?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Influenza vaccine has to be reformulated each year due to the ever-changing antigenicity of the influenza virus. However, few post-licensure studies of influenza vaccine are available in China. We aimed to measure the effectiveness of seasonal influenza vaccine during 2 consecutive seasons. Among children in Guangzhou aged 6 to 59 mo in 2010–2012, we matched each child with clinically diagnosed influenza to 3 healthy children. Cases with clinically diagnosed influenza were identified from surveillance system. Healthy controls were randomly sampled from the Children’s Expanded Programmed Immunization Administrative Computerized System. Conditional logistic regression was used to calculate vaccine effectiveness (VE). A total of 275 matched sets of subjects were included. VE levels against clinically diagnosed influenza for both seasons combined was 47.4% [95% confidence interval (CI), 8.5–69.8%] for full vaccination for children aged 6–35 mo, 33.6% (95% CI, 5.4–53.5%) for any vaccination for children aged 6–59 mo, respectively. VE by time since vaccination for any vaccination was 34.6% (95% CI, 4.7–55.2%) in 0–5 mo, and no protection was observed in 6–11 mo. Annual, full and timely vaccination should be encouraged for children.
Introduction
Influenza has been a major threat to global health since the 1918 influenza pandemic, which killed approximately 50 million people. Seasonal influenza causes more than 250,000 deaths worldwide each year.Citation1 Of the 20,000 children less than 5 y old hospitalized annually because of influenza in the United States, 80% are less than 2 y old, suggesting that younger children are more susceptible to life-threatening complications, pneumonia, respiratory failure, uncontrolled asthma and myocarditis.Citation2 During 2008–2011, an annual average of 92 677 seasonal influenza cases were reported in China (clinically diagnosed and laboratory confirmed cases, excluding those from abroad, Hong Kong, Taiwan and Macao; the reported number is much lower than the true one. Annual National Report of National Notifiable Infectious Disease, Ministry of Health of China, 2008–2011). In the peak of the influenza, less than 80% of clinical cases are due to influenza virus. Feng reported that influenza-associated excess mortality, for all causes, was 11.3(range: 7.3–17.8) deaths per 100 000 people per year in five cities in southern China.Citation3
The World Health Organization (WHO) contends that annual vaccination prior to the influenza epidemic peak is currently the principal strategy for reducing the influenza mortality and morbidity burden in the community. Because of the constant mutation of influenza viruses,Citation4 influenza vaccines have to be reformulated annually based on the global surveillance of circulating viral strains. Moreover, the short time available for vaccine manufacture and variation in the timing of influenza outbreaksCitation5,Citation6 make it necessary to estimate influenza vaccine effectiveness (VE) annually.
Guangzhou is located in eastern Asia, which is the largest trading city of southern China. This city has a registered population of over 7.94 million people and a floating population (who are defined as beginning to live in Guangzhou for less than 6 mo and are usually hard to included in the official census count) of over 4.67 million people. This city plays an important role in connecting mainland China with other countries in southeast Asia. With a subtropical monsoon climate, viral influenza strains that persist in this region could potentially serve as year-round reservoirs of global genetic diversity.Citation7 The increasing frequency of travel and trade throughout the worldCitation1,Citation8 makes Guangzhou a high-influenza area. In addition, the annual supply of influenza vaccine has increased steadily in China, from 16.9 million doses in 2004–2005 to 32.5 million doses in 2008–2009, an average annual increase of 18%.Citation9,Citation10 The influenza vaccination coverage among children aged 0–23 mo is 17.47% in 32 districts all over China (randomly selected from 32 provinces and municipalities autonomous regions) in July to August 2011.Citation11
Seasonal influenza vaccine is administered to people aged over 6 mo. It is provided free of charge for children and the elders in some areas in China. However, the influenza vaccination is in Chinese Non-National Immunization Program, vaccination in children has to be paid by their guardians; the cost is likely to be a barrier to vaccination for most Guangzhou families. There are so far no recommended influenza immunization schedule available in China, the actual schedule is recommended as follows: one dose of vaccine is recommended for children aged 6–35 mo who receive 2 doses of vaccine in the previous season; two doses of influenza vaccine are recommended for children aged 6–35 mo who receive ≤1 dose of vaccine in the previous season; the second dose should be given 4 weeks after the first dose. And one dose of vaccine is recommended annually for people over 3 y old (The instructions of seasonal influenza vaccine in China). The recommended composition of influenza virus vaccines for use in the 2010–2011 and 2011–2012 northern hemisphere influenza seasons was the same: A/California/7/2009 (H1N1)-like virus, A/Perth/16/2009 (H3N2)-like virus and B/Brisbane/60/2008-like virus (Recommendations for Influenza Vaccination Composition, WHO, 2012).
