Abstract
Passive immunization is a crucial parameter for prevention of human rabies. Presently as World Health Organization (WHO) strongly advocates local infiltration of rabies immunoglobulin in and around the bite wound, we feel that there is no basis for calculating the dose of immunoglobulin based on body weight. Keeping this in view we conducted both in vitro and in vivo studies to know whether the dose of immunoglobulin can be reduced and still obtain complete neutralization of the virus. In vitro neutralization studies were conducted using CVS strain of virus and BHK 21 cells. In vivo experiments were conducted in 4 weeks old Swiss albino mice by initial challenge with CVS followed by infiltration with increasing dilutions of either human rabies immunoglobulin (HRIG) and equine rabies immunoglobulin (ERIG). In vitro studies showed that a dose of 100 FFD 50 of CVS was neutralized by increasing dilution of both HRIG and ERIG and 100% neutralization was observed with HRIG and ERIG in as low quantities as 0.025 IU. In mice studies there was 100% survival of mice infiltrated with 0.025 IU of both HRIG and ERIG compared with 100% mortality in mice infiltrated with normal saline. These results suggest that it is possible to reduce the dose of rabies immunoglobulins by at least 16 times the presently advocated dose. These findings needs to be further evaluated using larger animal models and street viruses prevalent in nature but cannot serve as recommendations for use of RIG for passive immunization in humans
Introduction
Human rabies is 100% fatal but is preventable if the state of the art modern prophylactic measures are instituted soon after the exposure has occurred. It is estimated that annually about 55,000 human deaths occur due to rabies mostly in developing countries of Asia and Africa where dogs constitute more than 95% of transmitting vectors.Citation1 The virus is present in the saliva of rabid dogs which is inoculated in to the wound thus initiating an exposure. Passive immunization is a very important parameter in post exposure prophylaxis for two reasons: (1) the virus is present at the site of bite for varying periods of time thus amenable to neutralization by passively administered antibodies, and (2) active immunization with vaccines requires a minimum of 10–14 d for producing adequate levels of virus neutralizing antibodies. The importance of passive immunization was scientifically proved by studies by Habel and Koprowsky conducted five to six decades ago.Citation2,Citation3 Further studies by Atanasiu et al. in 1956 established the dosage of rabies immune globulin.Citation4 World Health Organization strongly recommended use of rabies immunoglobulin in 1973.Citation5 Most of the early studies conducted with regard to dosage schedule of RIG was at a time when unpurified anti rabies serum prepared in horses were used and systemic inoculation was in practice. Hence the dosage schedule was calculated based on body weight of the patient giving due consideration for biological half life of heterologous proteins and extent of distribution and dilution in the body.Citation4 Keeping this in mind, a dosage of 40 IU/kg body weight for Equine rabies immunoglobulin (ERIG) and 20 IU/kg body weight of human rabies immunoglobulin (HRIG) was advocated.Citation6 However, since 1992 the WHO is strongly advocating local infiltration of RIGs as much as anatomically feasible, keeping in view the unreliable blood levels reached after intramuscular injection.Citation7 Moreover, the currently available ERIGs are highly purified and enzyme refined products containing only antigen binding F(ab’)2 components and thus much more efficacious and safer