Abstract
Rotavirus is the most common cause of severe gastroenteritis in children under 5 y of age. Estimates of disease burden in Japan suggest that between 26,500 and 78,000 children in this age group need hospitalization each year, resulting in a direct medical cost of 10 to 24 billion Yen. Since being introduced in routine infant immunization schedules in the United States in 2006, the oral live pentavalent rotavirus vaccine RV5 (RotaTeq™) has contributed to dramatic reductions in the incidence of rotavirus gastroenteritis (RVGE) and in health care resource utilization. This was a multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of a 3-dose regimen of RV5 in healthy infants, age 6 to 12 weeks, at 32 sites across Japan. The results indicate that RV5 was significantly efficacious in preventing any severity [74.5% (95% confidence interval [CI]: 39.9%, 90.6%; p < 0.001)], moderate-to-severe [80.2% (95% CI: 47.4%, 94.1%)], and severe [100% (95% CI: 55.4%, 100%)] RVGE caused by viruses with serotypes contained in the vaccine. The observed cases of RVGE included rotavirus types G1 (n = 19), G3 (n = 9), G9 (n = 5) and one unspecified G serotype with P1A[8]. No G2 or G4 RVGE cases were observed, and this study was not powered to evaluate efficacy against individual serotypes. RV5 was generally safe and well tolerated in Japanese infants. These results are comparable to those observed in clinical studies conducted in other developed countries. Introduction of the vaccine in Japan may reduce disease burden and associated health care costs.
Introduction
Rotavirus is the most common cause of severe gastroenteritis in children less than 5 y of age in both developing and developed countries.Citation1 In 2008, rotavirus was estimated to account for 453,000 annual deaths (95% CI: 420,000–494,000) in children less than 5 y of age, with most deaths occurring in the developing world.Citation2
In the Rotavirus Efficacy and Safety Trial (REST) (Merck protocol V260–006) a total of nearly 70,000 infants were evaluated including half who received the live pentavalent rotavirus vaccine, RV5 (RotaTeqTM, Merck and Co., Inc.). This pivotal trial demonstrated a vaccine efficacy of 74.0% against any severity rotavirus gastroenteritis (RVGE) and 98.0% against severe RVGE, caused by viruses with serotypes G1, G2, G3 and G4.Citation3 In addition, hospitalizations were reduced by 95% for RVGE caused by any rotavirus serotype through the first 2 y of life after the third dose.Citation3 RV5 was generally well tolerated with respect to clinical adverse experiences. Of note, no increased risk of intussusception was observed for the vaccine compared with placebo.Citation3
RV5 has proven to be highly effective during routine use in the United States since licensure in 2006.Citation4-Citation8 Multiple rotavirus surveillance systems in the US have demonstrated a delay in the rotavirus season and dramatic reductions in gastroenteritis-related health care resource utilization and incidence of RVGECitation7,Citation9,Citation10 since introduction of RV5 to the routine infant immunization schedule in 2006.Citation11
In 2007, a universal RV5 vaccine program was initiated in Queensland, Australia. RV5 coverage in the first eligible birth cohort was 89.6% for at least 1 dose and 73.1% for 3 doses. The program provided immediate and sustained reductions in rotavirus-associated hospitalizations for people less than 20 y of age and also appeared to impact hospitalizations for acute gastroenteritis (AGE) that was not identified as due to rotavirus in children less than 5 y of age based on evaluation of medical records for International Classification of Diseases (ICD) codes for AGE including rotavirus in the primary or any secondary diagnostic field.Citation12 The observed vaccine effectiveness for 3 doses of RV5 was 89.3% to 93.9% (any/primary diagnosis) for rotavirus hospitalizations.Citation12 In addition to this direct evidence of protection from RV5, there is also evidence of indirect herd protection.Citation13 Similarly, clinical study results from developing countries in Asia and Africa demonstrate that RV5 was efficacious, immunogenic, and generally well tolerated in healthy infants, although efficacy results were lower than those observed in developed countries.Citation14 Of note, the efficacy of RV5 against severe RVGE was 51.0% in developing countries in Asia (Bangladesh, Vietnam) and 64.2% in Africa (Ghana, Kenya, Mali).Citation14,Citation15
Each year in Japan, approximately 26,500 to 78,000 children less than 5 y of age are hospitalized owing to RVGE, resulting in a direct medical cost of at least 10 billion YenCitation16,Citation17 in addition to an estimated 800,000 doctor office visits each year for children less than 6 y of age.Citation18 Of note, epidemiological data regarding RVGE hospitalizations in Japan are limited with a broad range of estimated incidence, perhaps due to temporal and regional differences in the available studies. Recently, the economic burden of RVGE in Japan was calculated to be 24 billion Yen, which includes direct and indirect costs of hospitalizations and outpatient visits.Citation19 The aim of this study was to evaluate the efficacy and safety of RV5 in healthy Japanese infants.
