Abstract
This was a retrospective study to determine the validity of institutional reference intervals for interpreting biochemistry and hematology results in healthy adults in the context of clinical trials of preventive vaccines. An example population of 974 healthy adults participating in clinical trials at the Jenner Institute, Oxford, UK, between 1999 and 2009 was studied. Methods for calculating the central 95% ranges and determining the coefficients of within person variation were demonstrated. Recommendations have been made as to how these data can be usefully applied to the interpretation of blood results in healthy adult subjects for the purposes of clinical trial inclusion decisions and post-vaccination safety monitoring.
Introduction
The accurate interpretation of biochemical and hematological blood analyses are an important aspect of clinical trials of preventive vaccines, both for trial inclusion decisions and post-intervention safety evaluation. Reference intervals (RIs) are typically derived from historical literature values, laboratory instrument manufacturers or appropriate statistical analysis of routine clinical laboratory patient data, all of which can introduce sampling bias.Citation1,Citation2 Blood results are affected by individual and laboratory factors, such as differences in age, gender, ethnicity, laboratory methods and laboratory instruments.Citation3-Citation5 While the gold standard is to minimize laboratory and population differences by computing institutional RIs, there are inherent problems with obtaining a sufficient sample size for such analyses, so validation of externally obtained RIs is recommended.Citation6 In clinical trials of preventive vaccines, abnormalities in blood results from healthy young adults are usually defined by comparison with all age institutional or published RIs.Citation7-Citation10 There is a need to validate the RIs used in clinical trials to ensure they are applicable to the trial population. The data set of blood results from vaccine clinical trial programmes in healthy young adults at the Jenner Institute, Oxford, UK, is sufficiently large for calculation of the central 95% ranges for comparison with the institutional RIs. One of the interventions, MVA85A, a candidate tuberculosis vaccine, has been administered to over 100 healthy adults in a series of early clinical trials.Citation11 Retrospective analyses of blood results obtained from asymptomatic adult subjects at the Jenner Institute and subjects vaccinated with MVA85A were conducted and the literature reviewed in order to identify and account for discrepancies between the study population and the institutional RI. The main aim of these analyses was to develop recommendations for the interpretation of blood results obtained from healthy adult clinical trial participants in Oxford, UK.
Results
Clinical trials, subjects and sample results
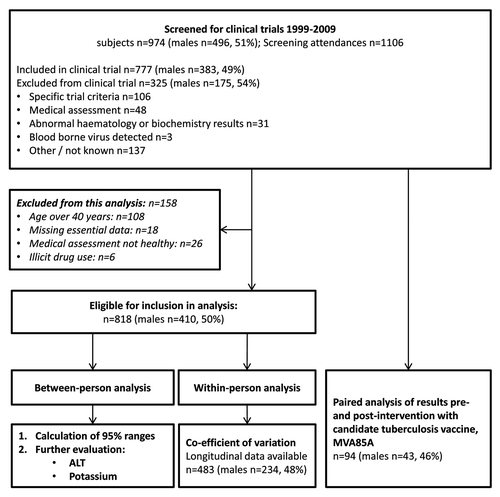
Between 1999 and 2009, 38 clinical trials of candidate malaria, tuberculosis, influenza and hepatitis C vaccines in healthy adult subjects were conducted (unpublished trial by Porter et al., NCT00548444).Citation12-Citation37 In total, 1106 screening appointments were attended by 974 subjects, of which 70% were enrolled in a clinical trial (). Subjects aged 18 to 60 y were screened for inclusion in clinical trials, with a median female age of 25.3 y (IQR 9.1). The median age of male subjects was 26.3 y (IQR 9.7). Ethnicity data was not routinely collected for all trials, but in a subset of 115 subjects participating in TB vaccine clinical trials, 80–100% of subjects were born in Europe.Citation12,Citation13,Citation19,Citation28
Between-person variation
The number of subjects aged 40 y and under who were included in the between-person analyses was 818, of which 410 (50.1%) were male (). Female subjects’ median age was 24.7 y (IQR 6.6), and the median age of males analyzed was 25.1 y (IQR 6.7).
Fewer than 20 datapoints from samples run on the Bayer Axon were within each analyte and gender subgroup, which was insufficient for between-instrument comparisons. Samples run on the Bayer Axon were therefore excluded from further analysis. No clinically relevant differences in results between the Abbott Aeroset and Siemens ADVIA 2400 were identified and results by these two different instruments were combined for subsequent analyses (data not shown).
For all analytes, except alanine aminotransferase (ALT) in males, there were no differences in results between age tertiles using Kruskal-Wallis (data not shown). Results for subjects aged 40 y and under were combined for subsequent analyses, except for ALT results in males. The proportions of results outside the institutional RI were determined, and the calculated 95% ranges for each analyte were compared with the institutional RI (). ALT and potassium results were examined in further detail.