In China, the level of protection of influenza vaccines is mostly estimated by pre-licensing study; true protection in the general population can be estimated only from post-licensure effectiveness studies.Citation5,Citation12,Citation13 However, few post-licensure effectiveness studies are available in China, especially in pediatric populations.Citation14 Therefore, we conducted a matched case-control study to estimate the effectiveness of seasonal influenza vaccine against clinically diagnosed influenza in 2010–2011 and 2011–2012. We also examined whether VE was similar in the two seasons. Finally, because the influenza vaccine had the same composition in both seasons, we estimated VE by time since vaccination.
Results
Demographic characteristics of study subjects
During the 2010–2012 seasons we included 275 (73.3% of 375) clinically diagnosed influenza cases from the Children’s Expanded Programmed Immunization (EPI) Administrative Computerized System, 36 (73.5% of 49) in 2010–2011 season, 239 (73.3% of 326) in 2011–2012 season, respectively. A total of 100 (26.7% of 375) cases were excluded because of missing vaccination records or loss to follow-up. The included cases did not differ from excluded cases with respect to age and area of residence (p = 0.170, p = 0.997, respectively). However, the included cases were more likely to be male than the excluded cases (65.2% vs. 49.5%, p = 0.050).
A total of 275 matched sets of cases and controls (1100 total subjects) were included during the 2 consecutive influenza seasons in the study. Cases mainly were male and lived in rural areas (). The median age of influenza onset was 29.5 mo.
Table 1. Demographic characteristics of study subjects [n(%)]
The controls had a higher percentage of full vaccination or vaccination than the cases except partial vaccination (21.1% vs. 12.8% and 18.2% vs. 10.7% in 2010–2012, respectively) ().
Vaccine effectiveness
VE in both seasons combined was 47.4% [95% confidence interval (CI), 8.5–69.8%] for full vaccination for children aged 6–35 mo, 33.6% (95% CI, 5.4–53.5%) for any vaccination for children aged 6–59 mo, no protective effect was observed for partial vaccination (). VE for vaccination was 55.8% (95% CI, -32.6 to 85.2%) in 2010–2011 season, 30.1% (95% CI, -1.9 to 52.0%) in 2011–2012 season.
Table 2. Influenza vaccine effectiveness by vaccination status and age group in 2010–2012
VE by time since vaccination for any vaccination was 34.6% (95% CI, 4.7–55.2%) in 0–5 mo, 26.1% (95%CI, -92.3 to 71.6%) in 6–11 mo.
Discussion
We estimated influenza VE using a matched case-control study with 275 clinically diagnosed influenza cases and 825 healthy matched controls identified during the 2010–2012 influenza seasons. We found a relatively low protection for full vaccination or vaccination in 2010–2012 against clinically diagnosed influenza for children aged 6 to 59 mo. VE by time since vaccination in 0–5 mo is 34.6%, with no protective effect noted for 6–11 mo. A contributing factor to the lack of statistical power was the small sample size in the VE for children aged 36–59 mo (11 cases), and in 6–11 mo (5 cases).
A post-licensure study of influenza vaccine in China which was similar to us was based on laboratory-confirmed cases and healthy children without acute respiratory infection (ARI) symptoms (e.g., cough or sore throat) in 2009 and 2010.Citation14 The authors reported influenza VE of 51.8% and 57.8% for full vaccination in 2009 and 2010, respectively. The VE in our study is lower than theirs.
We believe that several factors may play a role in the variable levels of VE. The major determinant of the different VEs is the degree to which circulating and vaccine influenza strains match. A better match would likely result in a higher VE. Szilagyi et al. published a case-cohort study of influenza VE in 2003–2004 and 2004–2005 in several United States counties.Citation13 No statistically significant VE was noted for vaccinated children. They attributed the lack of protective effect to a poor match between circulating and vaccine influenza strains. Based on viral surveillance data in Guangzhou (unpublished), the A(H1N1, H3N2) and B subtypes for in 2010–11 accounted for 83.52% and 16.48%, respectively; in 2011–12 accounted for 48.25% and 51.75% respectively. Unfortunately, we could not obtain data on the exact circulating influenza strains in Guangzhou to estimate the match degree between the circulating and vaccine strains in two seasons. The different approaches used in observational studies could also affect the VE estimates.Citation15 Kelly et al. reported influenza VE of 58% when using influenza negative controls, while VE of 68% when using those with another virus present as controls.Citation16 The ever-changing antigenicity and interseasonal variation of the influenza virus, along with the characteristics of the study population,Citation12 also should be considered.
