Abstract
An intense effort has been launched to develop improved anthrax vaccines that confer rapid, long lasting protection preferably with an extended stability profile amenable for stockpiling. Protective antigen (PA)-based vaccines are most favored as immune responses directed against PA are singularly protective, although the actual protective mechanism remains to be unraveled. Herein we show that contrary to the prevailing view, an efficacious PA-based vaccine confers protection against inhalation anthrax by preventing the establishment of a toxin-releasing systemic infection. Equally importantly, antibodies measured by the in vitro lethal toxin neutralization activity assay (TNA) that is considered as a reliable correlate of protection, especially for PA protein-based vaccines adjuvanted with aluminum salts appear to be not absolutely essential for this protective immune response.
Introduction
Anthrax is both a toxemic and an invasive bacteremic disease caused by B. anthracis and the pathogenesis is primarily dictated by four plasmid encoded virulence factors that constitute a tripartite exotoxin and a poly-γ-d-glutamic acid capsule that deflects complement deposition and phagocytosis. Protective antigen (PA) is the receptor binding component of the tripartite exotoxin which forms binary complexes either with the lethal factor (LF) or the edema factor (EF), in generating lethal toxin (LT) or edema toxin (ET) respectively.Citation1 These toxins are released in copious amounts during the vegetative growth of B. anthracis and are readily detectable in the serum of an infected host.Citation2 Both purified LT and ET when administered intravenously or intraperitoneally in microgram quantities have proven to be deadly in in vivo animal studies attesting to their pivotal role in the overwhelming systemic pathophysiology associated with B. anthracis infection in non-immune susceptible hosts.Citation1 A large body of evidence has amassed to indicate that an immune response to PA is both necessary and sufficient to protect against anthrax.Citation3,Citation4 The currently licensed Biothrax/AVA vaccine, an alum-adsorbed cell free filtrate of an acapsular strain (V770-NP1-R) of B. anthracis composed predominantly of PA requires a primary series of three doses administered at 0, 1 and 6 mo and subsequent booster dose. For biodefense purposes a vaccine that induces rapid, long lasting immunity with an extended stability profile amenable for stockpiling is desirable. This vaccine or other PA protein-based anthrax vaccine candidates such as recombinant PA (rPA) adjuvanted with aluminum salts elicit high levels of PA-directed toxin neutralizing antibodies and these toxin-neutralizing antibodies are considered to be pivotal in protecting the vaccinees from the lethal effects of toxins released by vegetatively growing B. anthracis upon spore germination.Citation3-Citation5 Contrary to this view, we present evidence that in vaccinated animals exposed to a lethal dose of virulent spores, the arrest of the infection precedes extensive vegetative bacterial multiplication and the release of copious amounts of toxins, and equally importantly, antibodies measured by the in vitro lethal toxin neutralization activity assay (TNA) are dispensable for this early protective immune response.
Results and Discussion
During the past decade, there has been an intense search for an improved anthrax vaccine and an array of new candidate vaccines have been developed for evaluation and comparison with the licensed AVA. In assessing candidate vaccines in conformity with the FDA animal rule there has been an evolving perspective that perhaps antibodies measured by the in vitro lethal toxin neutralization activity assay (TNA) which is a function-based assay that is species neutral is a better predictor or a correlate of protection than the serum anti-PA IgG titer for a given vaccine candidate. The underlying premise being that the toxins released from vegetatively growing B. anthracis as a result of the germination of inhaled spores requires neutralization for the survival of vaccinated subjects.Citation6-Citation8
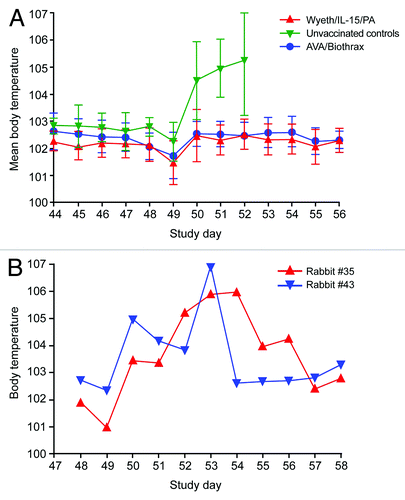
Recently we reported that a cohort of rabbits immunized with a vaccinia-based PA vaccine created by the integration of the pag gene and an immune enhancing cytokine IL-15 into the genome of licensed smallpox vaccine (Wyeth/IL-15/PA), conferred sterile protection against an inhalation challenge exceeding 200 LD50 of fully virulent Ames strain (A0462; pXO1+ pXO2+) spores.Citation9 The absence of bacteremia was also observed in the positive comparator group vaccinated with AVA suggesting perhaps PA based vaccines protect vaccinated subjects from inhalation anthrax by aborting the early events of the establishment of B. anthracis infection in the respiratory tract.Citation9 To explore this intriguing possibility in depth, first we examined the daily body temperature fluctuations in the rabbits that were subjected to the inhalation spore challenge as progressively rising body temperature is a consistent clinical manifestation in rabbits infected with B. anthracis via the inhalation route and parallels bacterial growth and toxin release.Citation10 The mean body temperature of the three groups of rabbits were similar prior to the inhalation spore challenge, for example, 24 h before the spore challenge, the mean body temperature of the Wyeth/IL-15/PA group was 102.14°F (SD 0.62), whereas the AVA group and the control group displayed a mean body temperature of 102.07°F (SD 0.58) and 102.80°F (SD 0.34) respectively.