Results
A total of 762 infants were randomized to the RV5 or placebo group and 761 received at least 1 dose of vaccine. Comparable numbers of participants from each group completed the study (96.6% in RV5 and 96.1% in placebo, respectively) (). Additionally, baseline characteristics of the RV5 and placebo groups were similar (). Consistent with the protocol design and global product label at the time the study was conducted, all subjects in both treatment groups received their first dose of RV5 or placebo between 6 to 12 weeks of age. The mean age at dose 2 for subjects in the RV5 group and in the placebo group was 12.5 ± 2.6 weeks and 12.4 ± 2.5 weeks, respectively. Similarly, the mean age at dose 3 for subjects in the RV5 group and in the placebo group was 18.7 ± 3.7 weeks and 18.5 ± 3.7 weeks, respectively.
Figure 1. Study disposition. Data are represented as n (%) unless otherwise noted. aIncludes 1 subject who was randomized but did not receive the study vaccine by the investigator’s decision. bCompleted: the number of subjects who continued follow-up until the last study visit (visit 5) regardless of the number of vaccinations received. cSubjects who had <3 vaccinations or <28 d between vaccinations, who received oral poliovirus vaccine or Bacille Calmette-Guérin within 27 d of any dose. dSubjects were classified as unevaluable owing to wild-type rotavirus-positive test results prior to 14 d post dose 3, incomplete clinical and/or laboratory results or stool samples collected out of the day range (e.g., within 7 d after the onset of symptoms).
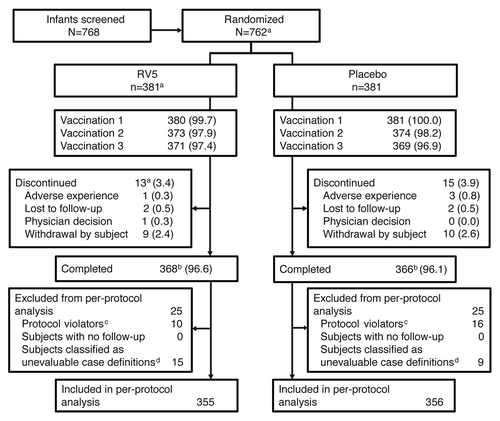
Table 1. Baseline characteristics of participants
The study was designed as a case-driven study with a sample size intended to provide a minimum total number of 30 cases of RVGE due to any of the vaccine-related types. Results of the primary endpoint, efficacy of RV5 against RVGE of any severity caused by viruses with serotypes contained in the vaccine, i.e., G1, G2, G3, G4 and G serotypes associated with P1A[8] (e.g., G9), occurring at least 14 d post dose 3 relative to placebo, are shown in . Of note, the predominant serotype seen was G1; there were few cases of RVGE due to G3 and G9 and none due to G2 or G4 rotavirus observed in this study. The overall vaccine efficacy for the primary endpoint was 74.5% (95% CI: 39.9%, 90.6%; p < 0.001). Median follow-up was 191 d and 189 d for the RV5 and placebo groups, respectively.
Table 2. Vaccine efficacy estimates against rotavirus gastroenteritis at least 14 d post dose 3
Results of secondary endpoints demonstrate that the vaccine efficacy was 80.2% (95% CI: 47.4%, 94.1%) against moderate-to-severe and 100% (95% CI: 55.4%, 100%) against severe cases of RVGE caused by viruses with serotypes contained in the vaccine, i.e., G1, G2, G3, G4, and G serotypes associated with P1A[8] (e.g., G9), occurring at least 14 d post dose 3 (). Moreover, efficacy estimates of the vaccine against RVGE due to any naturally occurring rotavirus (regardless of serotype) occurring at least 14 d following the third dose was 75.3% (95% CI: 42.2%, 90.9%) for any severity RVGE and was 81.0% (95% CI:49.6%, 94.3%) and 100% (95% CI: 55.2%, 100%) for moderate-to-severe and severe RVGE, respectively ().
