Abstract
Vaccine formulations on the basis of nano- (NP) or microparticles (MP) can solve issues with stabilization, controlled release, and poor immunogenicity of antigens. Likewise transcutaneous immunization (TCI) promises superior immunogenicity as well as the advantages of needle-free application compared with conventional intramuscular injections. Thus the combination of both strategies seems to be a very valuable approach. However, until now TCI using particle based vaccine formulations has made no impact on medical practice. One of the main difficulties is that NPs and MPs cannot penetrate the skin to an extent that would allow the application of the required dose of antigen. This is due to the formidable stratum corneum (SC) barrier, the limited amount of antigen in the formulation and often an insufficient immunogenicity. A multitude of strategies are currently under investigation to overcome these issues. We highlight selected methods presenting a spectrum of solutions ranging from transfollicular delivery, to devices disrupting the SC barrier and the combination of particle based vaccines with adjuvants discussing their advantages and shortcomings. Some of these are currently at an experimental state while others are already in clinical testing. All methods have been shown to be capable of transcutaneous antigen delivery.
Introduction
Infectious diseases impose a serious threat to public health worldwide. Among the strategies to fight infections only vaccination has the potential to eradicate the disease (the World Health Organization, WHO, certified the eradication of smallpox in 1979). New, safe, efficient, and cheap vaccination strategies are desperately needed to meet the needs of a growing population in developing countries as well as the challenges of fast spreading infectious diseases due to global traffic (e.g., 2009 swine flu pandemics).
Particle based vaccine formulations for TCI address several key issues in vaccination today. First of all TCI has been shown to induce superior immune responses as compared with systemic vaccines and to hold the potential to convey mucosal immunity. This is highly desirable to prevent microbial pathogens from entering the body through mucosal surfaces and thus block disease at a very early stage. This will also help to reduce the risk of horizontal transmission from infected individuals to susceptible hosts.
Many of the current strategies for TCI (e.g., micro-needles, Gene gun, PowderJect, skin abrasion), reduce the protective SC barrier for a significant time to facilitate the absorption of the vaccine. This makes them suboptimal for mass vaccination campaigns under critical hygienic conditions. Particle based formulations are an interesting alternative to this. Needle free strategies are at the forefront to combat vaccination-related transmitted diseases due to sharing of needles or needle stick accidents.
Over the past decade, particulate carriers have emerged as an attractive delivery strategy for antigens and adjuvants. Particle based formulations combine several desirable aspects which make them very attractive for antigen encapsulation. Vaccine antigens are often difficult biological entities, including DNA, peptides, proteins, attenuated viruses, microorganism fragments. (Nano)encapsulation can improve the stability, facilitate absorption and also increase antigenicity by mimicking the size of microorganisms.
The co-delivery of adjuvants is tantamount to increase the immunogenicity of the antigen and may allow a reduction of the antigen dose. In the context of vaccination so-called dose sparing allows reaching more people when limited amount of vaccine antigen is available such as in global pandemics. Furthermore, vaccines with higher efficiency are also needed to enable protection of immunosuppressed and elderly patients. Importantly, by choosing the right adjuvant it is possible to polarize the immune response in a predetermined direction and convey mucosal immunity.
However, until now TCI using particle based vaccine formulations has made no impact on medical practice. One of the main difficulties is that NPs cannot penetrate the skin to an extent that would allow the application of the required dose of antigen. This is due to the formidable stratum corneum (SC) barrier, the limited amount of antigen in the formulation and often an insufficient immunogenicity. A multitude of strategies are currently under investigation to overcome these issues. We highlight selected methods presenting a spectrum of solutions ranging from transfollicular delivery, to devices disrupting the SC barrier and the combination of particle based vaccines with adjuvants discussing their advantages and shortcomings. Some of these are currently at an experimental state while others are already in clinical testing. All methods have been shown to be capable of transcutaneous antigen delivery.
