follow-up did not provide their blood samples for immunogenic analysis because their parents declined to have them bled again. Potential bias in age, gender and baseline immunity was not introduced (data not shown) partially because the 114 toddlers equally distributed in the three arms (35%, 36% and 47% of participants in each arm).
To the best of our knowledge, this was the first study comparing three licensed 2010–2011 TIVs head-to-head in Chinese population. Our study showed that all the three TIVs had good immunogenicity in various age groups. Overall, the three TIVs had very similar immunogenicity regarding most of the indicators. The immunogenicity against H3N2 and B strains did not show statistical difference among the three arms, regardless of the participants’ age. Although statistical differences in immunogenicity against H1N1 among the three arms were found, multiple comparison only found statistical higher sercoconversion rate elicited by GSK’s TIV than Pasteur’s TIV. The underlying causes contributing to the differences were not clearly known. Since the three TIVs were manufactured using the same seed virus and contained the same content of hemaglutinin of each strain, the antigens itself were less possible to contribute to the differences in immunogenicity. Furthermore, the difference in immunogenicity did not necessarily mean clinical relevance.
In conclusion, our study found that the three 2010–2011 seasonal trivalent influenza vaccines manufactured by different companies had good immunogenicity and safety profile in Chinese toddlers, school-aged children and older adults and were generally similar with respect to immunogenicity and reactogenicity.
Materials and Methods
We conducted a double-blind and randomized trial including three arms to assess the immunogenicity and safety of the 2010–2011 TIV manufactured by GlaxoSmithKline and used other two 2010–2011 TIVs manufactured by Sanofi Pasteur and Sinovac Biotech as active controls in Chinese healthy population.
Participants
A total of 900 participants were enrolled and stratified into three age groups with 300 each (toddlers: 6 to 36 mo; school-aged children: 6 to 12 y; and older adults: ≥60 y). The participants were recruited in two sites: toddlers recruited in Sanhe city of Hebei province; children and older adults recruited in Baotou city of Inner Mongolia autonomous region. Eligible participants were generally healthy or had stable chronic medical conditions (for older adults only). The exclusion criteria included: history of allergic reaction to any component of the study vaccines or previous influenza vaccine; history of systemic hypersensitivity to hens’ eggs; history of Guillain Barré syndrome following any influenza vaccination; any immunodeficient or immunocompromised conditions; receipt of cytotoxic or immunosuppressive drugs within the past 6 mo; receipt of blood-derived product within the past 3 mo; receipt of any vaccine within one month prior to study entry with exception of pediatric routine vaccination; receipt of 2010–2011 seasonal TIV; participation in any other study with a non-approved drug during the study. Acute febrile disease and other self-limiting illness were the temporary exclusion criteria.
The study was conducted in accordance with Good Clinical Practice and the Helsinki Declaration and registered with the ClinicalTrials.gov identifier NCT01654809. The written informed consent was obtained from all participants or their guardians. All relevant documents were approved by the ethical review committee of Beijing Chaoyang District Center for Disease Control and Prevention.
Vaccines
The vaccines used in this study were three 2010–2011 TIVs manufactured by GlaxoSmithKline, Sanofi Pasteur and Sinovac Biotech, which were commercially available on China market and produced by similar, established procedures in embryonated hens’ eggs using the WHO-recommended H1N1, H3N2 and B virus strains for the northern hemisphere which were A/California/7/2009-like (H1N1), A/Perth/16/2009-like (H3N2) and B/Brisbane/60/2008-like viruses.Citation7 Two formulations were used. Adult formulation contained 15 μg of hemagglutinin of each strain in 0.5 mL dose and pediatric formulation contained 7.5 μg of hemagglutinin of each strain in 0.25 mL dose. All the vaccines were non-adjuvanted, split-virion, prefilled formulation. A single lot of each vaccine was used. The vaccines were stored and shipped at 2 °C to 8 °C.
Procedures
The randomization was done by age stratification. All the vaccine doses used for each age stratum were subject to randomization in a ratio of 2:1:1 (GSK: Pasteur: Sinovac). A blocked (block size of 4) randomization list was generated by a statistician not involved in the rest of the trial using SAS (version 9.0). For the purpose of masking, all the randomized vaccine doses used for each age stratum were packaged in identical appearance and blindly labeled with sequential numbers which were the only identifier. Each participant within each age group was assigned a sequential number according to their sequence of enrolment. Participants received vaccines labeled with the same sequential numbers. Therefore, the participants were randomly assigned to receive one of the three TIVs in a ratio of 2:1:1. All investigators and participants were kept blinded to the treatment allocation.