As shown in , the body temperature of rabbits vaccinated with either Wyeth/IL-15/PA (n = 9) or AVA (n = 9) remained normal throughout the post-challenge observation period with mean body temperatures being 102.20 and 102.40°F respectively that were not significantly different. In contrast, all animals in the control group (n = 7) displayed rapidly rising body temperatures that continued unabated until their death within 4 d of spore challenge (100% mortality in this control group). The post-challenge elevation of body temperatures in this group was statistically significant (p < 0.001 in comparison to Wyeth/IL-15/PA group or p < 0.01 in comparison to AVA group; Tukey’s multiple comparison test). It is noteworthy that the body temperatures of the rabbits in all three groups were transiently lowered immediately after the inhalation spore challenge perhaps as a consequence of the anesthesia used during the manipulations. Moreover, all animals in the control group also had high bacterial counts in their blood as we reported previously.Citation9 We also included two rabbits (#35 and #43) that were immunized with a sub-optimal dose of a vaccine derived from a non-replicating MVA vaccinia virus (MVA/IL-15/PA). Both of these rabbits had serum anti-PA IgG tites of 20,000 just prior to the inhalation spore challenge, whereas the mean serum anti-PA IgG titers for the Wyeth/IL-15/PA and AVA groups were 515,600 ± 192,300(SD) and 275,600 ± 165,500(SD) respectively as reported previously.Citation9 In addition, we did not detect any TNA antibodies in rabbits #35 and #43 prior to the spore challenge. The TNA titers for the Wyeth/IL-15/PA and AVA groups have been reported previouslyCitation9 and had mean values of 613 ± 540(SD) and 1948 ± 1140(SD) respectively. The body temperatures of rabbits #35 and #43 were elevated rapidly and pyrexia (body temperature above 104°F) persisted for several days (). Consistent with their elevated body temperatures these two rabbits remained bacteremic up to 6 d post-challenge although both animals were able to make a full recovery from their pulmonary anthrax.
Figure 1. Daily body temperature fluctuations in rabbits before and after B. anthracis spore challenge. In panel (A), rabbits were vaccinated with Wyeth/IL-15/PA (n = 9), AVA/Biothrax (n = 9) or control Wyeth/IL-15 (n = 7) on study day 0 and 28. Rabbits were given 2 doses of vaccine 4 weeks apart (study day 0 and 28), subcutaneously with the respective vaccinia virus recombinants (Wyeth/IL-15/PA or MVA/IL-15/PA) at a dose of 1x107 pfu. AVA/Biothrax vaccine was given intramuscularly with the same schedule as for vaccinia recombinants (50 microliters of AVA/Biothrax vaccine mixed with 450 microliters of PBS). Rabbits were challenged (study day 49) with a mean dose of 255 LD50 of B. anthracis (Ames strain, A0462; pXO1+; pXO2+; 1LD50 = 105,000 CFU) spores obtained from BEI Resources by oro-nasal inhalation under appropriate biosafety containment at the Southern Research Institute. In panel (B), two rabbits, #35 and #43 were vaccinated with a suboptimal dose of MVA/IL-15/PA. All animals were then subjected to an inhalation spore challenge of B. anthracis Ames strain with a mean challenge dose of 255 LD50 on study day 49. The body temperatures were measured (in °F) once daily before the inhalation challenge and 8 hourly (3 times a day) during the post-challenge observation period via a subcutaneously implanted programmable temperature transponder.