Absorption Routes of Nanoparticles Applied to the Skin
One of the main challenges in TCI is that the vaccine antigens need to overcome the SC barrier in order to reach the Langerhans cells (LCs) in the epidermis which act as antigen presenting cells (APCs). Intact human skin is widely impermeable to solid NPs and MPs. Ultra-flexible liposomes are an exception to this rule. Due to the addition of edge activators such as surfactants and/or ethanol they are able to change shape and squeeze through the lipid channels of the SC, supposedly following an osmotic gradient.Citation1,Citation2 Ultra-flexible liposomes have been used foremost for encapsulating protein antigens.Citation3,Citation4 By including positively charged lipids in the formulation they become amenable to complexing nucleotide based drugs and thus may be an alternative for DNA vaccination.Citation5-Citation7 For a more extensive overview the reader is referred to some recent reviews.Citation8,Citation9 Furthermore, ultra-small NPs with sizes less than 10 nm can enter the SC to a low and highly variable extent.Citation10 The toxicity and lack of biodegradability of quantum dots, metal or metal oxide NPs notwithstanding, ultra-small NPs are not suitable for drug delivery and vaccination purposes due to the extremely small amount of drug or antigen which can be loaded onto these particles. Also due to their low and variable degree of penetration such formulations would probably require some active means of penetration enhancement to enable delivery of a suitable dose.
Nanoparticles for Transfollicular Vaccination
Polymeric NPs as well as lipid carriers which are often in the size range of a few 100 nm are especially interesting delivery systems for the purpose of TCI. Nonflexible NPs which are applied onto the skin migrate into the hair follicle openings by a size-selective mechanism ().Citation11 The hair follicle is a promising target for TCI without compromising the skin barrier.Citation12 Interestingly, the trans-follicular route is a common pathway for the invasion of allergens such as pollen grains.Citation13 First evidence for the importance of the transfollicular route for transcutaneous vaccine delivery came from differences observed between hairy and nude mice.Citation14 At least for DNA vaccines the hair cycle state also seems to determine the successful transfection.Citation15 Inside the hair follicles antigen uptake by the rich pool of peri-follicular antigen presenting cells (APCs) is facilitated due to the absence of a SC barrier in the lower follicular orifice.Citation16 Therefore this delivery strategy does not require application of any further measures which would reduce the skin barrier. It seems that transfollicular delivery elicits a CD8+ biased response which is recommendable for combating intracellular microbes and developing vaccines against cancer and virus infections.Citation17 We showed that transfollicular delivery of the nano-encapsulated model antigen ovalbumin (OVA) elicited similar proliferation of OVA specific CD4+ T cells as an i.m. injection of the same dose of soluble antigen in an adoptive transfer model.Citation12 Nano-encapsulation into polymeric NPs improved the delivery of OVA into the hair follicles on excised pig ears by a factor of 2.5–3 compared with OVA in solution and protected OVA from cleavage or aggregation so that it maintained its biological activity to a high degree during storage and shipping.Citation12 As an important outcome of the study we observed in vitro as well as in vivo an intrinsic adjuvanticity of the NPs which was also influenced by the polymer material used.Citation12 The effect can probably be enhanced and directed by the combination or co-encapsulation of adjuvants into the formulation.
Figure 1. Schematic representation of methods to enable or facilitate TCI: (A) transfollicular delivery, (B) mechanical dermabrasion, (C) jet injection of liquids or powders, (D) microneedles (Images are not drawn to scale).
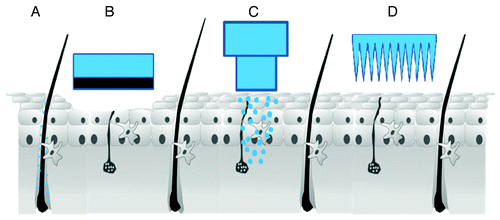
As hair follicles cover only 0.1% of the skin surface trans-follicular delivery has long been discussed to be negligible. It was demonstrated however that the capacity of hair follicles is similar to that of the SC.Citation18 Lademann et al. showed that NPs penetrated more efficient into the hair follicles than solutions and form a depot in the follicle that persists for more than one week.Citation19 Still a big issue with transfollicular application is the loss of formulation on the skin surface. Consequently a higher amount of vaccine would be required to achieve delivery of the required dose, which will need to be optimized in the future to make transfollicular vaccination a real alternative.