than previous un purified ARS.Citation8 In spite of these developments, the dosage schedule of RIGs have not been revised and it appears that we are administering greater than required quantities of RIGs. It becomes all the more important to re evaluate the dose as highly effective and potent human or murine monoclonal antibodies (Mabs) will be available in near future.Citation9,Citation10 Indeed a newly produced recombinant human Mab has been administered to humans in a dose of 10 IU/Kg body weight.Citation11 In a study conducted by Muhamuda et al., it was found that murine Mabs to rabies glycoprotein were at least 2000 times more potent than ERIG in terms of activity per milligram of protein.Citation12 Considering all these points, it seems illogical to calculate the dose based on body weight. To best of our knowledge, there are no studies done earlier which correlates the quantity of virus and the amount of RIG required to neutralize the same . Such type of studies may help us in calculating the dose of RIG required. Keeping this in mind we designed this study using both in vitro and in vivo experiments to determine the feasibility of reducing the dose of RIG. The preliminary results indicate that there is a direct correlation between the quantity of virus and dose of RIG required to neutralize and an optimal quantity of virus can be neutralized both in vivo and in vitro by reduced concentrations of RIGs
Results
In vitro tests
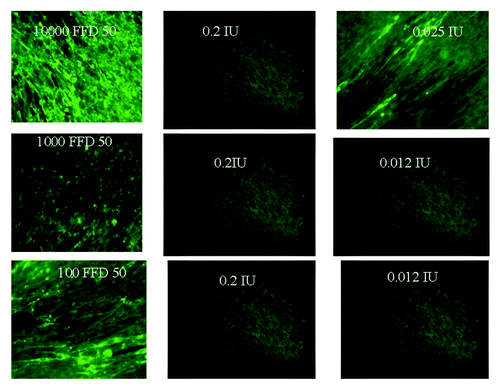
Initial experiments showed that there is a correlation between quantity of virus and amount of HRIG and ERIG required to neutralize (). A concentration of 10 4 FFD50 was 100% neutralized by dilutions of HRIG (0.2 and 0.05/100 ul) and 50% neutralization was observed in higher dilutions (0.025 IU/100ul). On the other hand a dose of 103 FFD50 as well as 102 FFD50 of CVS was 100% neutralized by HRIG in concentrations as low as 0.2 IU to as high as 0.012 IU/100 ul. ( and ). Similarly in the case of ERIG 100% neutralization was observed with 103FFD50 of CVS in concentrations ranging from 0.4 IU to 0.05 IU and 50% neutralization occurred with 0.025 IU/100 ul (). This concentration of ERIG neutralized 100% if virus dose was reduced to 102 FFD50
Table 1. Comparison of in vitro Neutralization of different concentrations of CVS with decreasing concentration of HRIG.
Table 2. Comparison of in vitro Neutralization of different concentrations of CVS with decreasing concentration of ERIG.
In vivo tests
These tests were conducted using one dilutions of virus (103 LD50) as our earlier experiments had indicated that a minimum of 103 LD50 is to be injected peripherally by intramuscular route to produce 100% mortality in weanling mice. We used this route to simulate natural exposure and RIGs were infiltrated locally in to the site of injection after 1 h of challenge. The results of these experiments are depicted in . It can be seen that in the control mice inoculated with 103 LD50 of virus there was 100% mortality while 100% survival was observed in mice infiltrated with decreasing quantities of HRIG (from 0.2 to 0.025 IU) or ERIG (from as high as 0.4 IU to as low as 0.025 IU/100 ul) and 80% survival was observed with a dilution as high as 0.012 IU of both HRIG and ERIG. These mice were absolutely normal and remained healthy up to 1 mo observation period. The control mice inoculated with normal saline developed typical signs of rabies in 5–6 d and died by 7th day. The death was confirmed to be due to rabies encephalitis by doing a FAT on brain samples by standard technique (one from each dilution).
Table 3. In vivo Neutralization of 10 3 LD 50 of CVS with varying Concentrations of HRIG and ERIG.