As another determinant of the germination of inhaled spores and the vegetative growth of B. anthracis in the vaccinated rabbits, we measured the serum LF levels utilizing an ultra sensitive method with a lower limit of detection of 5 picograms of LF per milliliter.Citation11 As shown in , only one animal in the Wyeth/IL-15/PA vaccinated group had any measurable LF (13 picograms/ml) while several rabbits in the AVA group also revealed the presence of some measurable LF in their sera. The two rabbits, #35 and #43, that were pyrexic and bacteremic too had measurable levels of serum LF although the levels were vastly different (11565 vs. 20 picograms/ml). Similar to this observation of wide variations in the serum LF levels, highly disparate serum PA levels have been reported in infected rabbits.Citation10 The LF secreted by vegetatively growing B. anthracis persists in the serum for several days and in non-immune rabbits infected with B. anthracis spores, the serum LF levels reach around 10–35 µg per milliliter by 48 h post-infection.Citation12 Therefore, the detection of only trace amounts of circulatory LF, if any, along with the absence of pyrexia and bacteremia, collectively imply that in rabbits vaccinated with either Wyeth/IL-15/PA or AVA, the inhaled spores failed to establish a productive infection although some limited germination of spores would likely have happened, as in certain animals trace levels of LF were still detectable. Strengthening this notion, when we examined the serum anti-PA IgG titers pre- and 14 d post-challenge, there was a 2.89 ± 1.36(SD) fold rise in the Wyeth/IL-15/PA group vs. 4.89 ± 4.26(SD) fold rise in the AVA group and the differences were not significant (p = 0.198). In contrast, the two rabbits #35 and #43 vaccinated with non-replicating vaccinia vaccine that had an anti-PA IgG titer of 20,000 before the spore challenge displayed a titer of 2,560,000 (128-fold rise) 14 d post-challenge.
Table 1. Post challenge serum LF levels in rabbits
To better understand how efficacious vaccines impact in vivo growth events of B. anthracis, we opted to use a mouse model that would also allow us to visualize in real time the dynamic early events following the inhalation of B. anthracis spores by in vivo imaging. To this end, we vaccinated groups of A/J mice with three different anthrax vaccines including AVA, rPA, and an attenuated vaccinia-based PA vaccine molecularly adjuvanted with IL-15 (LC16M8/IL-15/PA). All three vaccines were capable of inducing high levels of anti-PA IgG levels (). For anti-PA IgG titer calculations, the data from the two experiments shown in were combined and the-mean anti-PA IgG titers for the three vaccines were 223 000 ± 179 600 (SD) for AVA; 308 000 ± 199 600 (SD) for rPA, and 344 000 ± 214 200 (SD) for LC16M8/IL-15/PA. However, the anti-PA IgG titer differences were not significant among the groups (p = 0.1355) although the highest levels of anti-PA IgG were seen with LC16M8/IL-15/PA.
Table 2. Sero-status of mice prior to challenge and their survival after a 20LD50 inhalation spore challenge of B. anthracis Sterne strain
Both rPA and AVA vaccines contain the PA protein with aluminum salts that enhances antibody responses with a preferential Th2 bias. With LC16M8/IL-15/PA vaccine, the in vivo expression of the PA takes place in an inflammatory milieu in the presence of co-expressed proinflammatory cytokine IL-15 that dictates the anti-PA immune response in the vaccinated mice. Moreover, the PA polypeptide expressed in eukaryotic cells is glycosylatedCitation9,Citation13,Citation14 and could potentially influence the immune responses generated by LC16M8/IL-15/PA as well. When we assessed the subclass specificities of anti-PA IgG, the aluminum salt-adjuvanted protein vaccines elicited a predominantly Th2-biased IgG1 response compared with a lower IgG1 response elicited by LC16M8/IL-15/PA (). In contrast, a superior Th1-biased IgG2a response was evident in the mice vaccinated with LC16M8/IL-15/PA. Thus, LC16M8/IL-15/PA induces a more balanced Th1/Th2 type immune response with demonstrably high levels of both IgG1 and IgG2a anti-PA antibodies, whereas rPA is almost exclusively Th2-skewed with hardly any detectable IgG2a antibodies. Possibly because of residual bacillary components, AVA elicited some levels of anti-PA IgG2a antibodies in the vaccinated mice. However, the role of vaccine-induced IgG subclass specificities in the protection remains unclear as all three vaccines were equally effective.