Table 1. Study population blood results and the standard laboratory reference intervals (RI)
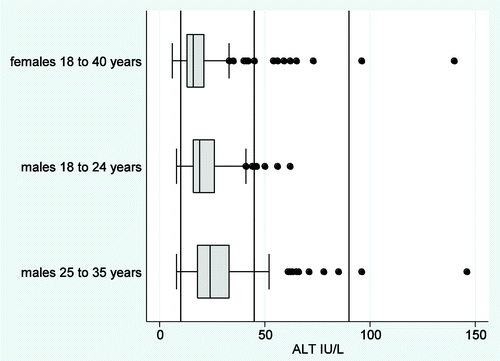
The calculated 95% range for ALT in female subjects was the same as the institutional RI. For male subjects, there were differences in ALT results between age tertiles (Kruskal-Wallis p = 0.0001) and a trend of increasing ALT result with increasing age (non-parametric trend p = 0.001) (). Since there were fewer than 120 data points within each age tertile, 95% ranges of ALT results for two age quantiles were calculated. For males aged 18 to 24 y, the calculated 95% ranges were similar to the institutional RI. For males aged 25 to 35 y, 12% of results were above the upper limit and the calculated upper limit was more than 1.5 times greater than the institutional upper limit for ALT. Repeat samples were available in 29 of the 37 (78%) subjects 18 to 40 y with an elevated ALT. Of these subjects, 11 (38%) had repeat samples within the institutional RI limits; 14 (48%) subjects’ repeating ALT results were mildly abnormal (up to twice the upper limit); and four (14%) subjects’ repeating ALT results were moderately abnormal (more than twice the upper limit).
Figure 2. ALT results for females aged 18 to 40 y; males aged 18 to 24 y and males aged 25 to 35 y are shown in a box plot. Boxes contain median lines and whiskers show the interquartile ranges. Dots are individual outliers.
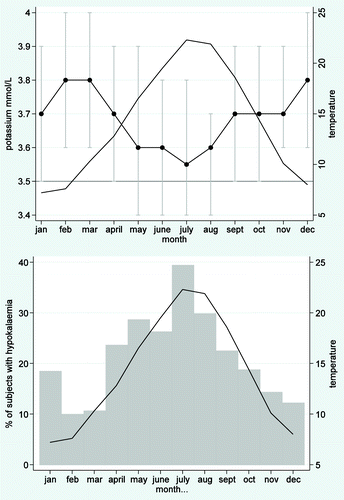
One quarter of all potassium results were below the lower limit for the institutional RI and the calculated upper and lower limits were lower than those for the institutional RI. Median monthly potassium was lower in the summer than the winter months () and correlated negatively with mean maximum monthly temperature (Spearman rho = −0.76, p < 0.001). The frequency of below range potassium results was highest in the summer months ().
Figure 3. Potassium and environmental temperature. (A) Median monthly potassium and mean monthly maximum temperature. Median potassium results for each month are shown as connected points, with capped bars indicating the interquartile ranges. The line represents the mean monthly maximum temperature in Oxford, UK, from 1979–2000. The nadir median monthly potassium results corresponded with the summer months and the peak environmental temperature. A negative association between monthly median potassium and mean temperature was found (Spearman rho = -0.76, p < 0.001). (B) Proportion of results indicating hypokalaemia and mean monthly temperature. The vertical bars indicate the percentage of samples with potassium results below the lower limit of the RI (3.5 mmol/L) each month. The line connects the mean maximum temperature for each month. Low potassium results were more frequent in the summer months than the winter months.
Within-person variation
Analytes showing the greatest within-person variability were bilirubin, ALT, white cells and urea (). The most stable analytes with least within-person variability were sodium, MCV, hemoglobin and albumin. Trends in within-person variation were comparable to previously published figures from large data sets. Pre-and post-intervention results for 94 subjects who participated in clinical trials of a candidate tuberculosis vaccine, MVA85A, were also analyzed (). The median age of female subjects was 26.8 y (IQR 9.2) and male subjects’ median age was 26.5 y (IQR 11.9). The median interval between pre-intervention screening samples and vaccination with MVA85A was 28 d (IQR 44). The median interval between vaccination and post-intervention samples was 7 d (IQR 0). One post-intervention sodium result (109 mmol/L) for one subject was excluded from the analysis. This result was deemed aberrant as there were no associated symptoms of hyponatraemia and sodium was within the normal range (139 mmol/L) on a sample taken 2 d later. The repeat sodium result of 139mmol/L was included. Albumin and bilirubin results were lower post-intervention compared with pre-intervention but no results fell below the lower limit of the institutional RI. Hemoglobin and MCV were transiently reduced one week post-intervention. Hemoglobin fell below the lower limit of the institutional RI one week post-intervention in five (6%) of subjects, all of whom were female, but in all cases returned to normal by 12 weeks post-intervention.
Table 2. Within-person variation and comparison of results pre-and post-MVA85A vaccination
Proposed criteria for interpreting blood results in clinical trials
Background evidence from published literature or guidelines from recognized sources for each analyte, with relevance for young, asymptomatic adult subjects, are summarized in . Cautionary ranges and expected variation from baseline are shown in .