Discussion
Rabies immunoglobulin administration is an integral part of the post-exposure prophylaxis and most treatment failures in patients exposed to rabid animals have been attributed to non administration of RIGs.Citation13,Citation14 In developing countries of Asia, usage of RIGs is limited to less than 5% of category III patients while more than 60% of all bites are category III exposures.Citation15 Even though highly purified and enzyme refined ERIGs are produced both in public sector and private sector, and incidence of anaphylaxis is extremely low (less than 0.001%)Citation16 most treating physicians are still reluctant to administer ERIG. Use of ERIG is limited to select specialized anti rabies centers. Some of the reasons for underutilization of RIGs are lack of awareness in general public as well as medical fraternity about the importance of passive immunization in preventing rabies and non availability of the product in most medical pharmacies. On the other hand human rabies immunoglobulins are very expensive and cannot be afforded by low socio-economic category of people who constitute majority of animal bite victims in these countries. Another major factor that needs consideration is the huge quantity of RIGs (depending on the body weight) that is administered and the associated pain and discomfort to the patients. Many a times it is not possible to infiltrate the calculated quantity of RIGs due to anatomical site of the wound (such as in finger tip). In such cases most of the calculated dose of RIG is administered intramuscularly. It is known that the systemically administered RIGs do not reach required protective levels in the circulation and hence the effect of IM administration is doubtful. Also, if huge doses of RIGs are inoculated systemically there is a possibility of circulating RIG interfering with active immune response to rabies vaccine administered concomitantly.Citation17 Therefore the current recommendation is to infiltrate as much as possible of calculated dose in to and around the wound. Hence, calculating the dose of RIG based on body weight may be not necessary, considering the fact that currently available RIGs are highly purified and potent. The recommendations of WHO for passive immunization are still based on earlier experiments using un purified equine antirabies serum. When highly purified and enzyme refined ERIGs and F(ab’)2 products became available in early eighties in many countries, there was a scope for revising the current dosage recommendation . However, as there were no evidences based on at least animal studies, this aspect of passive immunization was never discussed till the WHO expert committee meeting in 2008 at Annecy, France. During this meeting there was a serious discussion about possibility of revising the dosage schedule and it was opined that some data should be generated using animal experiments as human studies cannot be done.Citation18 Keeping this in mind we designed our studies in mice and we used CVS strain of rabies virus as this gives highly consistent and reproducible results. If such animal experiments are attempted in future in non human primates, another aspect that needs to be considered is the quantum of virus that may be inoculated in to the wound after natural exposure. Some of the earlier studies conducted have shown that the quantum of virus present in salivary glands of rabid dogs vary from as low as 102 to as high as 104 LD50 per gm.Citation19,Citation20
From our in vitro experiments it is evident that the extent of neutralization directly correlates with the amount of virus used to infect the cells. Further it has been shown clearly that a dose of 104 FFD50 of virus has been neutralized 100% by RIG in quantities as low as 0.025 IU and even with dilutions as high as 0.012 IU, 50% neutralization of virus has occurred. On the other hand a virus dose of 103 FFD50 has been neutralized 100% in all dilutions of both HRIG and ERIG ( and ).
Similarly in experiments conducted in mice, it was clearly shown that an optimal dose of virus (103 LLD50) was 100% neutralized by infiltration of both HRIG and ERIG in doses as low as 0.025 IU/100 ul (). Unlike the situation after natural exposure in humans, we did not administer post-exposure vaccination to challenged mice following RIG infiltration. In our experiments, RIG alone could protect the mice clearly indicating the value of RIG s in passive immunization. Our preliminary objective was to know to what extent RIG dose can be reduced to obtain 100% survival. To that extent we are successful as even 10 times reduction of RIG dosage (based on body weight of the mice) was able to protect 100% of challenged mice in contrast to 100% mortality in control mice inoculated with normal saline. However, one shortcoming of this study is that we have used 2nd international standard of RIG as a source of HRIG. This is due to non availability of commercial HRIG when we designed our experiments. Another shortcoming in the study is direct extrapolation of human dose of RIG s to dosage used in mice. This was done mainly to see if the dose calculation based on body weight is really applicable, but however, due to huge difference between the weight of humans and mice correct interpretation may not be possible.
To conclude, we have clearly shown that much reduced dosage of RIGs are required to neutralize fixed rabies virus in concentrations as high as 104 LD50. This study if first of its kind done anywhere in the world .It is too early to predict the future implications of this study, as this was done using infection of cell culture and in un natural host and using fixed virus CVS under stringent laboratory conditions. The study needs to be reproduced in larger animals including non human primates with street viruses prevalent in nature, as challenge experiments in humans are unethical and impossible. Therefore the results of this study cannot be extrapolated to situations in humans and current recommendations should be continued.. However, these preliminary findings should encourage more studies on similar lines specially keeping in mind that in future highly potent human and murine Mabs will be available for local infiltration.