Facilitated Absorption Methods
Particle based vaccines benefit from any form of skin pre-treatment which reduces or removes the SC or any mechanical or electrical application method which transfers them into the epidermis by force (for review see also ref. Citation20). Occlusion or chemical penetration enhancers, while enhancing permeation of molecules by several folds due to influencing molecular partition and/or diffusion coefficients, have very little effect on the permeability of particles.Citation21 Apart from facilitating invasion the barrier disruption additionally causes a non-specific immunostimulation.Citation22 Often these facilitating methods require special devices. Important points to consider in this context include (1) cost for devices, (2) ease and reproducibility of use also by non-trained personnel (e.g., in a pandemic or for mass vaccination in developmental countries lacking medical infrastructure), (3) severity and area of barrier disruption, time scale of barrier recovery which are connected to the risk of invasion of pathogens through the superficial wound. The severity of these issues varies depending on the type of vaccination which is aspired. Naturally the tendency to tolerate risk is higher with therapeutic vaccines than with prophylactic immunizations.
For example mechanical abrasion of the SC across a large area may be acceptable to improve the permeability of an HIV vaccine. In the DermaVir patch plasmid DNA encoding for 15 HIV antigens is complexed with mannosylated positively charged polyethylene imine to form particles with sizes of 80–400 nm.Citation23 Mannosylation in this case facilitates the uptake of the particles by APCs. The patch is applied onto skin sites (2–8 areas 80 cm2 each on the back and the thighs) which are previously being abraded with a DermaPrep medical sponge ().Citation24,Citation25 In phase II clinical trials the patch was applied to HIV infected individuals on combination antiretroviral therapy (cART).Citation24,Citation25 The treatment was well tolerated also in a dose escalation study; mostly side effects were limited to mild local irritation.Citation24,Citation25 The vaccination increased CD-8+ cell counts and may enable intermission of cART.Citation24
Devices such as jet-injectors () or gene guns may relatively easily be compatible with particle based vaccine formulations. The principle relies on the generation of a jet of liquid or powder formulation which is propelled into the skin with great force. Dry formulations have considerable stability advantages. Depending on volume and injection depth a small amount of pain or bleeding and a relatively strong local inflammation may occur.Citation26 Immunogenicity is reported to be higher or equal to classic vaccine application.Citation26,Citation27 The history of using jet-injectors for vaccination goes back to the 1950s.Citation28 Problems with cases of hepatitis B which were transmitted person-to-person by multi-dose injectors were overcome by next generation devices which may either be prefilled for single use or come with exchangeable cartridges.Citation29
As a third technology with great potential to facilitate the absorption of NPs microneedles (), either solid, hollow, or biodegradable, should be mentioned. They can be used for pretreatment (e.g., Derma Roller) or as a leave on patch which also delivers the formulation. Although not needle-free they reduce sharps associated issues. In contrast to intradermal injections (including the prefilled syringe system BD soluvia which is approved in Europe for influenza vaccination) microneedles only reach the epidermis, stay above the dermal nerve endings and avoid pain. The particles can indeed be integrated in the microneedle arrays. DeMuth et al. constructed a system made from biodegradable antigen loaded MPs.Citation30 MPs made of poly(lactide-co-glycolide) (PLGA) were embedded in water soluble poly(acrylic acid) (PAA) which actually forms the microneedles.Citation30 Upon insertion into the skin, PAA dissolves; the microneedles themselves disintegrate and leave a depot of MPs providing controlled release of the antigen.Citation30 This design may enable separate delivery of a fast (from the matrix) and a slowly available dose (from the MPs, in mice MP-associated fluorescence was demonstrated up to 10 d after microneedle insertion at the site of injection).Citation30 Likewise the system could be advanced to deliver initial and booster doses of the vaccine in a single application.