Table 3. Summary of available literature
Table 4. Recommendations
Discussion
The validity of the institutional RIs for the young adult study population was first evaluated by determining the proportion of results which were outside the range of the institutional RI. The institutional RIs were two sided, defining the upper and lower limits of 95% of results. No analyte had both 2.5% of results above the upper limits and 2.5% of results below the lower limits, as would be expected. For analytes such as albumin and ALP, this may reflect differences between the all adult reference populations, often patients, from which institutional RIs were derived and the population of young, asymptomatic adults volunteering to participate in vaccine clinical trials at the Jenner Institute, Oxford ().
One in four potassium results in the study were below the lower limit of the institutional RI for potassium. Potassium levels in ex-vivo blood samples are not stable when stored prior to analysis unless centrifuged at the point of care.Citation52 Seasonal pseudohypokalaemia has been described and is hypothesized to be caused by ex-vivo sodium-potassium ATPase-mediated increased uptake of potassium by metabolically active cells in warm conditions.Citation48,Citation53 We speculate that pseudhohypokalaemia due to storage of the samples at environmental temperature (centrally heated building in winter; no air conditioning in summer) prior to transport for analysis accounted for the downward shift potassium levels compared with the institutional RIs. Centrifuges have been installed in primary care practices, on the basis that correct results can only be ensured by centrifugation of samples in gel separator tubes.Citation53 In Scotland, source centrifugation of samples taken in primary care facilities successfully reduced the frequency of pseudohyperkalaemia.Citation54 We propose a prospective study to investigate the effect of centrifugation at source on potassium results.
Discrepancies between the study population and the institutional RIs were further evaluated by using a well-recognized method for defining RIs, which is the basis for current guidelines.Citation2,Citation6,Citation55,Citation56 The central 95% of results for each analyte, subgrouped by gender, were calculated following removal of outliers. This method aims to maximise sensitivity and specificity by obtaining information from the extremes of the sample, while avoiding outliers adversely influencing the analysis. The importance of the calculated 95% ranges is that there is a danger that a clinically significant result which falls outside the calculated range for this healthy adult population, but within the limits of the institutional RI for all adults, may be clinically significant, but would be overlooked if referring only to the institutional RIs.
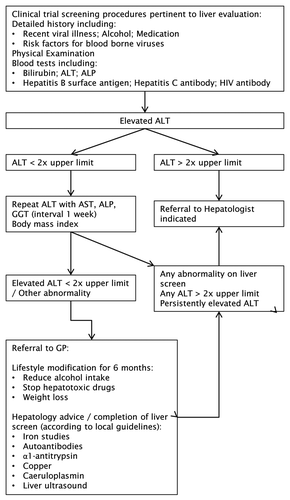
The calculated 95% range for ALT in males aged 25 to 40 y was higher than the institutional RI, reflecting increased levels in this subgroup, but adherence to the institutional RI for ALT is essential. Minimally elevated ALT levels are of clinical significance () and a number of groups have advocated reducing current upper normal limits.Citation4,Citation57 Current recommendations are for investigation of persistently elevated ALT of any degree above standard upper limits in asymptomatic individuals.Citation58 We have, therefore, proposed a clinical algorithm for the management of elevated ALT results in clinical trial populations ().
Understanding the expected degree of within-person variation for an analyte and the factors associated with variations is of particular importance for the interpretation of sequential results. Consistent with previous published data, bilirubin and ALT showed the greatest within-person variation. Transient fluctuations are common and may be caused by recent alcohol consumption, viral illnesses, concomitant medications and exercise patterns.Citation59 For these analytes, transient fluctuations need to be distinguished from persistent elevations, by repeat testing and possible causes of transient fluctuations documented, by relevant history taking. It is intuitive to expect that analytes which vary greatly are likely to be the most frequently reported as abnormal in post-vaccination safety evaluation. For example, in a recent study of two candidate tuberculosis vaccines, MVA85A and FP85A, post-MVA85A vaccination ALT was elevated in one subject.Citation12 The paired analysis of 94 pre- and post-MVA85A vaccination blood results, was reassuring in showing no frequent effect of MVA85A vaccination on ALT results.
The paired pre- and post-intervention analysis identified a transient reduction in hemoglobin and MCV in females, coinciding with the time in the trials when the greatest blood volumes were drawn. When the outcome measures of a clinical trial include the evaluation of vaccination induced immune responses, sizable blood samples (60–80 ml) are required from each participant at regular intervals. For trials such as these, we recommend adherence to national transfusion guidelines for the selection of blood donors, when setting trial inclusion criteria. In the UK, the minimum Hemoglobin levels for blood donors are 12.5 and 13.5 g/dl females and males, respectively.Citation60
The strengths of this study include the use of healthy young adult subjects, all of whom had a medical assessment. Subjects with pre-existing medical problems, alcohol excess or infection with blood borne viruses were excluded from the analysis. There were over 300 subjects per group, which was above the minimum recommended subgroup size (120 subjects) for non-parametric 95% range calculations.Citation6 Data used for between-person analyses were taken pre-intervention, i.e., subjects were maintaining their usual lifestyle. Most specimens were taken in the mornings with subjects at rest thereby removing diurnal variation and exertion induced changes as confounding pre-analytical factors. Paired samples for the same individuals were available and all samples were processed in the same laboratory.