Materials and Methods
Virus
We used BHK 21 cell adapted CVS-11 strain of rabies virus for both in vivo and in vitro experiments. The virus was titrated in BHK 21 cells (ATCC CCL 10) by fluorescent foci method and in weanling mice (Swiss Albino) by intra muscular inoculation to calculate the dose of the virus to be used in the experiments. The titer of the virus after infection in cell culture was 105 FFD 50/100 ul and in vivo experiments it was 105 LD50 by intra muscular inoculation.
Cell line used
All in vitro experiments were conducted using BHK 21 cells (ATCC CCL 10). The cells were grown and maintained as per standard procedures.
Experimental mice
Swiss albino mice four weeks old and weighing 10 g were obtained from Central Animal Research facility (CARF) of NIMHANS. The study was approved by institutional animal ethics committee. The mice were housed and looked after as per the institutional animal ethics committee guidelines in force.
Rabies immunoglobulin (RIG)
We used 2nd international standard (HRIG) having a potency of 30 IU /mL (obtained from National Institute of Biological Sciences) and commercial equine rabies immunoglobulin (ERIG) (Equirab batch No. A2712003 with a potency of 300 IU/mL produced by Bharat serum and vaccines)
Neutralization experiments using BHK 21 cells
The cells were grown to confluent monolayer in 96 well tissue culture plates (Cell star) using standard techniques. Different concentrations of CVS (ranging form 10000 FFD50 to 50 FFD50) were mixed with increasing dilutions of HRIG or ERIG (from 0.2 IU/100 ul to 0.012 IU/100 ul). The virus RIG mixture was kept at 37 C in a water bath for 1 h following which the mixture was added to the cell monolayer (100 Ul/well) and incubated for 1 h at 37 C. The monolayers were washed twice with medium, and replenished with maintenance medium and incubated for 24 h in a Co2 incubator (Nu Aier). The cells were fixed in cold acetone for 15 min and stained by fluorescent antibody technique using rabies conjugate (N Polyclonal, Light Diagnostics, Cat No. F199) in a working dilution of 1:40.
Neutralization experiments in mice
Mice were weighed individually and the average weight of mice was found to be 10 g. The recommended dose of HRIG and ERIG for humans is 20 IU and 40 IU per 1000 gs of body weight. Based on this the dose for mice were calculated to by 0.2 IU of HRIG and 0.4 IU of ERIG per mice for local infiltration. Groups of 10 mice were initially challenged with 103 LD50 per 0.1 mL by inoculating intramuscularly in the hind limb. After 1 h, increasing dilutions of either HRIG (0.2, 0.1, 0.05, 0.025, and 0.012 IU) or ERIG (0.4, 0.2, 0.1, 0.05, and 0.025 IU) was infiltrated in the same area where virus was inoculated. The control mice (10 in number) received 100 ul of normal saline infiltrated on the same area. The mice were kept for observation for 30 d.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- World Health Organzation. Expert consultation on rabies. First report. Tech Rep Ser 931, Geneva, WHO, 2005.
- Habel K. Seropprophylaxis in experimental rabies. Public Health Rep 1945; 60:545 - 60; http://dx.doi.org/10.2307/4585257; PMID: 19316026
- Koprowski H, Van Der Scheer J, Black J. Use of hyperimmune anti-rabies serum concentrates in experimental rabies. Am J Med 1950; 8:412 - 20; http://dx.doi.org/10.1016/0002-9343(50)90224-5; PMID: 15410721
- Atanasiu P, Bahmanyar M, Balatazard M. Rabies neutralizing antibody response to different schedules of serum and vaccine inoculation in non exposed persons. Bull World Health Organ 1955; 13:747 - 51; PMID: 13284556
- World Health Organization. Expert committee on rabies, 6th report. Tech Rep Ser 523, Geneva, WHO, 1973.