Intrinsic Adjuvant Effect—Influence of Particle Properties
While for most applications NPs should be inert, that means that complement activation is not desirable, an immuno-stimulatory effect of the nano-formulation may be a benefit for vaccination purposes. Various properties such as size, surface charge and material properties play a vital role in shaping an immune response. A wide variety of particulate systems, including liposomes, virosomes, nanocomplexes and polymer based carriers are being investigated, all showing a different immunological outcome. For example, a particle based vaccine formulation may be capable of provoking a strong humoral response but fail to generate cellular responses. This might be due to different particle properties.
It is widely accepted that particle based vaccine delivery systems are more immunogenic than soluble antigen as particulate system mimic the size and structure of a pathogen. Among others this is due to the enhanced uptake by APCs. Carrier size also plays an important role in determining the type of response induced.Citation31 Recently, Mottram et al. showed that small differences in particle size (20–200 nm) influence the cytokine balance after a single immunization, showing that particle size in the range of 40–49 nm induces type 1 (cellular) responses and larger particles with 93–123 nm inducing type 2 (humoral) responses.Citation32 These findings are especially noteworthy while considering strategies of immunization against viruses where cellular responses are required.
Surface charge of the particles is another vital property which not only determines the stability of particles but plays an important role in contributing the immuno-regulatory effect of the particulate systems. With respect to TCI, Rancan et al. showed that positively charged particulate systems seem to be taken up better by LCs due to electrostatic interactions with cell membrane eventually favoring their internalization.Citation33 Moreover, Ma et al. showed even the appropriate surface charge density is crucial to have an effective and efficacious immune-regulatory effect.Citation34
Finally, particle material may influence immunogenicity. The main function of a material is to stabilize the antigen by protecting it from the surrounding biological conditions, to slow down the clearance of antigens and to enhance delivery to APCs with design constraints such as biodegradability and biocompatibility. One strategy is to use materials extracted from microbial (e.g., total polar lipid extract from archae bacteria, or poly-γ-glutamic acidCitation35,Citation36) to formulate particulate carriers. Interestingly, the materials themselves do not show adjuvant properties when delivered as solution but when formulated as particulate carrier they upregulated cytokine responses and MHC molecules, suggesting that particulate formation is necessary for the interaction between materials and certain surface molecule on APCs.Citation35,Citation36
Coupling of Nanoparticles with Adjuvants
Adjuvants are commonly added to vaccine formulations to enhance immunogenicity of poorly immunogenic antigens such as subunit vaccines or to shape the type of immune response, e.g., to increase cellular responses. For the same reasons particulate vaccine formulations may be combined with adjuvants. A variety of adjuvants with different mode of actions are being used in clinical testing, marketed formulations as well as in research settings. An important consideration is the risk of side effects by adjuvants. This is especially true for prophylactic vaccines which are given to healthy people and therefore have to fulfill the strongest safety criteria. For example cholera toxin and E. coli heat labile toxine (LT) are both very effective adjuvants for TCI and are very effective in humans.Citation37 Nonetheless considering the application of LT as patch against travelers’ diarrhea even the appearance of skin rashes and discoloration at the patch site may lead to the termination in a late clinical phase. At present, the choice of adjuvant is a compromise between necessity for adjuvanticity and minimal level of side effects.Citation38 Nevertheless, combination of adjuvant, antigen and particulate carrier may reduce the dose of antigen as well as adjuvant required in the formulation and consequently minimize the risk of side effects of novel adjuvant candidates for prophylactic vaccines.Citation39,Citation40 Adjuvants may either be (1) simply co-administered with encapsulated antigen, (2) encapsulated in separate particles or (3) co-encapsulated with the antigen in the same particle. Alternatively the particle surface may be decorated with the adjuvant by physical or chemical association ().