Limitations were that the study was retrospective and single centered. Various environmental factors were not controlled. Recreational drug use was an exclusion criterion, but subjects taking certain prescription drugs (such as the oral contraceptive pill) or over-the-counter remedies (e.g., for coryzal and musculoskeletal symptoms) were not excluded. Subjects were not required to fast overnight prior to phlebotomy and recent exercise may have been taken. After phlebotomy, times to sample analysis were not controlled. At the point of laboratory analysis, samples were not frozen, so between-run variation could not be eliminated and replicate analyses of specimens were not performed. The trends in within-person variability were comparable to those previously published; suggesting factors which may have affected results remained consistent between sequential samples for individual subjects. Follow-up data were not available for subjects with significantly or persistently abnormal results who were excluded from participation and referred for primary care follow up. Ethnicity and socio-economic data, anthropometric measurements, iron studies in females and detailed smoking, alcohol, recent diet, exercise and menstrual history would strengthen the study.Citation61,Citation62
Taking the results of the analyses and the limitations of the study into consideration, recommendations for the interpretation of blood results in healthy adult subjects have been proposed (). Clearly, the calculated 95% ranges and coefficients of within-person variation are only valid for young, asymptomatic adults whose blood samples are analyzed at the Oxford University Hospitals NHS Trust laboratories, UK. However, the methods described are straightforward and could be applied to other data sets from healthy adult subjects. Key components of the clinical assessment conducting during clinical trial screening, pertinent to the interpretation of blood results, include ethnic origin; detailed alcohol, smoking, diet, exercise and medication history; recent mild illness; history of significant acute or chronic disease; height, weight and blood pressure measurements and urinalysis for blood, protein and glucose. When taking post-intervention blood tests for safety evaluation, concurrent history of recent symptoms, diet, exercise, medication and alcohol intake are recommended.
There is a recognized need to standardise safety reporting across clinical trials of vaccines and the Brighton Collaboration, a global research network, is developing case definitions and guidelines to this end.Citation63 This study provides important data on which to base further work to validate RIs for young, asymptomatic adults to enable the accurate interpretation of blood results in clinical trials.
Materials and Methods
Subject data set
A retrospective analysis of screening attendances, screening outcomes and laboratory results included all clinical trials recruiting healthy adult subjects at the Jenner Institute, Oxford, UK, between 1999 and 2009. Trials were conducted in accordance with the Declaration of Helsinki and ethical approval obtained. Informed consent was obtained before conducting any study procedures. Subjects were assessed by medical history, physical examination, laboratory assessment and corroborative medical history from General Practitioners, to exclude undisclosed medical conditions. Data from paper case report forms were entered onto an electronic database (Microsoft Excel). Accuracy of electronic data entry was verified by double data entry and crosschecking against paper records.
Laboratory results data set
Non-fasting phlebotomy was performed at the Centre for Clinical Vaccinology and Tropical Medicine, Oxford, during clinical trial clinics (07:30–11:00 a.m.). Biochemistry samples were taken in lithium heparin and hematology samples in potassium EDTA (EDTA) using the Beckton Dickinson (BD) vacutainer collection system. Laboratory specimens were collected within two hours of phlebotomy and transported two miles to the Oxford University Hospital NHS Trust Laboratories for same day analysis. Each blood tube was labeled with a unique study-specific identification (ID) number and laboratory reports were generated for these study IDs. Trial and subject-specific ID numbers were used to retrieve laboratory results electronically.
All methods and instrumentation used were commonly used standardised methods. Albumin (bromocresol green method), alkaline phosphatase (ALP, DEA buffered enzymatic), alanine aminotransferase (ALT, enzymatic method), bilirubin (vanadate oxidation method), creatinine (Jaffe method), sodium and potassium (both ion selective electrode methods) and urea (urease method) were undertaken on three different clinical chemistry analysers over the period studied. Samples analyzed before February 2000 utilized the Bayer Axon analyzer (Bayer Diagnostics); samples from March 2000 and March 2006 were analyzed on an Abbott Aeroset (Abbott diagnostics); and samples obtained from April 2006 to December 2009 were analyzed on a Siemens ADVIA 2400 (Siemens Diagnostics). RIs remained consistent despite these two changes of instrument as no clinically significant shifts in results were detected by comparison studies (T. James, personal correspondence). Haematological parameters were analyzed on the Sysmex XE-2100 hematology analyzer. The longitudinal stability of all assays was controlled through internal quality control and external quality assurance participation.
Laboratory data were excluded pre-analysis if medical assessment had revealed significant past medical or psychiatric history; illicit drug use; excessive alcohol intake or if infection with a blood borne virus was detected at screening.
Statistical analysis
Data were analyzed using Stata (Stata statistical software version 9.0, 2005, StataCorp, USA). Data were first visually reviewed to check for heavy skewing by individual results, using histograms and box plots. Continuous variables were not normally distributed and were summarized using median and interquartile range (IQR).
For between-person analyses, results for each analyte were filtered to include one pre-intervention result per subject and were sub-grouped by gender. Differences in the distribution of data between instrument subgroups were compared using Kolmogorov-Smirnov, before combining the data derived from different instruments. Subjects over 40 y of age were excluded and subjects 40 y and under were partitioned into age tertiles. Differences between age tertiles were evaluated by Kruskal-Wallis. Where differences were detected, a non-parametric test for trend across ordered groups was performed. Where no differences between age tertiles were detected, 95% ranges for combined data from subjects of all ages 40 y and under were calculated.