Figure 2. Subtype specificities of anti-PA IgG induced in A/J mice after immunization with recombinant PA, AVA/Biothrax and LC16M8/IL-15/PA vaccines. Groups of mice (n = 10) were immunized with AVA/Biothrax, rPA or LC16M8/IL-15/PA as described in the Materials and Methods section. Two doses of vaccines were administered 4 weeks apart and the sera from vaccinated mice were collected 25 d after the second immunization and pooled for each group. Pooled sera from each group were tested in triplicate in an ELISA with PA coated plates. In panel (A), PA bound IgG1 antibodies are shown whereas in panel (B), PA bound IgG2a antibodies are shown.
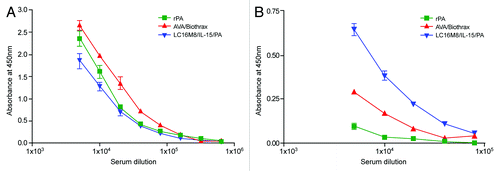
When we assessed PA-antibodies by the in vitro lethal toxin neutralization activity assay (TNA), although both AVA and rPA protein vaccines elicited high levels of TNA antibodies in the vaccinated mice [the mean titers for the two respective groups being 1345 ± 1161 (SD) and 3492 ± 2956 (SD)], paralleling the levels of anti-PA IgG in their sera, the TNA antibody levels in the mice vaccinated with LC16M8/IL-15/PA, were low or in some animals even non-existent () with a mean titer of 285 ± 722 (SD) for the entire group. The TNA titer difference between AVA and rPA groups was not significant, although the differences between either LC16M8/IL-15/PA and AVA or LC16M8/IL-15/PA and rPA were significant (p < 0.001). It should be noted that for TNA titer calculations, the data from two experiments shown in were combined. However, a significant positive correlation was found between anti-PA IgG ELISA titer and TNA titer for all three vaccines (AVA group, r = 0.4225; LC16M8/IL-15/PA group, r = 0.8492; rPA group, r = 0.8299). We note that the correlation was lower than what has been reported for AVA but the reason for this difference remains unclear.
The wide divergence of TNA antibody titer from anti-PA IgG titer in the LC16M8/IL-15/PA vaccinated mice provided us the opportunity to determine the relative contribution of anti-PA IgG vs. TNA antibodies in conferring protection against inhaled spores. In non-human primates and rabbits vaccinated with aluminum salt-adjuvanted PA protein vaccines such as AVA and rPA, the TNA antibodies provide a reliable cross-species prediction of survival to an aerosol challenge.Citation8 Although, both AVA and rPA also induce TNA antibodies in vaccinated mice whether these TNA antibody levels in mice correlate with protection has not been firmly established. The data shown in suggests the possible existence of such a correlate. When we challenged the vaccinated mice with a 20 LD50 dose of B. anthracis strain 7702, no mortality was seen in any of the three vaccinated groups (except one outlier in the LC16M8/IL-15/PA group) attesting to their equal potency in preventing inhalation anthrax, whereas all unvaccinated animals in the control group died. Because several mice in the LC16M8/IL-15/PA group lacked any detectable TNA antibodies and yet were protected from a lethal spore challenge, this finding along with similar observations made with certain DNA vaccines where non-human primates were protected in the absence of TNA antibodiesCitation15 raise concerns against the universal applicability of the TNA titer as a reliable correlate of protection against inhalation anthrax. It is also important to note that in the rabbit study, the #35 and #43 rabbits without any detectable toxin neutralizing antibodies were also capable of surviving a greater than 200 LD50 inhalation spore challenge as described above.
Because of the presence of PA protein on the spore surface and the demonstrable ability of anti-PA antibodies to impede spore germination, as well as enhance phagocytic and sporicidal activities of macrophages in in vitro studies, it has been suggested that these anti-spore activities of anti-PA antibodies could play a role in the protection during the initial stages of B. anthracis infection in vivo.Citation16,Citation17 To gain insights as to how these PA-based anthrax vaccines confer protection and impact the vegetative growth of B. anthracis infection, we subjected the vaccinated mice to a lethal inhalation spore challenge, with a derivative of B. anthracis 7702 (7702-lux strain) that emanates luminescence exclusively during the vegetative phase of growthCitation18 and utilized an in vivo bioluminescence imaging system to monitor the progression of infection both temporally and spatially (). The sero-status with respect to anti-PA IgG and TNA titers of each individual animal in the groups was measured just prior to the spore challenge and are shown in , experiment B. One day after the spore challenge, as has been previously reportedCitation18,Citation19 robustly growing B. anthracis was evident in nasally associated lymphoid tissues (NALT) in all animals in the unvaccinated control group, whereas in animals in the three vaccinated groups only a marginal growth was detected that was reminiscent of an abortive growth pattern associated with an aerosolic infection of Sterne strain 7702 lacking the pag gene (Δpag) described previously.Citation18 Nonetheless, this limited growth that was strictly confined to NALT was completely cleared by day 2 post-challenge in all of the animals in all three vaccinated groups except one mouse likely to be an outlier in the LC16M8/IL-15/PA group (one death in two separate experiments involving 20 mice), which had sustained growth in NALT without further dissemination but died on day 5 post-challenge. In contrast, progressively disseminating infection was seen in all of the unvaccinated control group animals resulting in 100% mortality. The existence of a dissemination bottleneck for inhaled Sterne spores has been demonstratedCitation20 in the A/J mouse model that operates downstream of NALT where germination first occurs in this model and the dissemination of vegetative bacilli beyond the NALT has been shown to be dependent on the expression of lethal toxin by vegetative bacilli.Citation18 It is apparent from , that the immune response induced by PA-based vaccines exerts its impact upstream of these impediments of dissemination since the luminescence signal in the NALT of vaccinated mice is much lower than that of unvaccinated mice 24 h post-inhalation spore challenge, the only time point at which we were able to detect any bacillary growth in the vaccinated mice.