Figure 2. Combinations of antigen and adjuvant in a particulate carrier: (A) adjuvants are co-administered with the encapsulated antigen, (B) antigen and adjuvant are encapsulated in separate particles, (C) antigen and adjuvant are co-encapsulated in the same particle, (D) the particle surface is decorated with the antigen and/or the adjuvant by physical or chemical association.
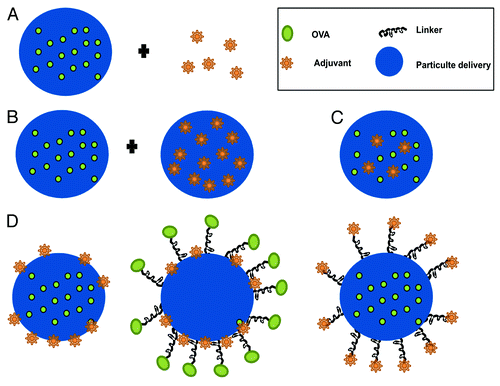
Recently, Bershteyn et al. showed that co-delivery of antigen with an adjuvant decorated on the surface of a particle generates humoral as well as cellular immune responses at ultra-low doses (at nanogram levels) of antigen and adjuvant.Citation41 In the same study, the authors have shown that co-loading of antigen and adjuvant in the same particle after a single immunization was significantly advantageous compared with antigen and adjuvant delivered on separate particles. But this advantage was lost after a boost immunization, suggesting that after boosting it does no longer matter whether antigen and adjuvant were on the same or separate particles. However, conflicting results exist in literature, whether it is beneficial to co-encapsulate adjuvant and antigen in the same particles or admix the adjuvant just prior to immunization (either in solution or separately encapsulated) in order to generate long lasting immune responses.Citation42,Citation43
Future studies also need to clarify which type of association between adjuvant and particle should be preferred. Conceivably this may depend on the type of adjuvant used. For example, an adjuvant which has receptors expressed on the surface of APCs (e.g., some sugars such as mannose) will probably benefit from being displayed on the particle surface. In contrast, encapsulation of intracellularly processed adjuvants will possibly generate a more robust immune response due to enhanced uptake by APCs and intracellular delivery compared with administration as solution.Citation44 Overall, combining adjuvant and antigen in particulate carriers either by (co-)encapsulation or admixing is an attractive approach to improve the efficacy of vaccines and reduce the cost of vaccination.
Conclusion
Altogether particle based vaccines hold great promise for TCI. However, their successful delivery is not without challenge. A product which makes it to the market probably needs to combine particles, antigen, adjuvant, and a device to achieve both a sufficient immunogenicity and sufficient permeation. This naturally increases the regulatory hurdles. At the same time production costs of the product should still be low to extend its affordability beyond the industrialized world. Some of the solutions presented in this overview raise hopes that this may indeed be achievable in the future.