The 95% range for each analyte was calculated using a standard method.Citation2,Citation6,Citation55,Citation56 Normality of data within each subgroup was assessed graphically by normal probability plots (for evaluating central normality) and normal quantile plots (for identifying deviations from normality at the tails of the data). The Shapiro–Wilk test for skewness and kurtosis and the Kolmogorov-Smirnov test against an empirical normal distribution were performed. Where data were not normally distributed, Box–Cox transformations within each group or subgroup were performed. The effect of normalizing transformation was assessed graphically and statistically as above. The upper (Q1) and lower (Q3) quartiles and IQR (Q1–Q3) of the transformed data were calculated. Data points lying outside the range (Q1–1.5 × IQR) to (Q3 + 1.5 × IQR) were identified as outliers and excluded.Citation2 Non-parametric 95% ranges were derived by obtaining the 2.5th and 97.5th percentiles of the raw data after exclusion of outliers. Parametric 95% ranges were derived by calculating mean ± 1.96 × standard deviations (SDs) of the raw data after exclusion of outliers.
The analysis of within-person variation included all clinical trial participants 40 y and under for whom at least two sequential results per analyte were available. If a subject had participated in more than one clinical trial, samples were taken from the first clinical trial in which they had participated. The first (r1) and last (r2) retrieved results for each subject were included in the analysis. This minimised any effect of intervention on the analysis, since the first samples for each subject were pre-intervention and the last samples were taken at least 12 weeks post-intervention. The within-person coefficient of variation was calculated using a described method.Citation62 The square of the coefficient of variation (CVCitation2) for each subject was calculated by [(within subject variance)/ (mean) Citation2], where within subject variance was [(r1-r2)Citation2 / 2] and mean result was [(r1+r2)/2]. The within-person coefficient of variation for the sample was obtained by calculating the root mean square; [(√(mean CVCitation2))*100]. The standard error was calculated by [(standard deviation of CVCitation2s)/ (√sample size)].
The seasonality of potassium results were evaluated by comparing median monthly potassium results against average monthly temperatures. Potassium results from subjects aged 40 y or under were analyzed. Data were grouped by the month the sample was obtained. Monthly average temperatures in Oxford over a 30-y period from 1971–2001 were obtained from the Meterological Office.Citation64 The association between median monthly potassium and mean monthly temperature was evaluated by Spearman’s rho correlation coefficient.
Paired pre- and post-intervention results were analyzed for the candidate tuberculosis vaccine MVA85A (ClinicalTrials.gov registration number of unpublished trial by Porter et al., NCT00548444).Citation12,Citation13,Citation19,Citation25,Citation28,Citation35 The latest pre-intervention and earliest post-intervention results available were included in the analysis. Results were excluded if samples were obtained greater than one month (31 d) post-intervention, since vaccine-related adverse events and cellular immune responses peak one to two weeks post-vaccination.Citation21,Citation29,Citation35,Citation65
Acknowledgments
We are grateful to all the subjects who volunteered to participate in clinical trials and to members of the clinical trial teams who performed recruitment and screening procedures.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The Jenner Institute clinical trials unit receives general research support funding from the UK National Institute for Health Research Oxford Biomedical Research Centre. Funders for specific clinical trials for which subjects were recruited are acknowledged in the cited publications. H.M. is a Wellcome Trust Senior Clinical Research Fellow. H.M., S.C.G. and A.V.S.H. are Jenner Institute Investigators. The funding agencies took no part in study design, data analysis, manuscript preparation or decision to publish.
Disclosure of Potential Conflicts of Interest
HM, SCG and AVSH are named inventors on a composition of matter patent for MVA85A, and are shareholders in a Joint Venture formed for the further development of this vaccine. HM, AVSH, SCG and GO are named as inventors on patent applications relating to induction of T-cell responses by vaccination.
Notes
† These authors contributed equally to this work.
References
- Friedberg RC, Souers R, Wagar EA, Stankovic AK, Valenstein PN, College of American Pathologists. The origin of reference intervals. Arch Pathol Lab Med 2007; 131:348 - 57; PMID: 17516737
- Shine B. Use of routine clinical laboratory data to define reference intervals. Ann Clin Biochem 2008; 45:467 - 75; http://dx.doi.org/10.1258/acb.2008.008028; PMID: 18753418
- Lacher DA, Hughes JP, Carroll MD. Biological variation of laboratory analytes based on the 1999-2002 National Health and Nutrition Examination Survey. Natl Health Stat Report 2010; 2010:1 - 7; PMID: 20540274
- Kariv R, Leshno M, Beth-Or A, Strul H, Blendis L, Kokia E, et al. Re-evaluation of serum alanine aminotransferase upper normal limit and its modulating factors in a large-scale population study. Liver Int 2006; 26:445 - 50; http://dx.doi.org/10.1111/j.1478-3231.2006.01197.x; PMID: 16629648
- Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol 1996; 49:664 - 6; http://dx.doi.org/10.1136/jcp.49.8.664; PMID: 8881919
- Horowitz GL, Altaie S, Boyd JC, Ceriotti F, Garg U, Horn P, et al. Defining, establishing, and verifying reference intervals in the clinical laboratory: Approved guideline. 3rd ed. Clinical and Laboratory Standards Institute (CLSI), 2008.