Our study has several limitations. First, we used clinical influenza as cases other than laboratory confirmation. Using clinical influenza as an endpoint is not as specific as laboratory confirmation,Citation15 which will misclassify non-influenza respiratory virus infections as influenza cases. The non-influenza respiratory virus infections are more likely to received vaccines since the influenza vaccine cannot provide protection against non-influenza respiratory virus, which may underestimate VE. According to the majority studies with laboratory-confirmed cases, more than 20% of the clinical influenza cases can be due to other respiratory viruses except influenza virus in the peak of influenza. Thus, it is expected that a higher VE would be estimated by using laboratory-confirmed cases, which would be more than 1.2 times of the estimate in this study.
Second, healthy controls from community may be less comparable with cases than test-negative controls in comorbidity and the use of health care services. Selection bias may introduce by selecting controls from the children’s EPI Administrative Computerized System, because the floating children who never received vaccines in Guangzhou are unlikely to be register in the system, that is to say, these children are unlikely to be included in our study, which may lead to a negative effect to the result.
Third, for incomplete data, we did not evaluate chronic medical conditions (e.g., chronic pulmonary, renal, hepatic, metabolic disorder, immunosuppressive conditions) and severity of influenza cases (cases with complications), which can lead to an underestimate VE, because influenza vaccine may more efficiently for those children. And household register is another potential confounder we did not evaluate, local and floating children may markedly different from socio-economic status, which could result in positive or negative biases.
Another limitation of this study is that the VE estimates based on cases from the influenza sentinel surveillance hospitals may not be representative of all the influenza cases in Guangzhou. Only 275 eligible cases were included in our study, which were only a very small proportion of influenza cases in Guangzhou. These cases were obtained from the surveillance system, which were reported by physician daily when the cases came to see a doctor. Not all cases of influenza are going to the hospital to seek treatment, a majority of cases would be treated at home. Additionally, only several hospitals in Guangzhou reported the influenza cases to the surveillance system as required. Even worse, part of cases in these hospitals may fail to report to the system. All these factors may denote a very restrictive selection of cases and a low completeness of the surveillance system in Guangzhou, which may make an impact on validity of VEs in our study.
Finally, there are 26.7% of cases missing vaccination records or loss to follow-up in our study. This may be due to children from neighboring cities who sought medical treatment in Guangzhou, or the floating children lived in Guangzhou. Missing vaccination records may be more frequent among unvaccinated children, which can result in a negative bias to the results.
There are several strengths to this study. Above all, by using information about cases’ clinical diagnoses from the surveillance system and vaccination records from the children’s EPI Administrative Computerized System, we eliminated the recall bias that is a common problem in traditional case-control studies. A second strength is that controls matched cases with respect to the street where cases lived, thus cases and controls may be comparable to exposure to influenza, regardless of underlying medical condition and family financial status, which improve the validity of the results. Additional advantage of our study design is that we used a random sampling method to select the healthy control in the EPI Administrative Computerized System, making it a relative representative sample from the system which covered the greatest part of pediatric population.
The majority of influenza transmission occurs among children, with their parents serving as bridges to the rest of the population,Citation17 including people with high-risk conditions. Vaccination can reduce the potential impact from influenza epidemicsCitation18 by increasing vaccinated individuals’ immunity, as well as providing the benefits of herd protection to the community by breaking the transmission of influenza or lessening the chances of susceptible coming in contact with infective individual.Citation19 Influenza vaccination can usually provide protection even when there is a poor match between circulating and vaccine influenza strains.Citation20 In addition, cost-effectiveness analyses support the benefits of vaccination.Citation21
Generally speaking, influenza vaccination is not fully effective until 2 weeks after vaccination. Only children who are vaccinated in time will avoid seasonal influenza. Therefore, timely vaccination is critical for children.
Clinical trials suggest that protection against viruses with similar antigenicity to vaccine strains persists for 6–8 mo.Citation22 In addition, influenza viruses mutate constantly.Citation4 These evidences support the need for annual vaccination against seasonal influenza.