Figure 3. Inhaled B. anthracis spores fail to establish systemic, toxin-releasing vegetative infections in mice immunized with PA-based anthrax vaccines. Groups of mice were immunized with Biothrax/AVA (AVA), recombinant PA (rPA) or LC16M8/IL-15/PA recombinant vaccinia virus (vac/IL-15/PA) as described in the Materials and Methods section and four weeks after the second dose of vaccine, mice were subjected to a 20 LD50 (4–8x106 spores) aerosolic inhalation spore challenge with a derivative of B.anthracis 7702 that emanates luminescence exclusively during the vegetative phase of growth (7702-lux strain). In vivo imaging was performed using an IVIS 100 imaging system from Xenogen and analyzed by using Living Image 2.5 software. The pseudo color scale represents light unit counts in photons/s/cm2 and the luminescence in unvaccinated mice in day 1 corresponds to 1–2 x105 CFU B. anthracis in NALT as previously reported.Citation18
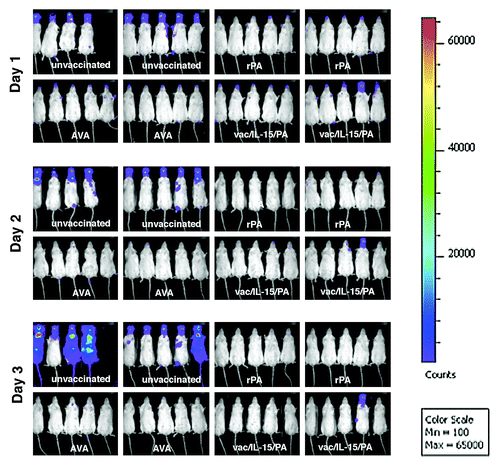
The true extent of progressive B. anthracis infection in a previously vaccinated immune host following an inhalation exposure to a lethal dose of infective spores has not been explored adequately or demonstrated convincingly. More importantly, in few studies where post-challenge bacteremia has been evaluated in vaccinated NHP or rabbits, the detection of vegetative bacteria in the blood has been infrequent and rare.Citation9,Citation21,Citation22 The very limited initial vegetative growth we observed with bioluminescence in the NALT of mice vaccinated with the three vaccines is consistent with the trace levels of LF we detected in rabbits vaccinated with Wyeth/IL-15/PA or AVA/Biothrax (see ). It is likely that in these vaccinated rabbits, just as in vaccinated mice, some minimal initial vegetative growth of B. anthracis would have occurred with the release of some trace amounts of exotoxins that we were able to capture with our ultra sensitive LF detection assay, although the bacillary biomass of this limited growth was not only insufficient to trigger a pyrogenic response, but was also rapidly cleared without any evidence of detectable bacteremia in these rabbits. The use of the A/J mouse model to confirm and further explore the very-early arrest of inhaled Ames spores that we observed in the vaccinated rabbits could be criticized because of the inherent limitations associated with this complement-deficient mouse model that utilizes an attenuated acapsular Sterne strain of B. anthracis, which may not truly recapitulate the early interactions of fully virulent inhaled spores with host effector responses of higher phylogenetic animal models. Nonetheless, our in vivo bioluminescence imaging data are concordant with what we observed in the vaccinated rabbits thus underscoring the utility of this A/J mouse model despite its inherent limitations to explore certain aspects of host-pathogen interactions in a less restrictive laboratory environment in a cost effective manner.