Of the 34 participants with cases of RVGE meeting the primary endpoint in this study, 19 were caused by serotype G1, 9 were caused by serotype G3, and 5 were caused by the G9 serotype; one was caused by an unspecified G serotype. All serotypes contained P1A[8] except for one case caused by the G1 serotype for which the P serotype was not able to be determined. As noted above, no cases of RVGE due to G2 or G4 rotavirus were observed in this study. Although the study was not powered to determine the individual efficacy against each of the types in the vaccine, the serotype-specific efficacy was demonstrated to be 81.4% (95% CI: 35.1%, 96.5%) against serotype G1, 20.0% (95% CI: < 0%, 84.1%) against serotype G3, and 100.0% (95% CI: < 0%, 100%) against serotype G9. The vaccine efficacy for G1 was statistically significant; for serotypes G3 and G9, there were too few cases for a statistically meaningful estimate.
A review of health care resource utilization shows 3 participants in the placebo group were hospitalized or made an emergency department visit owing to RVGE caused by viruses with serotypes contained in the vaccine, i.e., G1, G2, G3, G4, and G serotypes associated with serotype P1A[8] (e.g., G9), compared with 0 participants in the RV5 group. The efficacy estimate of RV5 against hospitalization and emergency department visits for RVGE was 100% (95% CI: < 0%, 100%) (p = 0.249). This estimate is not statistically significant owing to the low number of cases, although numerically fewer participants utilized health care resources in the RV5 group compared with placebo. To evaluate clinical evidence of dehydration and/or administration of rehydration therapy, dehydration positive was defined as a World Health Organization score ≥2 owing to RVGE, which included 1 participant in the RV5 group and 7 in the placebo group. The vaccine efficacy estimate against RVGE- related dehydration was 85.7% (95% CI: < 0%, 99.7%). Moreover, 4 participants in the placebo group required rehydration therapy and 0 required rehydration therapy in the RV5 group.
During this trial, no deaths occurred within 14 d following vaccination; 1 subject in the RV5 group died on day 36 post dose 2 owing to respiratory syncytial virus bronchiolitis, which was considered not related to the study vaccination by the site investigator. Importantly, there were no reports of intussusception during the course of this trial or in the follow-up period. No participants in the RV5 group were discontinued because of a clinical adverse experience. The most common (≥10% in any treatment group) clinical adverse experiences occurring during the 14-d follow-up period were diarrhea (12.1% RV5 and 12.3% placebo) and nasopharyngitis (9.7% RV5 and 8.7% placebo). The percentages of participants with prespecified (Tier 1 and Tier 2) clinical adverse experiences are given in . Of note, there were similar reports of clinical adverse experiences between the 2 study groups (49.7% RV5 and 50.1% placebo). The incidence of Tier 1 events, including diarrhea, elevated temperature (≥38.1 °C, rectal equivalent), behavioral change (irritability) and vomiting within 7 d following any vaccination was low and was not statistically different in the 2 treatment groups. The percentage of participants with vaccine-related clinical adverse experiences, defined as those clinical adverse experiences judged as related to the study vaccine by the investigator, was 14.5% in the RV5 group, which was higher than the placebo group (8.9%) based on the 95% confidence interval of the treatment group difference. All vaccine-related clinical adverse experiences in the RV5 group were mild or moderate and resolved without discontinuation. A total of 7 participants (1.8%) in the RV5 group and 9 participants (2.4%) in the placebo group had serious clinical adverse experiences within 14 d following any vaccination, all of which were considered not related to the study vaccination. Likewise in infants who were premature at birth, defined as gestation ≤ 36 weeks of age (n = 20 RV5, n = 11 placebo), the incidence of clinical adverse experiences were generally comparable between the groups. No specific clinical adverse experiences or vaccine-related adverse experiences were observed in this population.
Table 3. Comparison of the incidence of prespecified Tier 1 and Tier 2 clinical adverse experiences
Discussion
The epidemiology of naturally occurring rotavirus disease in humans shows that rotavirus types can vary across geographical areas and from one season to the next. One cannot predict which strains will predominate in any given rotavirus season. The primary endpoint prespecified in this study was efficacy against RVGE caused by rotavirus types represented in the vaccine, i.e., G1, G2, G3, G4 and G types associated with P1A[8]. From an epidemiological and historical basis, these combined types represent over 90% of the rotavirus observed globally to cause disease in humans.