- Richmond PC, Nissen MD, Marshall HS, Lambert SB, Roberton D, Gruber WC, et al. A bivalent Neisseria meningitidis recombinant lipidated factor H binding protein vaccine in young adults: results of a randomised, controlled, dose-escalation phase 1 trial. Vaccine 2012; 30:6163 - 74; http://dx.doi.org/10.1016/j.vaccine.2012.07.065; PMID: 22871351
- Riveau G, Deplanque D, Remoué F, Schacht AM, Vodougnon H, Capron M, et al. Safety and immunogenicity of rSh28GST antigen in humans: phase 1 randomized clinical study of a vaccine candidate against urinary schistosomiasis. PLoS Negl Trop Dis 2012; 6:e1704; http://dx.doi.org/10.1371/journal.pntd.0001704; PMID: 22802974
- US Department of Health and Human Services Food and Drug Administration. Guidance for Industry. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. 2007.
- Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med 2004; 351:1548 - 63; PMID: 15470219
- Rowland R, Brittain N, Poulton ID, Minassian AM, Sander CR, Porter DW, et al. A review of the tolerability of the candidate TB vaccine, MVA85A compared with BCG and Yellow Fever vaccines, and correlation between MVA85A vaccine reactogenicity and cellular immunogenicity. Trials in Vaccinology 2012; 1:27 - 35; http://dx.doi.org/10.1016/j.trivac.2012.07.001
- Rowland R, Pathan AA, Satti I, Poulton ID, Matsumiya MM, Whittaker M, et al. Safety and immunogenicity of an FP9-vectored candidate tuberculosis vaccine (FP85A), alone and with candidate vaccine MVA85A in BCG-vaccinated healthy adults: a phase I clinical trial. Hum Vaccin Immunother 2012; 9:50 - 62; http://dx.doi.org/10.4161/hv.22464; PMID: 23143773
- Meyer J, Harris SA, Satti I, Poulton ID, Poyntz HC, Tanner R, et al. Comparing the safety and immunogenicity of a candidate TB vaccine MVA85A administered by intramuscular and intradermal delivery. Vaccine 2013; 31:1026 - 33; http://dx.doi.org/10.1016/j.vaccine.2012.12.042; PMID: 23266342
- O’Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis 2012; 205:772 - 81; http://dx.doi.org/10.1093/infdis/jir850; PMID: 22275401
- Sheehy SH, Duncan CJ, Elias SC, Biswas S, Collins KA, O’Hara GA, et al. Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PLoS One 2012; 7:e31208; http://dx.doi.org/10.1371/journal.pone.0031208; PMID: 22363582
- Minassian AM, Satti I, Poulton ID, Meyer J, Hill AV, McShane H. A human challenge model for Mycobacterium tuberculosis using Mycobacterium bovis bacille Calmette-Guerin. J Infect Dis 2012; 205:1035 - 42; http://dx.doi.org/10.1093/infdis/jis012; PMID: 22396610
- Lillie PJ, Berthoud TK, Powell TJ, Lambe T, Mullarkey C, Spencer AJ, et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis 2012; 55:19 - 25; http://dx.doi.org/10.1093/cid/cis327; PMID: 22441650
- Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med 2012; 4:ra1; http://dx.doi.org/10.1126/scitranslmed.3003155; PMID: 22218690
- Pathan AA, Minassian AM, Sander CR, Rowland R, Porter DW, Poulton ID, et al. Effect of vaccine dose on the safety and immunogenicity of a candidate TB vaccine, MVA85A, in BCG vaccinated UK adults. Vaccine 2012; 30:5616 - 24; http://dx.doi.org/10.1016/j.vaccine.2012.06.084; PMID: 22789508
- Sheehy SH, Duncan CJ, Elias SC, Collins KA, Ewer KJ, Spencer AJ, et al. Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Mol Ther 2011; 19:2269 - 76; http://dx.doi.org/10.1038/mt.2011.176; PMID: 21862998
- Berthoud TK, Hamill M, Lillie PJ, Hwenda L, Collins KA, Ewer KJ, et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin Infect Dis 2011; 52:1 - 7; http://dx.doi.org/10.1093/cid/ciq015; PMID: 21148512
- Duncan CJ, Sheehy SH, Ewer KJ, Douglas AD, Collins KA, Halstead FD, et al. Impact on malaria parasite multiplication rates in infected volunteers of the protein-in-adjuvant vaccine AMA1-C1/Alhydrogel+CPG 7909. PLoS One 2011; 6:e22271; http://dx.doi.org/10.1371/journal.pone.0022271; PMID: 21799809