The current study provides an estimate of influenza VE under real-world conditions in the pediatric population. Based on our study and the statements above, we conclude that influenza vaccination offers relatively low protection against clinically diagnosed influenza for children aged 6 to 59 mo. To maximize the effectiveness of influenza vaccine, children should receive full, timely and annual vaccinations. More specifically, children should be fully vaccinated before the peak influenza season (March to July in southern China, however, it may change from year to year), even if the vaccine strains have not changed from the previous year.
Materials and Methods
Study population
Children aged 6 to 59 mo in Guangzhou who had been included in Children’s EPI Administrative Computerized System were eligible for inclusion in the study.
Study design
Based on annual influenza virus surveillance data in Guangzhou (unpublished), influenza epidemic period was defined as week 7 to week 24(from February 6th to June 11th in 2010–2011 and from February 12th to June 16th in 2011–2012) in our study. We conducted a matched case-control study in which cases with clinically diagnosed influenza were each matched to three healthy children based on date of birth (± one month), gender and the street where the cases lived.
Clinically diagnosed influenza cases were obtained from the surveillance system which collected the national notifiable diseases information. In Guangzhou, hospitals are required to report the influenza cases, however part of hospitals failed to do so. Those influenza cases who went to these cooperative hospitals for medical treatments were likely to be reported, and other hospitals’ influenza were unlikely to report.
Clinical diagnosis was defined as fever (>38°C) with an ARI symptom, combined with systemic symptom (e.g., headache, myalgia, or tiredness) or worsening chronic lung disease.
Controls were randomly sampled from the Children’s EPI Administrative Computerized System, which has been designed to manage immunization records of children under 5 y old in Guangzhou since 1997. By using the system, health care workers can record, retrieve and analyze all children’s vaccination information easily.Citation23 Children under 5 y old were required to register in Children’s EPI Administrative Computerized System whenever they received vaccine in Guangzhou. Healthy controls were defined as children without ARI symptoms before the date of the matched case’s clinical diagnosis, which was confirmed by phone call by physicians from Guangzhou Center for Disease Control and Prevention (Guangzhou CDC). We selected the controls from the Children’s EPI Administrative Computerized System randomly as follow steps: (1) Matched the children’ age, gender and the street cases lived in the system; (2) Chosen a page from the search result, the page was randomly selected by using random number table; (3) Started from the first child on that page and collected the children’s information until the objects were enough, without knowing theirs vaccination records before.
Influenza vaccination records for cases and controls were collected from the Children’s EPI Administrative Computerized System. The cases did not exist in that system were excluded from our study.
Study approval was obtained from the Guangzhou CDC ethics committee.
Vaccination status
Influenza vaccination status was categorized into vaccination (full or partial vaccination only for children aged 6–35 mo) or no vaccination.
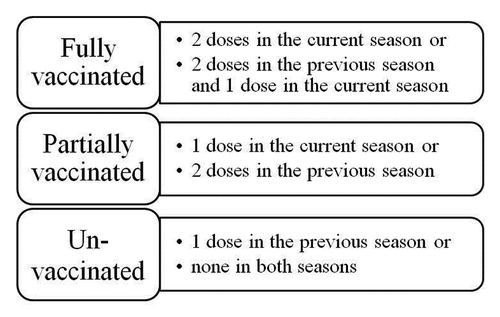
Children aged 6–35 mo: Children were considered fully vaccinated if they received 2 age-appropriate doses of seasonal influenza vaccine in the current influenza season and 4 or more weeks apart, with the second dose given ≥2 weeks before ARI symptom onset. Children were also considered fully vaccinated if they received 2 doses in the previous season and 1 dose in the current season. Children were counted as partially vaccinated if they received 1 dose in the current season or 2 doses in the season immediately before the study season. All other children were considered as not vaccinated ().
Children aged 36–59 mo: Children were considered vaccinated if they received 1 age-appropriate dose in the current season, others were considered as not vaccinated.