In this study, the RV5 vaccine was efficacious in preventing RVGE in Japan, decreasing severe disease and health care resource utilization. Results () of this study are similar to those obtained in clinical trials from other developed countries like REST, where vaccine efficacy was demonstrated to be 74.0% (95% CI: 66.8%, 79.9%), 81.7% (95% CI: 75.0%, 86.8%) and 98.0% (95% CI: 88.3%, 100%) against any, moderate-to-severe and severe RVGE, respectively, caused by viruses with serotypes G1, G2, G3 and G4 as a combined group.Citation3
Serotype-specific protection against rotavirus has also been demonstrated for RV5 in earlier larger studies. Based upon combined data from the pivotal Phase III studies (REST and the Finnish Extension study), the reduction up to 3 y post-vaccination in the rate of hospitalizations and emergency department visits for RVGE was 94.4% (95% CI: 91.6%, 96.2%) for genotypes G1–G4, 95.5% (95% CI: 92.8%, 97.2%) for genotype G1, 81.9% (95% CI: 16.1%, 98.0%) for genotype G2, 89.0% (95% CI: 53.3%, 98.7%) for genotype G3, 83.4% (95% CI: 51.2%, 95.8%) for genotype G4 and 94.2% (95% CI: 62.2%, 99.9%) for genotype G9.Citation20
The current study was designed as a case-driven study with a sample size intended to provide a minimum of 30 total primary endpoint cases of RVGE due to any of the vaccine-related types combined. However, this study was not powered to determine the individual efficacy against each of the types in the vaccine, and the feasibility of doing so is dependent upon observing a sufficient number of cases of each of the rotavirus types occurring naturally during the course of the study.
Globally, G1P1A[8] is the most common rotavirus type causing gastroenteritis in humans, and this was the predominant type observed in this study in Japan. Of the 34 participants with cases of RVGE meeting the primary endpoint in this study, 19 were caused by serotype G1, 9 were caused by serotype G3, and 5 were caused by the G9 serotype; 1 case was caused by an unspecified G serotype. All serotypes contained P1A[8] except for 1 case caused by the G1 serotype for which the P serotype could not be determined. No cases of RVGE due to G2 or G4 rotavirus were observed in this study. Serotype-specific efficacy was demonstrated to be 81.4% (95% CI: 35.1%, 96.5%) against serotype G1, 20.0% (95% CI: < 0%, 84.1%) against serotype G3, and 100.0% (95% CI: < 0%, 100%) against serotype G9. The vaccine efficacy for G1 was statistically significant; for serotypes G3 and G9, there were too few cases for a statistically meaningful estimate.
Additionally, in this study RV5 has demonstrated a good safety profile and was generally well tolerated in Japanese participants, including a small number of premature infants. The overall safety profile was consistent with the global trial REST. Of note, there was no report of intussusception during the course of this study, including the follow-up period, although this study was underpowered to demonstrate any association with intussusception.
RV5 is likely to provide a much needed benefit in Japan to reduce the burden of RVGE-related hospitalizations and emergency department visits. Furthermore, these results in Japan confirmed the potential for high vaccine efficacy in Asia, which is similar to other developed world settings and appears higher than the observed efficacy in developing settings in Asia, such as those observed in Bangladesh and Vietnam (Merck Protocol V260-015).Citation14 The methodology of the clinical trials in the developing world was different (case capture method), hence the data cannot be directly compared. Data from studies conducted in other countries support the concomitant use of RV5 with routine pediatric vaccines. However, at the time of this study, general guidelines in Japan did not recommend administering routine infant vaccines concomitantly with an investigational vaccine. Therefore, concomitant use of RV5 with routine pediatric vaccines in Japan was not evaluated in the present study, and is currently being investigated.
Globally, nearly all children are infected by rotavirus by 5 y of age, regardless of socioeconomic status.Citation21 Implementation of a rotavirus vaccination program in Japan will prevent severe cases of RVGE and reduce disease burden in infants and the burden to their families. Likewise, such a program could reduce the burden on health care workers and associated health care costs. Among RVGE hospitalizations which occur in children younger than 5 y of age in Japan, only 4% arise in those ≤6 mo of age,Citation22 by which time rotavirus vaccination would be completed, protecting against most cases of RVGE. Observational studies conducted in the United States, which has a similar socioeconomic setting to Japan, have shown sustained protection against RVGE by vaccination until 2 to 3 y of age.Citation10 Universal administration of an effective and safe vaccine may considerably reduce the burden associated with RVGE morbidity, mortality, and health care costs.