Acknowledgments
Financial support for AM came from German Academic Exchange Service (DAAD).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Cevc G, Blume G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta 1992; 1104:226 - 32; http://dx.doi.org/10.1016/0005-2736(92)90154-E; PMID: 1550849
- Gratieri T, Schaefer UF, Jing L, Gao M, Kostka KH, Lopez RF, et al. Penetration of quantum dot particles through human skin. J Biomed Nanotechnol 2010; 6:586 - 95; http://dx.doi.org/10.1166/jbn.2010.1155; PMID: 21329051
- Gupta PN, Mishra V, Rawat A, Dubey P, Mahor S, Jain S, et al. Non-invasive vaccine delivery in transfersomes, niosomes and liposomes: a comparative study. Int J Pharm 2005; 293:73 - 82; http://dx.doi.org/10.1016/j.ijpharm.2004.12.022; PMID: 15778046
- Paul A, Cevc G, Bachhawat BK. Transdermal immunisation with an integral membrane component, gap junction protein, by means of ultradeformable drug carriers, transfersomes. Vaccine 1998; 16:188 - 95; http://dx.doi.org/10.1016/S0264-410X(97)00185-0; PMID: 9607029
- Geusens B, Van Gele M, Braat S, De Smedt SC, Stuart MCA, Prow TW, et al. Flexible nanosomes (SECosomes) enable efficient siRNA delivery in cultured primary skin cells and in the viable epidermis of ex vivo human skin. Adv Funct Mater 2010; 20:4077 - 90; http://dx.doi.org/10.1002/adfm.201000484
- Mishra D, Mishra PK, Dubey V, Nahar M, Dabadghao S, Jain NK. Systemic and mucosal immune response induced by transcutaneous immunization using Hepatitis B surface antigen-loaded modified liposomes. Eur J Pharm Sci 2008; 33:424 - 33; http://dx.doi.org/10.1016/j.ejps.2008.01.015; PMID: 18359615
- Wang J, Hu JH, Li FQ, Liu GZ, Zhu QG, Liu JY, et al. Strong cellular and humoral immune responses induced by transcutaneous immunization with HBsAg DNA-cationic deformable liposome complex. Exp Dermatol 2007; 16:724 - 9; http://dx.doi.org/10.1111/j.1600-0625.2007.00584.x; PMID: 17697144
- Hansen S, Lehr C-M. Nanoparticles for transcutaneous vaccination. Microb Biotechnol 2012; 5:156 - 67; http://dx.doi.org/10.1111/j.1751-7915.2011.00284.x; PMID: 21854553
- Romero EL, Morilla MJ. Topical and mucosal liposomes for vaccine delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2011; 3:356 - 75; http://dx.doi.org/10.1002/wnan.131; PMID: 21360692
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol Sci 2006; 91:159 - 65; http://dx.doi.org/10.1093/toxsci/kfj122; PMID: 16443688
- Patzelt A, Richter H, Knorr F, Schäfer U, Lehr CM, Dähne L, et al. Selective follicular targeting by modification of the particle sizes. J Control Release 2011; 150:45 - 8; http://dx.doi.org/10.1016/j.jconrel.2010.11.015; PMID: 21087645
- Mittal A, Raber AS, Schaefer UF, Weissmann S, Ebensen T, Schulze K, et al. Non-invasive delivery of nanoparticles to hair follicles: A perspective for transcutaneous immunization. Vaccine 2013; In Press http://dx.doi.org/10.1016/j.vaccine.2012.12.048; PMID: 23290836
- Jacobi U, Engel K, Patzelt A, Worm M, Sterry W, Lademann J. Penetration of pollen proteins into the skin. Skin Pharmacol Physiol 2007; 20:297 - 304; http://dx.doi.org/10.1159/000108101; PMID: 17851273
- Fan H, Lin Q, Morrissey GR, Khavari PA. Immunization via hair follicles by topical application of naked DNA to normal skin. Nat Biotechnol 1999; 17:870 - 2; http://dx.doi.org/10.1038/12856; PMID: 10471927
- Shaker DS, Sloat BR, Le UM, Löhr CV, Yanasarn N, Fischer KA, et al. Immunization by application of DNA vaccine onto a skin area wherein the hair follicles have been induced into anagen-onset stage. Mol Ther 2007; 15:2037 - 43; http://dx.doi.org/10.1038/sj.mt.6300286; PMID: 17700542