Statistical analysis
Analyses were conducted separately for each season and for the combination of both seasons. Odds ratios (OR) were estimated using conditional logistic regression. Age of vaccination was considered as confounder,Citation24,Citation25 and was included in the adjusted analysis. Vaccination status was included as a categorical exposure, as defined above. VE was expressed as a percentage and was calculated by (1-AOR)*100.Citation26 VE estimates compared any, full and partial vaccination to no vaccination. VE by time since vaccination were used to determine the effects of time since vaccination to estimate whether VE waned over time. VE by time since vaccination was calculated by 0–5 mo or 6–11 mo since vaccination. Additional variables needed to be created to estimate the VE by time since vaccination, a set of each period during which vaccinated children could have developed influenza was createdCitation27. The values equal 1 for subjects whose status was vaccinated in a given period prior to onset of influenza and 0 for the else, including all unvaccinated subjectsCitation27. Age of vaccination was defined as the age when children received the second dose of vaccines if they received 2 doses of vaccines; or the age when children was vaccinated if they received only 1 dose. We compared subjects with respect to age, gender, area of residence and immunization coverage rate by using a two-sample t-test, χ2 test. Area of residence was defined by the streets where subjects lived, and was categorized into urban (Liwan, Yuexiu, Haizhu), rural (Baiyun, Huadu, Zengcheng, Conghua) and rural-urban continuum area (Tianhe, Luogang, Huangpu, Panyu, Nansha). SPSS statistical software (version 13.0, SPSS, Inc., Chicago, IL) was used for data validation and statistical analysis. p ≤ 0.05 was regarded as a statistically significant difference.
Funding
This work was supported by grants from the Guangdong Provincial Department of Science and Technology (2011B050300001 and 2012B091100045), Science and Information Technology of Guangzhou (2012J5100005).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We highly appreciate the help from the physicians from sentinel surveillance hospitals for this study and Guangzhou CDC. We are also indebted to the parents for their participation.
References
- Graham-Rowe D. Epidemiology: Racing against the flu. Nature 2011; 480:S2 - 3; http://dx.doi.org/10.1038/480S2a; PMID: 22158296
- Centers For Disease Control Prevention. Children, the Flu, and the Flu Vaccine.August 29, 2012. Available at: http://www.cdc.gov/flu/protect/child.htm. Accessed September 22, 2012.
- Feng L, Shay DK, Jiang Y, Zhou H, Chen X, Zheng Y, et al. Influenza-associated mortality in temperate and subtropical Chinese cities, 2003-2008. Bull World Health Organ 2012; 90:279 - 288B; PMID: 22511824
- Morens DM, Taubenberger JK, Fauci AS. The persistent legacy of the 1918 influenza virus. N Engl J Med 2009; 361:225 - 9; http://dx.doi.org/10.1056/NEJMp0904819; PMID: 19564629
- Treanor JJ, Talbot HK, Ohmit SE, Coleman LA, Thompson MG, Cheng PY, et al, US Flu-VE Network. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012; 55:951 - 9; http://dx.doi.org/10.1093/cid/cis574; PMID: 22843783
- Stöhr K, Bucher D, Colgate T, Wood J. Influenza virus surveillance, vaccine strain selection, and manufacture. Methods Mol Biol 2012; 865:147 - 62; http://dx.doi.org/10.1007/978-1-61779-621-0_9; PMID: 22528158
- Nelson MI, Holmes EC. The evolution of epidemic influenza. Nat Rev Genet 2007; 8:196 - 205; http://dx.doi.org/10.1038/nrg2053; PMID: 17262054
- Azziz Baumgartner E, Dao CN, Nasreen S, Bhuiyan MU, Mah-E-Muneer S, Al Mamun A, et al. Seasonality, timing, and climate drivers of influenza activity worldwide. J Infect Dis 2012; 206:838 - 46; http://dx.doi.org/10.1093/infdis/jis467; PMID: 22829641
- Feng L, Mounts AW, Feng Y, Luo Y, Yang P, Feng Z, et al. Seasonal influenza vaccine supply and target vaccinated population in China, 2004-2009. Vaccine 2010; 28:6778 - 82; http://dx.doi.org/10.1016/j.vaccine.2010.07.064; PMID: 20688038
- Palache A. Seasonal influenza vaccine provision in 157 countries (2004-2009) and the potential influence of national public health policies. Vaccine 2011; 29:9459 - 66; http://dx.doi.org/10.1016/j.vaccine.2011.10.030; PMID: 22024174
- Zheng J, Cao L, Guo S, A K, Wang L, Yu W, et al. Survey on the Immunization Status of Category B Vaccine among Children Aged 1 to 2 Years in China. Chinese Journal of Vaccines and Immunization 2012; 18:233 - 7 In Chinese.