Materials and Methods
Participants and study design
This was a randomized, double-blind, placebo-controlled, parallel-group, comparative, multicenter study (Merck protocol V260-029) to evaluate the efficacy and safety of the 3-dose regimen of oral RV5 in healthy Japanese infants 6 to 12 weeks of age at enrollment. The study was conducted from August 2008 to August 2009 at 32 sites across Japan. Sites were selected based on Merck Sharp and Dohme Corp. (MSD), a subsidiary of Merck and Co., Inc., standard operating procedures and in accordance with Good Clinical Practice (GCP). MSD provided site and study feasibility questionnaires to medical institutions with known experience in pediatric clinical research studies in Japan. Sites were selected based on the potential number of eligible subjects, site infrastructure, investigators’ experience, cost, and compliance to GCP. Of the sites selected for this study, 22 were hospitals with pediatric departments and 10 were pediatric clinics. Most of the subjects were born in these hospitals or in obstetric facilities near the study sites. Investigators recruited the subjects by introducing the clinical study during routine medical evaluations or by displaying posters and/or leaflets within the facilities. Participants were identified by their medical condition and interest in participation. All vaccinations were performed by the investigators.
Healthy infants, aged 6 through 12 weeks at the time of the first dose, who had not received any prior rotavirus vaccination, were eligible to partake in the study with written informed consent from parents or guardians who were available for follow-up for safety by telephone, e-mail, or clinic visit following vaccination. Infants were excluded if they had a history of congenital abdominal disorders, intussusception, abdominal surgery, RVGE, chronic diarrhea, growth disorders or active gastrointestinal illness. Infants were also excluded if they had received oral poliovirus vaccine (OPV) or Bacille Calmette-Guérin (BCG) within 27 d prior to the first dose of the study vaccine or placebo for consistency with Japanese guidelines for prophylactic vaccination. The study was approved by the institutional review board of each site and conducted according to good clinical practice standards and to the Declaration of Helsinki guidelines. Written informed consent was obtained from the parents or guardians of all participants. This study is registered with ClinicalTrials.gov, number NCT00718237.
Procedures
Infants were assigned in a 1:1 ratio using a computer-generated randomization schedule provided by Merck to receive 3 oral doses of 2 mL of RV5 or placebo at 28- to 70-d intervals between each dose with the third dose given by 32 weeks of age. Treatment groups were blinded against parents or guardians, investigators, study coordinators, study-related personnel, and the sponsor (including external agencies). Blinding was maintained using the randomization code. RV5 contains 5 human-bovine reassortant rotavirus strains: G1, G2, G3, G4, and P1A[8].Citation3 The placebo contained the same buffered solution as RV5 without the viral constituents. The first dose was administered at visit 1, between 6 and 12 weeks of age and the third (last) dose was administered by 32 weeks of age. With the exception of OPV and BCG, there were no restrictions on the concomitant administration of other approved routine pediatric vaccines (e.g., diphtheria, tetanus and pertussis vaccine; hepatitis B vaccine; Haemophilus influenzae type b vaccine) during this study. Simultaneous vaccination of RV5 and BCG has not been fully evaluated. A previous study demonstrated concomitant vaccination with OPV had negligible effect on the safety and assumed efficacy of concomitant use of RV5.Citation14 However, in the current study concomitant vaccination with either OPV or BCG was prohibited for consistency with Japanese guidelines for prophylactic vaccination. All other approved pediatric vaccines were provided as standard of care as determined by the investigator. The study vaccine or placebo was allowed to be administered without regard to feeding (i.e., fed or fasting conditions). Of note, subjects in the placebo group did not receive vaccine at the end of the study because they were not age-eligible for RV5; at the time the study was conducted, the approved package circular for RV5 required that all 3 doses be administered by 32 weeks of age with a minimum of 4 weeks between doses.