- Porter DW, Thompson FM, Berthoud TK, Hutchings CL, Andrews L, Biswas S, et al. A human Phase I/IIa malaria challenge trial of a polyprotein malaria vaccine. Vaccine 2011; 29:7514 - 22; http://dx.doi.org/10.1016/j.vaccine.2011.03.083; PMID: 21501642
- Whelan KT, Pathan AA, Sander CR, Fletcher HA, Poulton I, Alder NC, et al. Safety and immunogenicity of boosting BCG vaccinated subjects with BCG: comparison with boosting with a new TB vaccine, MVA85A. PLoS One 2009; 4:e5934; http://dx.doi.org/10.1371/journal.pone.0005934; PMID: 19529780
- Sander CR, Pathan AA, Beveridge NE, Poulton I, Minassian A, Alder N, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am J Respir Crit Care Med 2009; 179:724 - 33; http://dx.doi.org/10.1164/rccm.200809-1486OC; PMID: 19151191
- Thompson FM, Porter DW, Okitsu SL, Westerfeld N, Vogel D, Todryk S, et al. Evidence of blood stage efficacy with a virosomal malaria vaccine in a phase IIa clinical trial. PLoS One 2008; 3:e1493; http://dx.doi.org/10.1371/journal.pone.0001493; PMID: 18231580
- Sanderson F, Andrews L, Douglas AD, Hunt-Cooke A, Bejon P, Hill AV. Blood-stage challenge for malaria vaccine efficacy trials: a pilot study with discussion of safety and potential value. Am J Trop Med Hyg 2008; 78:878 - 83; PMID: 18541763
- Pathan AA, Sander CR, Fletcher HA, Poulton I, Alder NC, Beveridge NE, et al. Boosting BCG with recombinant modified vaccinia ankara expressing antigen 85A: different boosting intervals and implications for efficacy trials. PLoS One 2007; 2:e1052; http://dx.doi.org/10.1371/journal.pone.0001052; PMID: 17957238
- Webster DP, Dunachie S, McConkey S, Poulton I, Moore AC, Walther M, et al. Safety of recombinant fowlpox strain FP9 and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine 2006; 24:3026 - 34; http://dx.doi.org/10.1016/j.vaccine.2005.10.058; PMID: 16488059
- Walther M, Thompson FM, Dunachie S, Keating S, Todryk S, Berthoud T, et al. Safety, immunogenicity, and efficacy of prime-boost immunization with recombinant poxvirus FP9 and modified vaccinia virus Ankara encoding the full-length Plasmodium falciparum circumsporozoite protein. Infect Immun 2006; 74:2706 - 16; http://dx.doi.org/10.1128/IAI.74.5.2706-2716.2006; PMID: 16622207
- Dunachie SJ, Walther M, Vuola JM, Webster DP, Keating SM, Berthoud T, et al. A clinical trial of prime-boost immunisation with the candidate malaria vaccines RTS,S/AS02A and MVA-CS. Vaccine 2006; 24:2850 - 9; http://dx.doi.org/10.1016/j.vaccine.2005.12.041; PMID: 16434127
- Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, et al. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun 2006; 74:5933 - 42; http://dx.doi.org/10.1128/IAI.00590-06; PMID: 16988273
- Webster DP, Dunachie S, Vuola JM, Berthoud T, Keating S, Laidlaw SM, et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci U S A 2005; 102:4836 - 41; http://dx.doi.org/10.1073/pnas.0406381102; PMID: 15781866
- Walther M, Dunachie S, Keating S, Vuola JM, Berthoud T, Schmidt A, et al. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine 2005; 23:857 - 64; http://dx.doi.org/10.1016/j.vaccine.2004.08.020; PMID: 15603885
- McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 2004; 10:1240 - 4; http://dx.doi.org/10.1038/nm1128; PMID: 15502839
- Moorthy VS, McConkey S, Roberts M, Gothard P, Arulanantham N, Degano P, et al. Safety of DNA and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine 2003; 21:1995 - 2002; http://dx.doi.org/10.1016/S0264-410X(02)00771-5; PMID: 12706689
- McConkey SJ, Reece WH, Moorthy VS, Webster D, Dunachie S, Butcher G, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med 2003; 9:729 - 35; http://dx.doi.org/10.1038/nm881; PMID: 12766765
- Westgard QC. Desirable Specifications for Total Error, Imprecision, and Bias, derived from intra- and inter-individual biologic variation. Tools, Technologies and Training for Healthcare Laboratories. http://www.westgard.com/biodatabase1.htm, 2012.