- Baleeiro RB, Wiesmüller KH, Reiter Y, Baude B, Dähne L, Patzelt A, et al. Topical Vaccination with Functionalized Particles Targeting Dendritic Cells. J Invest Dermatol 2013; In Press http://dx.doi.org/10.1038/jid.2013.79; PMID: 23426134
- Combadière B, Vogt A, Mahé B, Costagliola D, Hadam S, Bonduelle O, et al. Preferential amplification of CD8 effector-T cells after transcutaneous application of an inactivated influenza vaccine: a randomized phase I trial. PLoS One 2010; 5:e10818; http://dx.doi.org/10.1371/journal.pone.0010818; PMID: 20520820
- Otberg N, Patzelt A, Rasulev U, Hagemeister T, Linscheid M, Sinkgraven R, et al. The role of hair follicles in the percutaneous absorption of caffeine. Br J Clin Pharmacol 2008; 65:488 - 92; http://dx.doi.org/10.1111/j.1365-2125.2007.03065.x; PMID: 18070215
- Lademann J, Richter H, Teichmann A, Otberg N, Blume-Peytavi U, Luengo J, et al. Nanoparticles--an efficient carrier for drug delivery into the hair follicles. Eur J Pharm Biopharm 2007; 66:159 - 64; http://dx.doi.org/10.1016/j.ejpb.2006.10.019; PMID: 17169540
- Mitragotri S. Mechanical disruption of skin barrier for vaccine delivery. Drug Deliv Syst 2012; 27:202 - 12; http://dx.doi.org/10.2745/dds.27.202
- Lopez RF, Seto JE, Blankschtein D, Langer R. Enhancing the transdermal delivery of rigid nanoparticles using the simultaneous application of ultrasound and sodium lauryl sulfate. Biomaterials 2011; 32:933 - 41; http://dx.doi.org/10.1016/j.biomaterials.2010.09.060; PMID: 20971504
- Karande P, Mitragotri S. Transcutaneous immunization: an overview of advantages, disease targets, vaccines, and delivery technologies. Annu Rev Chem Biomol Eng 2010; 1:175 - 201; http://dx.doi.org/10.1146/annurev-chembioeng-073009-100948; PMID: 22432578
- Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, et al. Control of HIV despite the discontinuation of antiretroviral therapy. [2] N Engl J Med 1999; 340:1683 - 4; http://dx.doi.org/10.1056/NEJM199905273402114; PMID: 10348681
- Gudmundsdotter L, Wahren B, Haller BK, Boberg A, Edbäck U, Bernasconi D, et al. Amplified antigen-specific immune responses in HIV-1 infected individuals in a double blind DNA immunization and therapy interruption trial. Vaccine 2011; 29:5558 - 66; http://dx.doi.org/10.1016/j.vaccine.2011.01.064; PMID: 21300092
- Lisziewicz J, Bakare N, Calarota SA, Bánhegyi D, Szlávik J, Újhelyi E, et al. Single DermaVir immunization: dose-dependent expansion of precursor/memory T cells against all HIV antigens in HIV-1 infected individuals. PLoS One 2012; 7:e35416; http://dx.doi.org/10.1371/journal.pone.0035416; PMID: 22590502
- Williams J, Fox-Leyva L, Christensen C, Fisher D, Schlicting E, Snowball M, et al. Hepatitis A vaccine administration: comparison between jet-injector and needle injection. Vaccine 2000; 18:1939 - 43; http://dx.doi.org/10.1016/S0264-410X(99)00446-6; PMID: 10699344
- Matheï C, Van Damme P, Meheus A. Hepatitis B vaccine administration: comparison between jet-gun and syringe and needle. Vaccine 1997; 15:402 - 4; http://dx.doi.org/10.1016/S0264-410X(96)00196-X; PMID: 9141211
- Millar JD, Morris L, Macedo Filho A, Mack TM, Dyal W, Medeiros AA. The introduction of jet injection mass vaccination into the national smallpox eradication program of Brazil. Trop Geogr Med 1971; 23:89 - 101; PMID: 5573585
- Abb J, Deinhardt F, Eisenburg J. The risk of transmission of hepatitis B virus using jet injection in inoculation. J Infect Dis 1981; 144:179; http://dx.doi.org/10.1093/infdis/144.2.179; PMID: 7276631