Subjects were seen in person at several scheduled study visits for receipt of vaccination (visits 1–3) in addition to a vaccination completion follow-up visit (visit 4) and a final completion visit (visit 5). Parents or guardians were contacted by telephone or e-mail on days 8 and 15 following each dose of vaccine/placebo to discuss AGE and associated health care utilization as well as other clinical adverse experiences. Visits 2 and 3 occurred 28 to 70 d after the prior vaccine dose was received and visit 4 occurred 14 to 24 d post dose 3. After visit 4, the occurrence of gastroenteritis was monitored biweekly during the rotavirus season (January 1 through June 30) and every 4 weeks thereafter via telephone or e-mail until the end of the study period (visit 5). After each vaccination visit, parents or guardians were instructed to complete a vaccine report from the time of each vaccination (day 1) through day 14. The report card required the parent or guardian to record certain specific symptoms including body temperature (rectal whenever possible) and the frequency of episodes of vomiting and diarrhea every day for 7 d following each vaccination. Other adverse experiences (including behavioral changes) and concomitant drugs and/or vaccination administered were also recorded for 14 d following each vaccination. An acute gastroenteritis report card (AGRC) as well as a stool-sampling kit was also provided following each vaccination for completion in the event a subject showed symptoms of an AGE. Parents or guardians were instructed to complete the AGRC, which documented body temperature, symptoms and use of any medication, daily, until symptom resolution. They were also instructed to collect a stool sample within 3 d and not later than 7 d after the onset of symptoms. A description of symptoms that could be present in the event of potential AGE that were provided to parents included 1 or more watery stools or 3 or more looser-than-normal stools within a 24-h period and/or forceful vomiting. The stool sample was to be kept refrigerated and submitted directly to the study site within 72 h of collection. Samples were stored frozen at or below –20 °C at the study site. Stool samples were screened for the presence of rotavirus antigen by enzyme immunoassay at the Cincinnati Children’s Hospital Medical Center. All positive samples were further tested using polymerase chain reactions to identify the rotavirus serotype (i.e., G and P types using VP7 and VP4 assays, respectively) and to distinguish between vaccine virus strain and wild-type rotavirus (VP6 assay) at PPD Vaccine and Biologics Labs, a group of Pharmaceutical Product Development (PPD), LLC. All clinical adverse experiences were collected for 14 d following each dose. Information on deaths, serious vaccine-related clinical adverse experiences, and events of clinical interest (intussusception) occurring during the entire study period were also recorded. A case was defined as ≥ 3 watery or looser-than-normal stools with a 24-h visit and/or single forceful vomiting and detection of wild-type RV in a stool specimen taken within 7 d after the onset of symptoms. Follow-up was continued until the end of the first RV infection season after enrollment of the first subject. The last visit occurred after the number of subjects with RVGE (number of events) reached the target number (30) necessary for primary evaluation.
Determination of RVGE severity
The RVGE severity scoring system was adapted from that used by Duffy et al. and calculated based on the frequency and duration (number of days) of diarrhea, vomiting and elevated temperature (≥38.1 °C, rectal equivalent) and behavioral change (irritability).Citation23 This scoring system was validated and shown to correlate strongly with physician assessment of the severity of gastroenteritis symptoms.Citation24 A score >8 and ≤16 points was designated moderate and >16 points was designated severe.
Study outcomes
The primary efficacy endpoint was the incident rate of RVGE of any severity caused by viruses with serotypes contained in the vaccine, i.e., G1, G2, G3, G4, and G serotypes associated with P1A[8] (e.g., G9), occurring at least 14 d following the third dose. RVGE was defined by both of the following criteria: ≥3 watery or looser-than-normal stools observed within a 24-h period and/or forceful vomiting (handled as AGE) and wild-type rotavirus detected in a stool sample collected within 7 d after the onset of symptoms. The primary safety endpoint was the safety of RV5 with respect to all clinical adverse experiences occurring within 14 d of any dosing in healthy Japanese infants.
Secondary endpoints included the incidence rate of moderate-to-severe and severe RVGE caused by viruses with serotypes contained in the vaccine, i.e., G1, G2, G3, G4, and G serotypes associated with P1A[8] (e.g., G9), occurring at least 14 d following the third dose. Another secondary measure was the incidence rate of RVGE caused by any naturally occurring rotavirus (regardless of serotype) occurring at least 14 d following the third dose.
The incidence rate of RVGE caused by an individual rotavirus serotype occurring at least 14 d following the third dose was a tertiary endpoint (descriptive), as was the presence of health care resource utilization for RVGE caused by viruses with serotypes contained in the vaccine, i.e., G1, G2, G3, G4, and G serotypes associated with P1A (e.g., G9), including hospital admission or outpatient treatment in an emergency department. The presence of clinical evidence of dehydration and/or the administration of rehydration therapy for episodes of RVGE caused by viruses with serotypes G1, G2, G3, G4, and G serotypes associated with P1A was also a predetermined objective.
Statistical analysis
The study was designed as a case-driven study with a sample size intended to provide a minimum required total number of primary endpoint cases of RVGE due to any of the vaccine-related rotavirus types, i.e., serotypes G1, G2, G3, G4, and G serotypes associated with P1A. The power to satisfy the primary efficacy hypothesis at the end of the study was based on results from the REST trial. The incidence of RVGE in Japan was estimated based on studies conducted in Asia because the true incidence in Japan was unknown. Assuming the incidence of RVGE in the placebo group was 10% and the vaccine efficacy was 73.8%, then at least 30 cases of RVGE would be expected if there were 298 subjects enrolled in each group. These values yield 92% power to detect an RV5 efficacy estimate higher than 0%; the planned number of subjects was set at 744 subjects or 372 per group, assuming an exclusion rate of 20% owing to protocol violations and loss of cases due to RVGE infection. Therefore, the critical point estimate of RV5 efficacy would be 57.1% with 9 subjects in the RV5 group and 21 subjects in the placebo group. Of note, the study was not powered to assess efficacy according to individual rotavirus serotypes, nor was it powered for safety measures, including intussusception.