- Kubota K, Kadomura T, Ohta K, Koyama K, Okuda H, Kobayashi M, et al. Analyses of laboratory data and establishment of reference values and intervals for healthy elderly people. J Nutr Health Aging 2012; 16:412 - 6; http://dx.doi.org/10.1007/s12603-011-0355-3; PMID: 22499467
- Schiele F, Henny J, Hitz J, Petitclerc C, Gueguen R, Siest G. Total bone and liver alkaline phosphatases in plasma: biological variations and reference limits. Clin Chem 1983; 29:634 - 41; PMID: 6831690
- Elinav E, Ben-Dov IZ, Ackerman E, Kiderman A, Glikberg F, Shapira Y, et al. Correlation between serum alanine aminotransferase activity and age: an inverted U curve pattern. Am J Gastroenterol 2005; 100:2201 - 4; http://dx.doi.org/10.1111/j.1572-0241.2005.41822.x; PMID: 16181369
- Piton A, Poynard T, Imbert-Bismut F, Khalil L, Delattre J, Pelissier E, et al, MULTIVIRC Group. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. Hepatology 1998; 27:1213 - 9; http://dx.doi.org/10.1002/hep.510270505; PMID: 9581673
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002; 346:1221 - 31; http://dx.doi.org/10.1056/NEJMra011775; PMID: 11961152
- Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004; 328:983; http://dx.doi.org/10.1136/bmj.38050.593634.63; PMID: 15028636
- Claridge LC, Armstrong MJ, Booth C, Gill PS. Gilbert’s syndrome. BMJ 2011; 342:d2293; http://dx.doi.org/10.1136/bmj.d2293; PMID: 21508045
- Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med 2006; 354:2473 - 83; http://dx.doi.org/10.1056/NEJMra054415; PMID: 16760447
- Vidt DG, Ridker PM, Monyak JT, Schreiber MJ, Cressman MD. Longitudinal assessment of estimated glomerular filtration rate in apparently healthy adults: a post hoc analysis from the JUPITER study (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). Clin Ther 2011; 33:717 - 25; http://dx.doi.org/10.1016/j.clinthera.2011.05.004; PMID: 21704236
- Sodi R, Davison AS, Holmes E, Hine TJ, Roberts NB. The phenomenon of seasonal pseudohypokalemia: effects of ambient temperature, plasma glucose and role for sodium-potassium-exchanging-ATPase. Clin Biochem 2009; 42:813 - 8; http://dx.doi.org/10.1016/j.clinbiochem.2009.01.024; PMID: 19232334
- Sinclair D, Briston P, Young R, Pepin N. Seasonal pseudohyperkalaemia. J Clin Pathol 2003; 56:385 - 8; http://dx.doi.org/10.1136/jcp.56.5.385; PMID: 12719461
- Gardner MD, Scott R. Age- and sex-related reference ranges for eight plasma constituents derived from randomly selected adults in a Scottish new town. J Clin Pathol 1980; 33:380 - 5; http://dx.doi.org/10.1136/jcp.33.4.380; PMID: 7400337
- Wise RP, Bonhoeffer J, Beeler J, Donato H, Downie P, Matthews D, et al, Brighton Collaboration Thrombocytopenia Working Group. Thrombocytopenia: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2007; 25:5717 - 24; http://dx.doi.org/10.1016/j.vaccine.2007.02.067; PMID: 17493712
- Jackson C, Best N, Elliott P. UK Biobank Pilot Study: stability of haematological and clinical chemistry analytes. Int J Epidemiol 2008; 37:Suppl 1 i16 - 22; http://dx.doi.org/10.1093/ije/dym280; PMID: 18381388
- Masters PW, Lawson N, Marenah CB, Maile LJ. High ambient temperature: a spurious cause of hypokalaemia. BMJ 1996; 312:1652 - 3; http://dx.doi.org/10.1136/bmj.312.7047.1652; PMID: 8664721
- Turner HE, Peake RW, Allison JJ. Seasonal pseudohyperkalaemia: no longer an issue?. Ann Clin Biochem 2012; 49:94 - 6; http://dx.doi.org/10.1258/acb.2011.011122; PMID: 22067093
- Horn PS, Pesce AJ. Reference intervals: an update. Clin Chim Acta 2003; 334:5 - 23; http://dx.doi.org/10.1016/S0009-8981(03)00133-5; PMID: 12867273
- Harris EK, Boyd JC. On dividing reference data into subgroups to produce separate reference ranges. Clin Chem 1990; 36:265 - 70; PMID: 2302771
- Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002; 137:1 - 10; http://dx.doi.org/10.7326/0003-4819-137-1-200207020-00006; PMID: 12093239
- Cobbold JF, Anstee QM, Thomas HC. Investigating mildly abnormal serum aminotransferase values. BMJ 2010; 341:c4039; http://dx.doi.org/10.1136/bmj.c4039; PMID: 20675393
- Sherwood P, Lyburn I, Brown S, Ryder S. How are abnormal results for liver function tests dealt with in primary care? Audit of yield and impact. BMJ 2001; 322:276 - 8; http://dx.doi.org/10.1136/bmj.322.7281.276; PMID: 11157530
- UK Blood Transfusion & Tissue Transplantation Services. Whole Blood and Components: Donor Selection Guidelines: Haemoglobin Estimation. http://www.transfusionguidelines.org.uk/index.aspx?Publication=WB&Section=5&PageID=3702&AZLetter=H, 2013.
- Fraser CG. Clinically useful limits (CUL) criteria best based on within-subject biologic variation. Am J Clin Pathol 1989; 92:256 - 7; PMID: 2756946
- Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 1989; 27:409 - 37; http://dx.doi.org/10.3109/10408368909106595; PMID: 2679660
- Brighton collaboration. https://brightoncollaboration.org/public/who-we-are.html, 2013.
- MetOffice. UK climate averages. http://www.metoffice.gov.uk/climate/uk/averages/19712000/sites/oxford.html 2012.
- Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, et al. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol 2005; 174:449 - 55; PMID: 15611270