Toxemia is a pivotal driver of anthrax pathogenesis and as such, passive administration of LT neutralizing antibodies confers protection from death against inhalation anthrax, if administered sufficiently early in the disease process in several animal models.Citation23 However, in the case of protection afforded by passively transferred toxin neutralizing antibodies, the amount of circulating toxin-neutralizing antibodies required to achieve survival is much greater than what is needed in active vaccination, thus implying the involvement of humoral effector mechanisms other than toxin neutralization in the vaccine-induced acquired immune response. For PA protein based vaccines such as AVA and rPA, it is likely that the antibodies measured by TNA are inclusive of these other effectors. On the other hand, with vector delivered (LC16M8/IL-15/PA as was shown here) or certain DNA-based PA vaccines as have been shown previously,Citation15,Citation24,Citation25 there appears to be a more pronounced and preferential induction of effectors other than antibodies measured by the in vitro lethal toxin neutralization activity assay (TNA) thus allowing the separation and dissociation of these antibody entities with their exclusive protective capabilities.Citation15,Citation24,Citation25 It is also possible that vector-delivered PA vaccines induce potent cell mediated immune responses that can effectively contribute to the elimination of inhaled spores or arrest their germination. The exact mechanisms by which these vector-delivered PA vaccines confer protection against inhaled B. anthracis spores especially in the absence of TNA antibodies will be a focus of our future endeavors.
Importantly, this study provides evidence utilizing two animal models involving rabbits and A/J mice that an efficacious PA-based vaccine confers protection against inhalation anthrax by preventing the establishment and dissemination of a toxin-releasing systemic infection and, of equal significance, that antibodies measured by the in vitro lethal toxin neutralization activity assay (TNA) are not an absolute element essential in this protective immune response, although they are likely to be pivotal for PA protein vaccines. A correlate of protection is consequently dependent on the vaccine type and its formulation. The evidence presented here thus underscores the importance of looking beyond the current emphasis on the antibodies measured by the in vitro lethal toxin neutralization activity assay (TNA) in identifying anthrax vaccines with improved efficacy against the inhalation form of anthrax that is most lethal and is of greatest concern, especially if B. anthracis is weaponized as a bioterror agent.
Materials and Methods
Vaccines, rabbits, mice, and bacterial strains
Recombinant PA (rPA) protein and AVA/Biothrax vaccine were from BEI Resources. The construction of LC16M8/IL-15/PA vaccinia recombinant was identical to the method described previouslyCitation9 and detailed in the Supplemental Materials section. Ten weeks old-female New Zealand white rabbits were from Myrtle’s Rabbitry. Eight-week-old female A/J mice were from the National Cancer Institute. B. anthracis Sterne strains 7702 (pXO1+;pXO2-) and its luminescent derivative (7702-lx strains) have been described previously.Citation18
Anti-PA serological assessment
An ELISA was used to determine the titers of anti-PA IgG antibodies.Citation4 The J774A.1 cell-based toxin neutralization assay was performed essentially as described by Li et al.Citation26 and a detailed description is given in the Supplemental Materials section.
Serum Lethal Factor quantification by mass spectrometry
Serum LF activity was detected and quantitated by a validated mass spectrometry method based on the targeted cleavage of a peptide substrate by LF endoproteinase activity that yield two specific peptide products and has been described in detail previously.Citation11
Immunization, aerosol challenge and in vivo imaging
Immunization of rabbits has been reported previously.Citation9 For immunization of mice with AVA/Biothrax, 25 microliters were mixed with 75 microliters of saline per mouse; for rPA vaccination, 15 nanograms of rPA in 0.3% aluminum hydroxide gel were used in a total volume 100 microliter per mouse. Both rPA and AVA/Biothrax were administered intramuscularly; LC16M8/IL-15/PA was used at a dose of 1x108 pfu per mouse and given subcutaneously. Two doses of vaccines were administered 4 weeks apart and mice were challenged 4 weeks later by exposure to aerosolized spores using a nose-only exposure system as described previously with a dose of 20 LD50 (4–6x106 spores) of B. anthracis Sterne strain 7702.Citation18 In vivo imaging was done using an IVIS 100 imaging system from Xenogen and analyzed by using Living Image 2.5 software as described earlier.Citation18 All of the animal experiment protocols were approved by the Institutional Animal Care and Use Committee.