Children and older adults were given one dose (0.5 mL) and toddlers were give two doses (0.25 mL per dose) on day 0 and day 28. Vaccines were administered intramuscularly in deltoid region. Participants were kept to observe immediate adverse events (AEs) for 30 min after injection. The solicited AEs (the specified AEs to be recorded as endpoints in the clinical study and its occurrence and intensity were actively solicited from the participants or their parents during a specified follow-up period) were recorded daily within 7 d after vaccination by using diary cards, which would be reviewed by researchers to assure their completeness and accuracy. Participants were also asked to report any unsolicited AEs (any AEs reported in addition to those solicited during the clinical study) within 28 or 21 d after each dosing. Adverse events were coded and graded according to the scales issued by the Division of Microbiology and Infectious Diseases pediatric and adult toxicity table.Citation18,Citation19 For the toddlers too young to complain about injection-site pain, headache and myalgia, the following procedures were used to collect these events: mild, minor reaction on touch or no limitation of activity; moderate, cries/protests on touch or observable impairment of activity; severe, cries when limb/head is moved or marked impairment of activity. The event fatigue was not solicited in toddlers. Auxillary temperatures of 37.1–37.5 °C, 37.6–39.0 °C and higher than 39.0 °C were graded as mild, moderate and severe fever, respectively.
For the immunogenic assessment, blood samples were collected from children and older adults on day 0 (immediately before vaccination), day 7 and day 28, and from toddlers on day 0, day 35 (7 d after the second dose) and day 49 (21 d after the second dose).
Serological assay
All serum samples were assayed by hemagglutination-inhibition (HI) method against the homologous strains. The assay was done in accordance with established procedures as reported previously.Citation20 Briefly, serum samples were treated with receptor-destroying enzyme (cholera filtrate, Sigma) at 36 °C for 16 h before titration, and then tested in 2-fold dilution starting with a 1:10 dilution. The titers were expressed as the reciprocal of the highest dilution that showed complete inhibition of hemagglutination. All samples were blindly assayed in duplicate and double-checked. For the purpose of calculation, HI titers below 1:10 were assigned an arbitrary value of 1:5.
Statistical analysis
The sample size was estimated to fulfill the European Union Committee for Medicinal Products for Human Use (EU CHMP) criteria for the evaluation of seasonal TIV.Citation17 A sample size of 61 participants per group was needed to give a power of at least 80% on the basis of the assumption of 85% for seroprotection rate in vaccinated participants to fulfill the criteria (70%) for seroprotection rate issued by the EU CHMP. This study used the TIV of GSK as experimental vaccine and the TIVs of Pasteur and Sinovac as active controls; therefore, a ratio of 2:1:1 (GSK: Pasteur: Sinovac) in sample size was set to maintain a balance between experimental vaccine and active controls. To allow for incompliance and drop-out, a sample size of 150 participants per group in each age strata was determined for the experimental vaccine group and 75 participants for the active control groups.
The safety analysis was expressed as the incidence and severity of injection-site and systemic adverse events, and the incidence of serious adverse events (SAE). The incidence and grade of an adverse event were calculated based on the highest intensity if same symptom with two or more intensity was reported. The safety analysis was performed on the total vaccinated cohort.
In line with the licensure criteria set out by the EU CHMP, the immunogenic outcomes used in this study were HI antibody responses to each vaccine strain including seroconversion rate and seroprotection rate.Citation17 Seroconversion rate is defined as the percentage of vaccines who have a titer before vaccination of less than 1:10 and a titer after vaccination of 1:40 or more, or a titer before vaccination of 1:10 or more and at least 4-fold increase after vaccination. HI titer ≥ 1:40 is considered as seroprotection. The antibody titers were transformed into logarithmic scale for the calculation of geometric mean titer (GMT).
The results of immunogenicity and safety were presented with point estimates and two-sided 95% confidence intervals (CI). Chi-square test or Fisher Exact test was used for analyzing categorical data when relevant. Student’s t-test or ANOVA was used for analyzing continuous data when relevant. The statistical analysis was conducted by an independent statistician. Hypothesis testing was two-sided with α value of 0.05. Bonferroni adjustment was used for multiple comparison, by which the α’ value was adjusted by comparison time.