- World Health Organization. Expert committee on rabies 7th report. Tech Rep Ser 709 WHO, Geneva, 1984.
- World Health Organization. Expert committee on rabies 8th report. Tech Rep Ser 824 Geneva, WHO, 1992.
- Quiambao BP, Dy-Tioco HZ, Dizon RM, Crisostomo ME, Teuwen DE. Rabies post-exposure prophylaxis with purified equine rabies immunoglobulin: one-year follow-up of patients with laboratory-confirmed category III rabies exposure in the Philippines. Vaccine 2009; 27:7162 - 6; http://dx.doi.org/10.1016/j.vaccine.2009.09.036; PMID: 19925947
- Müller T, Dietzschold B, Ertl H, Fooks AR, Freuling C, Fehlner-Gardiner C, et al. Development of a mouse monoclonal antibody cocktail for post-exposure rabies prophylaxis in humans. PLoS Negl Trop Dis 2009; 3:e542; http://dx.doi.org/10.1371/journal.pntd.0000542; PMID: 19888334
- Bakker AB, Python C, Kissling CJ, Pandya P, Marissen WE, Brink MF, et al. First administration to humans of a monoclonal antibody cocktail against rabies virus: safety, tolerability, and neutralizing activity. Vaccine 2008; 26:5922 - 7; http://dx.doi.org/10.1016/j.vaccine.2008.08.050; PMID: 18804136
- Gogtay N, Thatte U, Kshirsagar N, Leav B, Molrine D, Cheslock P, et al, SII RMab author group. Safety and pharmacokinetics of a human monoclonal antibody to rabies virus: a randomized, dose-escalation phase 1 study in adults. Vaccine 2012; 30:7315 - 20; http://dx.doi.org/10.1016/j.vaccine.2012.09.027; PMID: 23010601
- Muhamuda K, Madhusudana SN, Ravi V. Use of neutralizing murine monoclonal antibodies to rabies glycoprotein in passive immunotherapy against rabies. Hum Vaccin 2007; 3:192 - 5; http://dx.doi.org/10.4161/hv.3.5.4386; PMID: 17637573
- Hemachudha T, Mitrabhakdi E, Wilde H, Vejabhuti A, Siripataravanit S, Kingnate D. Additional reports of failure to respond to treatment after rabies exposure in Thailand. Clin Infect Dis 1999; 28:143 - 4; http://dx.doi.org/10.1086/517179; PMID: 10028088
- Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NS, Ashwath Narayana DH, Abdul Rahman S, et al. Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Int J Infect Dis 2007; 11:29 - 35; http://dx.doi.org/10.1016/j.ijid.2005.10.007; PMID: 16678463
- Fang LX, Ping F, Ping Y, Hui BG, Yan YX. Socioeconomic status is a critical risk factor for human rabies post-exposure prophylaxis. [d] Vaccine 2010; 28:6847 - 51; http://dx.doi.org/10.1016/j.vaccine.2010.08.034; PMID: 20723628
- Wilde H, Chutivongse S. Equine rabies immune globulin: a product with an undeserved poor reputation. Am J Trop Med Hyg 1990; 42:175 - 8; PMID: 2316787
- Archer BG, Dierks RE. Effects of homologous or heterologous antiserum on neutralizing-antibody response to rabies vaccine. Bull World Health Organ 1968; 39:407 - 17; PMID: 5303907
- World Health Organization. Human and dog rabies prevention and control. Report of the WHO/Bill Gates Foundation Consultation. 7-9 October 2009, Annecy, France WHO/HTM/NTD/NZD/2010.1.
- Fekadu M, Shaddock JH, Baer GM. Excretion of rabies virus in the saliva of dogs. J Infect Dis 1982; 145:715 - 9; http://dx.doi.org/10.1093/infdis/145.2.715; PMID: 7077094
- Vaughn JB Jr., Gerhardt P, Newell KW. Excretion of street rabies virus in the saliva of dogs. JAMA 1965; 193:363 - 8; http://dx.doi.org/10.1001/jama.1965.03090050039010; PMID: 14313891