Statistical analyses
Data analyses were performed with Prism, version 5.04 (GraphPad Software). Due to non-Gaussian distribution of data, ELISA and TNA titers were analyzed using Kruskal-Wallis, with Dunn’s post test for differences among the groups vaccinated with respective vaccines. Correlation analysis between ELISA and TNA titers for each group was performed with the Spearman method. For all statistical analyses, P-values < 0.05 were considered significant.
| Abbreviations: | ||
| TNA | = | toxin neutralization activity assay |
| PA | = | protective antigen |
| rPA | = | recombinant PA |
| AVA | = | anthrax vaccine adsorbed |
| NALT | = | nasal-associated lymphoid tissues |
Additional material
Download Zip (106 KB)Acknowledgments
This work was in part supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, NIH and by a 3-y competitive research funding award to L.P.P from the Trans-NIH/FDA Intramural Biodefense Program. We gratefully acknowledge the receipt of reagents from the Biodefense and Emerging Infections Research Resources Repository (BEI Resources, NIAID) for this study. We also express our gratitude to Drs Anne Boyer and John Barr at the CDC for assistance with LF measurements in the rabbit sera.
Conflict-of-interest statement
The authors declare no competing financial interests.
Author contributions
TJM, P-YP, TH, TWA and LPP designed research and analyzed data. AV assisted with TNA, GML assisted with mouse studies, HY provided novel reagents. LPP supervised the study and wrote the paper.
References
- Hicks CW, Sweeney DA, Cui X, Li Y, Eichacker PQ. An overview of anthrax infection including the recently identified form of disease in injection drug users. Intensive Care Med 2012; 38:1092 - 104; http://dx.doi.org/10.1007/s00134-012-2541-0; PMID: 22527064
- Mabry R, Brasky K, Geiger R, Carrion R Jr., Hubbard GB, Leppla S, et al. Detection of anthrax toxin in the serum of animals infected with Bacillus anthracis by using engineered immunoassays. Clin Vaccine Immunol 2006; 13:671 - 7; http://dx.doi.org/10.1128/CVI.00023-06; PMID: 16760326
- IOM Committee to Assess the Safety and Efficacy of the Anthrax Vaccine. The Anthrax Vaccine: Is It Safe? Does It Work? Washington DC: National Academies Press; 2002.
- Detailed Safety Review of Anthrax Vaccine adsorbed January 2012, Complied by the Military Vaccine (MILVAX) Agency, US army Medical Command, Falls Church, Virginia http://www.anthrax.osd.mil/documents/854avasafetyrvw.pdf
- Wright JG, Quinn CP, Shadomy S, Messonnier N. Use of anthrax vaccine in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Morb Mortal Wkly Rep 2010; 59:1 - 30; PMID: 20075837
- Weiss S, Kobiler D, Levy H, Marcus H, Pass A, Rothschild N, et al. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect Immun 2006; 74:394 - 8; http://dx.doi.org/10.1128/IAI.74.1.394-398.2006; PMID: 16368995
- Chitlaru T, Altboum Z, Reuveny S, Shafferman A. Progress and novel strategies in vaccine development and treatment of anthrax. Immunol Rev 2011; 239:221 - 36; http://dx.doi.org/10.1111/j.1600-065X.2010.00969.x; PMID: 21198675
- Fay MP, Follmann DA, Lynn F, Schiffer JM, Stark GV, Kohberger R, et al. Anthrax vaccine-induced antibodies provide cross-species prediction of survival to aerosol challenge. Sci Transl Med 2012; 4:ra126; http://dx.doi.org/10.1126/scitranslmed.3004073; PMID: 22972844
- Merkel TJ, Perera PY, Kelly VK, Verma A, Llewellyn ZN, Waldmann TA, et al. Development of a highly efficacious vaccinia-based dual vaccine against smallpox and anthrax, two important bioterror entities. Proc Natl Acad Sci U S A 2010; 107:18091 - 6; http://dx.doi.org/10.1073/pnas.1013083107; PMID: 20921397
- Comer JE, Ray BD, Henning LN, Stark GV, Barnewall RE, Mott JM, et al. Characterization of a therapeutic model of inhalational anthrax using an increase in body temperature in New Zealand white rabbits as a trigger for treatment. Clin Vaccine Immunol 2012; 19:1517 - 25; http://dx.doi.org/10.1128/CVI.00292-12; PMID: 22837095