- Eick-Cost AA, Tastad KJ, Guerrero AC, Johns MC, Lee SE, Macintosh VH, et al. Effectiveness of seasonal influenza vaccines against influenza-associated illnesses among US military personnel in 2010-11: a case-control approach. PLoS One 2012; 7:e41435; http://dx.doi.org/10.1371/journal.pone.0041435; PMID: 22859985
- Szilagyi PG, Fairbrother G, Griffin MR, Hornung RW, Donauer S, Morrow A, et al, New Vaccine Surveillance Network. Influenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case-cohort study. Arch Pediatr Adolesc Med 2008; 162:943 - 51; http://dx.doi.org/10.1001/archpedi.162.10.943; PMID: 18838647
- Yang Z, Dong Z, Fu C. Seasonal influenza vaccine effectiveness among children aged 6 to 59 months in southern China. PLoS One 2012; 7:e30424; http://dx.doi.org/10.1371/journal.pone.0030424; PMID: 22291953
- Orenstein EW, De Serres G, Haber MJ, Shay DK, Bridges CB, Gargiullo P, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol 2007; 36:623 - 31; http://dx.doi.org/10.1093/ije/dym021; PMID: 17403908
- Kelly H, Jacoby P, Dixon GA, Carcione D, Williams S, Moore HC, et al, WAIVE Study Team. Vaccine Effectiveness Against Laboratory-confirmed Influenza in Healthy Young Children: A Case-Control Study. Pediatr Infect Dis J 2011; 30:107 - 11; http://dx.doi.org/10.1097/INF.0b013e318201811c; PMID: 21079528
- Medlock J, Galvani AP. Optimizing influenza vaccine distribution. Science 2009; 325:1705 - 8; http://dx.doi.org/10.1126/science.1175570; PMID: 19696313
- Jordan R, Connock M, Albon E, Fry-Smith A, Olowokure B, Hawker J, et al. Universal vaccination of children against influenza: are there indirect benefits to the community? A systematic review of the evidence. Vaccine 2006; 24:1047 - 62; http://dx.doi.org/10.1016/j.vaccine.2005.09.017; PMID: 16298026
- Paul Y. Herd immunity and herd protection. Vaccine 2004; 22:301 - 2; http://dx.doi.org/10.1016/j.vaccine.2003.07.016; PMID: 14670308
- Nichol KL. Efficacy and effectiveness of influenza vaccination. Vaccine 2008; 26:Suppl 4 D17 - 22; http://dx.doi.org/10.1016/j.vaccine.2008.07.048; PMID: 19230153
- Fairbrother G, Cassedy A, Ortega-Sanchez IR, Szilagyi PG, Edwards KM, Molinari NA, et al, New Vaccine Surveillance Network (NVSN). High costs of influenza: Direct medical costs of influenza disease in young children. Vaccine 2010; 28:4913 - 9; http://dx.doi.org/10.1016/j.vaccine.2010.05.036; PMID: 20576536
- Centers For Disease Control Prevention. Prevention and Control of Influenza with Vaccines. July 29, 2010. Available at:http://www.cdc.gov/mmwr/preview/mmwrhtml/rr59e0729al.htm. Accessed October 29, 2012.
- Fu C, Liang J, Wang M. Matched case-control study of effectiveness of live, attenuated S79 mumps virus vaccine against clinical mumps. Clin Vaccine Immunol 2008; 15:1425 - 8; http://dx.doi.org/10.1128/CVI.00122-08; PMID: 18667635
- Kissling E, Valenciano M, I-MOVE case-control studies team. Early estimates of seasonal influenza vaccine effectiveness in Europe, 2010/11: I-MOVE, a multicentre case-control study. Euro Surveill 2011; 16:19818; PMID: 21435329
- Valenciano M, Kissling E, Ciancio BC, Moren A. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioner networks. Vaccine 2010; 28:7381 - 8; http://dx.doi.org/10.1016/j.vaccine.2010.09.010; PMID: 20851086
- Fu C, Wang M, Liang J, Xu J, Wang C, Bialek S. The effectiveness of varicella vaccine in China. Pediatr Infect Dis J 2010; 29:690 - 3; http://dx.doi.org/10.1097/INF.0b013e3181d7380e; PMID: 20216242
- Niccolai LM, Ogden LG, Muehlenbein CE, Dziura JD, Vázquez M, Shapiro ED. Methodological issues in design and analysis of a matched case-control study of a vaccine’s effectiveness. J Clin Epidemiol 2007; 60:1127 - 31; http://dx.doi.org/10.1016/j.jclinepi.2007.02.009; PMID: 17938054