The efficacy estimate of the vaccine was determined for the per-protocol population and defined as (1 − Rvaccine/Rplacebo) × 100%, where R is the incidence rate of RVGE in the respective groups and used an exact conditional 1-sided test for a binomial proportion given the total number of observed cases (α = 0.025). Ninety-five percent CIs for vaccine efficacy were calculated based on the exact 95% CIs for the binomial probability.
Although the study was not powered for safety measures, certain clinical adverse experiences were prespecified as Tier 1 and Tier 2 events. Diarrhea, elevated temperature (≥38.1 °C, rectal equivalent), behavioral change (irritability), and vomiting occurring within 7 d following any dose of the study vaccine were considered Tier 1 events. Tier 2 events included the categories of clinical adverse experiences consisting of the percentage of subjects with a clinical adverse experience, a vaccine-related clinical adverse experience, a serious adverse experience, a vaccine-related serious adverse experience, discontinued because of a clinical adverse experience, and discontinued because of a vaccine-related clinical adverse experience. P-values (Tier 1 only) and 95% CIs (Tier 1 and Tier 2) were provided for between-treatment differences in the percentage of subjects with events; these analyses were performed using the Miettinen and Nurminen method.
| Abbreviations: | ||
| AGE | = | acute gastroenteritis |
| BCG | = | Bacille Calmette-Guérin |
| CI | = | confidence interval |
| OPV | = | oral poliovirus vaccine |
| REST | = | Rotavirus Efficacy and Safety Trial |
| RV5 | = | RotaTeq™ |
| RVGE | = | rotavirus gastroenteritis |
Acknowledgments
We are indebted to the infants and their parents or guardians who participated in this study. We would like to express our deepest appreciation to the study investigators who had committed to the enrollment of subjects into this study. The authors gratefully acknowledge Max Ciarlet’s contribution to the design of the study. We acknowledge Tonya Goodman and Karen Collins, Arbor Communications, Inc., for manuscript preparation and editorial assistance on behalf of Merck Sharp and Dohme Corp., a subsidiary of Merck and Co., Inc.
Disclosure of Potential Conflicts of Interest
The study was designed by Merck and Co., Inc. (including MSD, K.K., formerly Banyu Pharmaceutical Co., LTD), with substantial input from site investigators. Merck had direct oversight or participation in every stage of the study. All authors had full access to the data after study completion and unblinding, and the corresponding author had final responsibility for the decision to submit for publication.
All investigators at the study clinical sites were funded through their institution to perform the study protocol. Dr. Iwata reports having received lecture fees and grant support from MSD and GlaxoSmithKline. Drs. Nakata and Kuroki report having received lecture fees from Merck and GlaxoSmithKline. Drs. Ukae, Koizumi and Morita have no conflicts of interest. Dr. Tanaka and Mr. Shizuya are employees of MSD, K.K., a group of Merck Sharp and Dohme Corp., a subsidiary of Merck and Co., Inc. Ms. Brown and Dr. Lawrence are employees of Merck and Co., Inc. Dr. Schödel was an employee of Merck and Co., Inc., while this clinical trial was ongoing. His current affiliation is Philimmune LLC.