| Abbreviations: | ||
| TIV | = | trivalent influenza vaccine |
| AE | = | adverse event |
| SAE | = | serious adverse event |
| HI | = | hemagglutination-inhibition |
| GMT | = | geometric mean titer |
| SCR | = | seroconversion rate |
| SPR | = | seroprotection rate |
| CI | = | confidence intervals |
| GSK | = | GlaxoSmithKline |
| WHO | = | World Health Organization |
| EU CHMP | = | European Union Committee for Medicinal Products for Human Use |
Acknowledgments
This study was funded by an unrestricted research grant from Beijing Centers for Diseases Control and Prevention that participated in study design. We appreciated all the investigators from Sanhe Center for Disease Control and Prevention in Hebei province and Baotou Center for Disease Control and Prevention in Inner Mongolia autonomous region for their contribution to the study. We appreciated Dr. Yuan-Zheng Qiu for his help in the manuscript writing and revision.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Bridges CB, Katz JM, Levandowski RA, Cox NJ. Inactivated influenza vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Amsterdam: Elsevier Inc, 2008:259-90.
- Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al, Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56:RR-6 1 - 54; PMID: 17625497
- World Health Organization. WHO position paper on influenza vaccines. Wkly Epidemiol Rec 2005; 33:279 - 87
- World Health Organization. DG Statement following the meeting of the Emergency Committee. http://www.who.int/csr/disease/swineflu/4th_meeting_ihr/en/index.html (accessed September 18, 2012)
- Wu J, Xu F, Lu L, Lu M, Miao L, Gao T, et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. N Engl J Med 2010; 363:2416 - 23; http://dx.doi.org/10.1056/NEJMoa1006736; PMID: 21158658
- World Health Organization. H1N1 in post-pandemic period. http://www.who.int/mediacentre/news/statements/2010/h1n1_vpc_20100810/en/index.html (accessed September 18, 2012)
- World Health Organization. Recommended viruses for influenza vaccines for use in the 2010-2011 northern hemisphere influenza season. Wkly Epidemiol Rec 2010; 85:81 - 92; PMID: 20210260
- Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2010; 375:56 - 66; http://dx.doi.org/10.1016/S0140-6736(09)62003-1; PMID: 20018364
- Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med 2009; 361:2414 - 23; http://dx.doi.org/10.1056/NEJMoa0908535; PMID: 19846844
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197 - 201; http://dx.doi.org/10.1126/science.1176225; PMID: 19465683
- Langley JM, Scheifele DW, Quach C, Vanderkooi OG, Ward B, McNeil S, et al. Safety and immunogenicity of 2010-2011 H1N12009-containing trivalent inactivated influenza vaccine in children 12-59 months of age previously given AS03-adjuvanted H1N12009 pandemic vaccine: a PHAC/CIHR Influenza Research Network (PCIRN) study. Vaccine 2012; 30:3389 - 94; http://dx.doi.org/10.1016/j.vaccine.2012.03.046; PMID: 22469860
- Loebermann M, Anders G, Brestrich G, Fritzsche C, Klammt S, Boršo D, et al. Safety and immunogenicity of a trivalent single dose seasonal influenza vaccine containing pandemic A(H1N1) antigen in younger and elderly subjects: a phase III open-label single-arm study. Vaccine 2011; 29:1228 - 34; http://dx.doi.org/10.1016/j.vaccine.2010.11.092; PMID: 21167116
- Tregnaghi MW, Stamboulian D, Vanadía PC, Tregnaghi JP, Calvari M, Fragapane E, et al. Immunogenicity, safety, and tolerability of two trivalent subunit inactivated influenza vaccines: a phase III, observer-blind, randomized, controlled multicenter study. Viral Immunol 2012; 25:216 - 25; PMID: 22691101
- Künzel W, Engelmann H, D’Hondt E. Immune response to influenza vaccination. Lancet 1994; 343:173; http://dx.doi.org/10.1016/S0140-6736(94)90962-8; PMID: 7904017
- Künzel W, Glathe H, Engelmann H, Van Hoecke C. Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine 1996; 14:1108 - 10; http://dx.doi.org/10.1016/0264-410X(96)00061-8; PMID: 8911005
- Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine 1994; 12:993 - 9; http://dx.doi.org/10.1016/0264-410X(94)90334-4; PMID: 7975853
- European Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96). European Agency for the Evaluation of Medicinal Products, 1997.
- Division of Microbiology and Infectious Diseases. DMID pediatric toxicity tables. http://www.niaid.nih.gov/LabsAndResources/resources/DMIDClinRsrch/Documents/dmidpedtox.pdf (accessed September 19, 2012)
- Division of Microbiology and Infectious Diseases. DMID adult toxicity tables. http://www.niaid.nih.gov/LabsAndResources/resources/DMIDClinRsrch/Documents/dmidadulttox.pdf (accessed September 19, 2012)
- World Health Organization. WHO manual on animal influenza diagnosis and surveillance. Geneva: The World Health Organization 2002.