- Demuth PC, Garcia-Beltran WF, Ai-Ling ML, Hammond PT, Irvine DJ. Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv Funct Mater 2013; 23:161 - 72; http://dx.doi.org/10.1002/adfm.201201512; PMID: 23503923
- Singh M, Chakrapani A, O’Hagan D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev Vaccines 2007; 6:797 - 808; http://dx.doi.org/10.1586/14760584.6.5.797; PMID: 17931159
- Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, et al. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm 2007; 4:73 - 84; http://dx.doi.org/10.1021/mp060096p; PMID: 17274665
- Rancan F, Gao Q, Graf C, Troppens S, Hadam S, Hackbarth S, et al. Skin penetration and cellular uptake of amorphous silica nanoparticles with variable size, surface functionalization, and colloidal stability. ACS Nano 2012; 6:6829 - 42; http://dx.doi.org/10.1021/nn301622h; PMID: 22797484
- Ma YF, Zhuang Y, Xie XF, Wang C, Wang F, Zhou DM, et al. The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses. Nanoscale 2011; 3:2307 - 14; http://dx.doi.org/10.1039/c1nr10166h; PMID: 21499635
- Higa LH, Schilrreff P, Perez AP, Iriarte MA, Roncaglia DI, Morilla MJ, et al. Ultradeformable archaeosomes as new topical adjuvants. Nanomedicine 2012; 8:1319 - 28; http://dx.doi.org/10.1016/j.nano.2012.02.008; PMID: 22366598
- Uto T, Akagi T, Hamasaki T, Akashi M, Baba M. Modulation of innate and adaptive immunity by biodegradable nanoparticles. Immunol Lett 2009; 125:46 - 52; http://dx.doi.org/10.1016/j.imlet.2009.05.008; PMID: 19505507
- Frech SA, Dupont HL, Bourgeois AL, McKenzie R, Belkind-Gerson J, Figueroa JF, et al. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet 2008; 371:2019 - 25; http://dx.doi.org/10.1016/S0140-6736(08)60839-9; PMID: 18554712
- Gupta RK, Relyveld EH, Lindblad EB, Bizzini B, Ben-Efraim S, Gupta CK. Adjuvants--a balance between toxicity and adjuvanticity. Vaccine 1993; 11:293 - 306; http://dx.doi.org/10.1016/0264-410X(93)90190-9; PMID: 8447157
- Diwan M, Elamanchili P, Cao M, Samuel J. Dose sparing of CpG oligodeoxynucleotide vaccine adjuvants by nanoparticle delivery. Curr Drug Deliv 2004; 1:405 - 12; http://dx.doi.org/10.2174/1567201043334597; PMID: 16305402
- Wilson KD, de Jong SD, Tam YK. Lipid-based delivery of CpG oligonucleotides enhances immunotherapeutic efficacy. Adv Drug Deliv Rev 2009; 61:233 - 42; http://dx.doi.org/10.1016/j.addr.2008.12.014; PMID: 19232375
- Bershteyn A, Hanson MC, Crespo MP, Moon JJ, Li AV, Suh H, et al. Robust IgG responses to nanograms of antigen using a biomimetic lipid-coated particle vaccine. J Control Release 2012; 157:354 - 65; http://dx.doi.org/10.1016/j.jconrel.2011.07.029; PMID: 21820024
- Fischer S, Schlosser E, Mueller M, Csaba N, Merkle HP, Groettrup M, et al. Concomitant delivery of a CTL-restricted peptide antigen and CpG ODN by PLGA microparticles induces cellular immune response. J Drug Target 2009; 17:652 - 61; http://dx.doi.org/10.1080/10611860903119656; PMID: 19622019
- Schlosser E, Mueller M, Fischer S, Basta S, Busch DH, Gander B, et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine 2008; 26:1626 - 37; http://dx.doi.org/10.1016/j.vaccine.2008.01.030; PMID: 18295941
- Bal SM, Hortensius S, Ding Z, Jiskoot W, Bouwstra JA. Co-encapsulation of antigen and Toll-like receptor ligand in cationic liposomes affects the quality of the immune response in mice after intradermal vaccination. Vaccine 2011; 29:1045 - 52; http://dx.doi.org/10.1016/j.vaccine.2010.11.061; PMID: 21129393