References
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 2009; 200:Suppl 1 S9 - 15; http://dx.doi.org/10.1086/605025; PMID: 19817620
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136 - 41; http://dx.doi.org/10.1016/S1473-3099(11)70253-5; PMID: 22030330
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al, Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23 - 33; http://dx.doi.org/10.1056/NEJMoa052664; PMID: 16394299
- Bégué RE, Perrin K. Reduction in gastroenteritis with the use of pentavalent rotavirus vaccine in a primary practice. Pediatrics 2010; 126:e40 - 5; http://dx.doi.org/10.1542/peds.2009-2069; PMID: 20587671
- Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis 2010; 201:1617 - 24; http://dx.doi.org/10.1086/652403; PMID: 20402596
- Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics 2010; 125:e208 - 13; http://dx.doi.org/10.1542/peds.2009-1246; PMID: 20100757
- Centers for Disease Control and Prevention (CDC). Reduction in rotavirus after vaccine introduction--United States, 2000-2009. MMWR Morb Mortal Wkly Rep 2009; 58:1146 - 9; PMID: 19847149
- Boom JA, Tate JE, Sahni LC, Rench MA, Hull JJ, Gentsch JR, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 2010; 125:e199 - 207; http://dx.doi.org/10.1542/peds.2009-1021; PMID: 20083525
- Centers for Disease Control and Prevention (CDC). Delayed onset and diminished magnitude of rotavirus activity--United States, November 2007-May 2008. MMWR Morb Mortal Wkly Rep 2008; 57:697 - 700; PMID: 18583958
- Tate JE, Mutuc JD, Panozzo CA, Payne DC, Cortese MM, Cortes JE, et al. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J 2011; 30:Suppl S30 - 4; http://dx.doi.org/10.1097/INF.0b013e3181ffe3eb; PMID: 21183838
- Parashar UD, Alexander JP, Glass RI, Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:RR-12 1 - 13; PMID: 16902398
- Field EJ, Vally H, Grimwood K, Lambert SB. Pentavalent rotavirus vaccine and prevention of gastroenteritis hospitalizations in Australia. Pediatrics 2010; 126:e506 - 12; http://dx.doi.org/10.1542/peds.2010-0443; PMID: 20732946
- Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr Infect Dis J 2011; 30:Suppl S25 - 9; http://dx.doi.org/10.1097/INF.0b013e3181fefdee; PMID: 21183837
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615 - 23; http://dx.doi.org/10.1016/S0140-6736(10)60755-6; PMID: 20692031
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606 - 14; http://dx.doi.org/10.1016/S0140-6736(10)60889-6; PMID: 20692030
- Kamiya H, Nakano T, Inoue M, Kamiya H, Abd TT, Patel M, et al. A retrospective evaluation of hospitalizations for acute gastroenteritis at 2 sentinel hospitals in central Japan to estimate the health burden of rotavirus. J Infect Dis 2009; 200:Suppl 1 S140 - 6; http://dx.doi.org/10.1086/605028; PMID: 19817592
- Nakagomi T, Nakagomi O, Takahashi Y, Enoki M, Suzuki T, Kilgore PE. Incidence and burden of rotavirus gastroenteritis in Japan, as estimated from a prospective sentinel hospital study. J Infect Dis 2005; 192:Suppl 1 S106 - 10; http://dx.doi.org/10.1086/431503; PMID: 16088792
- Yokoo M, Arisawa K, Nakagomi O. Estimation of annual incidence, age-specific incidence rate, and cumulative risk of rotavirus gastroenteritis among children in Japan. Jpn J Infect Dis 2004; 57:166 - 71; PMID: 15329449
- Sato T, Nakagomi T, Nakagomi O. Cost-effectiveness analysis of a universal rotavirus immunization program in Japan. Jpn J Infect Dis 2011; 64:277 - 83; PMID: 21788701
- RotaTeq™, oral solution. Summary of product characteristics. EMEA/H/C/0669/IB/042. 16-Feb-2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000669/WC500054181.pdf. Accessed April 19, 2013.
- Vesikari T, Van Damme P, Giaquinto C, Gray J, Mrukowicz J, Dagan R, et al. European society for paediatric infectious diseases/European society for paediatric gastroenterology, hepatology, and nutrition evidence-based recommendations for rotavirus vaccination in Europe. J Pediatr Gastroenterol Nutr 2008; 46:Suppl 2 S38 - 48; http://dx.doi.org/10.1097/MPG.0b013e31816f7a10; PMID: 18460971
- Kamiya H, Nakano T, Kamiya H, Yui A, Taniguchi K, Parashar U, Rotavirus Epidemiology Study Group. Rotavirus-associated acute gastroenteritis hospitalizations among Japanese children aged <5 years: active rotavirus surveillance in Mie Prefecture, Japan. Jpn J Infect Dis 2011; 64:482 - 7; PMID: 22116326
- Duffy LC, Byers TE, Riepenhoff-Talty M, La Scolea LJ, Zielezny M, Ogra PL. The effects of infant feeding on rotavirus-induced gastroenteritis: a prospective study. Am J Public Health 1986; 76:259 - 63; http://dx.doi.org/10.2105/AJPH.76.3.259; PMID: 3004238
- Mast TC, Cai B, Heaton P, Staus WL. Development and validation of an acute gastroenteritis report card and scoring system to measure the clinical severity of acute gastroenteritis in infants. 22nd Annual Meeting of the European Society for Paediatric Infectious Diseases; 2004 May 26–28. Tampere, Finland. Abstract 155. Available at: http://meetings.espid.org/espid2004/program/session1.asp?SessionId=P07&SSessionDate=5/27/2004. Accessed March 17, 2013.