- Kuklenyik Z, Boyer AE, Lins R, Quinn CP, Gallegos-Candela M, Woolfitt A, et al. Comparison of MALDI-TOF-MS and HPLC-ESI-MS/MS for endopeptidase activity-based quantification of Anthrax lethal factor in serum. Anal Chem 2011; 83:1760 - 5; http://dx.doi.org/10.1021/ac1030144; PMID: 21302970
- Molin FD, Fasanella A, Simonato M, Garofolo G, Montecucco C, Tonello F. Ratio of lethal and edema factors in rabbit systemic anthrax. Toxicon 2008; 52:824 - 8; http://dx.doi.org/10.1016/j.toxicon.2008.08.011; PMID: 18812184
- Lee JS, Hadjipanayis AG, Welkos SL. Venezuelan equine encephalitis virus-vectored vaccines protect mice against anthrax spore challenge. Infect Immun 2003; 71:1491 - 6; http://dx.doi.org/10.1128/IAI.71.3.1491-1496.2003; PMID: 12595467
- McConnell MJ, Hanna PC, Imperiale MJ. Cytokine response and survival of mice immunized with an adenovirus expressing Bacillus anthracis protective antigen domain 4. Infect Immun 2006; 74:1009 - 15; http://dx.doi.org/10.1128/IAI.74.2.1009-1015.2006; PMID: 16428747
- Keitel WA, Treanor JJ, El Sahly HM, Evans TG, Kopper S, Whitlow V, et al. Evaluation of a plasmid DNA-based anthrax vaccine in rabbits, nonhuman primates and healthy adults. Hum Vaccin 2009; 5:536 - 44; http://dx.doi.org/10.4161/hv.5.8.8725; PMID: 19458488
- Stepanov AV, Marinin LI, Pomerantsev AP, Staritsin NA. Development of novel vaccines against anthrax in man. J Biotechnol 1996; 44:155 - 60; http://dx.doi.org/10.1016/0168-1656(95)00092-5; PMID: 8717399
- Welkos S, Little S, Friedlander A, Fritz D, Fellows P. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 2001; 147:1677 - 85; PMID: 11390699
- Loving CL, Khurana T, Osorio M, Lee GM, Kelly VK, Stibitz S, et al. Role of anthrax toxins in dissemination, disease progression, and induction of protective adaptive immunity in the mouse aerosol challenge model. Infect Immun 2009; 77:255 - 65; http://dx.doi.org/10.1128/IAI.00633-08; PMID: 18955474
- Weiner ZP, Glomski IJ. Updating perspectives on the initiation of Bacillus anthracis growth and dissemination through its host. Infect Immun 2012; 80:1626 - 33; http://dx.doi.org/10.1128/IAI.06061-11; PMID: 22354031
- Plaut RD, Kelly VK, Lee GM, Stibitz S, Merkel TJ. Dissemination bottleneck in a murine model of inhalational anthrax. Infect Immun 2012; 80:3189 - 93; http://dx.doi.org/10.1128/IAI.00515-12; PMID: 22753373
- Peterson JW, Comer JE, Baze WB, Noffsinger DM, Wenglikowski A, Walberg KG, et al. Human monoclonal antibody AVP-21D9 to protective antigen reduces dissemination of the Bacillus anthracis Ames strain from the lungs in a rabbit model. Infect Immun 2007; 75:3414 - 24; http://dx.doi.org/10.1128/IAI.00352-07; PMID: 17452469
- Pitt MLM, Ivins BE, Estep JE, Farchaus J, Friedlander AM. Comparison of the efficacy of purified protective antigen and MDPH to protect non-human primates from inhalation anthrax. Salisbury Med Bull Suppl 1996; 87:130
- Human Genome Sciences Inc. Anti-Infectives Advisory Committee Briefing Document: Raxibacumab, Treatment of Inhalation Anthrax, BLA 125349, September 21, 2009 (Food and Drug Administration, Silver Spring, MD, 2009).
- Luxembourg A, Hannaman D, Nolan E, Ellefsen B, Nakamura G, Chau L, et al. Potentiation of an anthrax DNA vaccine with electroporation. Vaccine 2008; 26:5216 - 22; http://dx.doi.org/10.1016/j.vaccine.2008.03.064; PMID: 18462850
- Livingston BD, Little SF, Luxembourg A, Ellefsen B, Hannaman D. Comparative performance of a licensed anthrax vaccine versus electroporation based delivery of a PA encoding DNA vaccine in rhesus macaques. Vaccine 2010; 28:1056 - 61; http://dx.doi.org/10.1016/j.vaccine.2009.10.111; PMID: 19896452
- Li H, Soroka SD, Taylor TH Jr., Stamey KL, Stinson KW, Freeman AE, et al. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J Immunol Methods 2008; 333:89 - 106; http://dx.doi.org/10.1016/j.jim.2008.01.007; PMID: 18304568