Abstract
Haemagglutination inhibition (HI) and neutralization (NT) titers as well as haemagglutinin (HA) specific antibody responses were examined in 50 healthy adults aged between 22 and 69 y old after two intranasal administrations of an inactivated whole virus vaccine derived from A/Victoria/210/2009 virus (45 μg HA per dose) at 3 week intervals. Serum HI titers after two-doses of the nasal vaccine showed >2.5-fold rise in the ratio of geometric mean titer upon vaccination, >40% of subjects with a ≥4-fold increase in titer and >70% of subjects with a titer of ≥1:40, all parameters associated with an effective outcome of vaccination in the criteria defined by the European Medicines Agency. Serum neutralizing antibody responses correlated with HI antibody responses, although NT titers were about 2-fold higher than HI titers. These high levels of serum responses were accompanied by high levels of HI and neutralizing antibody responses in nasal mucus as measured in concentrated nasal wash samples that were about 10 times diluted compared with natural nasal mucus. Serum and nasal HI and neutralizing antibody responses consisted of HA-specific IgG and IgA antibody responses, with IgG and IgA antibodies being dominant in serum and nasal responses, respectively.
Introduction
Currently available inactivated vaccines, usually whole virus vaccines or sub-virion vaccines, such as detergent-disrupted split-viruses or purified surface glycoprotein vaccines, are injected via the non-mucosal route.Citation1 These vaccines induce serum IgG antibodies, which are highly protective against homologous virus infections but less effective against heterologous virus infections. However, it has been shown that secretory IgA (S-IgA) and IgG antibodies in the respiratory tract largely contribute to the protective immunity induced by influenza virus infection.Citation2,Citation3 Moreover, S-IgA antibodies are more cross-reactive against variant influenza viruses than serum IgG antibodies and therefore provide more effective protection against a heterologous virus.Citation4-Citation9 Thus, intranasal administration of an inactivated influenza vaccine that induces both S-IgA and IgG antibody responses is expected to outperform the protective efficacy of intramuscular or subcutaneous vaccines.Citation10 Of note, intranasal vaccination would have several additional advantages, since this type of vaccination is needle-free which enables easier administration, is more readily accepted by the recipients, reduces the problems associated with needle waste and prevents the risk of disease transmission through needle reuse.Citation11
Several trials have been conducted to augment the induction of both S-IgA and IgG antibodies using intranasal administration of an inactivated influenza vaccine, either with or without an extrinsic adjuvant.Citation12-Citation21 In several clinical trials, antibody responses were mainly evaluated based on haemagglutination inhibition (HI) titers of the serum and haemagglutinin (HA)-specific IgA and IgG antibody titers estimated by enzyme-linked immunosorbent assay (ELISA) in nasal wash samples. Currently, serum HI titers are used for the evaluation of the efficacy of the seasonal influenza vaccines,Citation22-Citation24 because levels of protection against viruses that are homologous to the vaccine strain, correlate well with these serum HI titers.Citation25 However, both serum and nasal antibody responses are involved in protection,Citation26,Citation27 and together might result in better correlates for protection against heterologous influenza strains. Therefore, it would be useful to measure HI titers in both serum and nasal mucus in humans. In addition, although neutralizing capacity is considered to be a more functional criterion for protection than HI or HA-specific binding, neutralizing antibody responses in nasal wash samples have rarely been assessed. Previous studies show that HI titers may be lower or higher than the corresponding neutralization (NT) titers, depending on the strain of influenza A or B virus used.Citation28 Other studies show that HI assays using anti-sera failed to detect the H5N1 virus.Citation29,Citation30 Thus, the efficacy of antibody responses following nasal vaccination should preferentially be assessed by characterizing the HI and NT titers in serum and nasal mucus.
In a previous study, it was shown that neutralizing antibody responses in both serum and nasal mucus were induced in five healthy adults after intranasal administration of a split-virus vaccine derived from A/Uruguay/716/2007 (H3N2) virus (45 μg HA per dose).Citation31 Neutralizing antibody titers were measured in nasal wash samples, which typically contain about 1/10 the amount of IgA antibody found in natural nasal mucus.Citation31,Citation32 Virus-specific neutralizing antibody responses were detected in nasal mucus samples from 4 out of 5 subjects, with a rise in NT titer of ≥ 4-fold after the second vaccination.Citation31 Nasal mucus NT titers appeared to reflect the absolute titers of nasal mucus antibodies and these titers were not affected by the slight variability in the recovery of total antibodies from nasal mucus of different subjects.
Inactivated influenza whole virus vaccines are more immunogenic than split-product vaccines when administered intranasally to mice.Citation33,Citation34 Similar results were found for humans in clinical trials showing that intranasally administered whole virus vaccines cause enhanced production of both local HA-specific IgA antibodies and serum HI antibodies.Citation14-Citation16 The higher immunogenicity of the whole virus vaccine may be explained by the adjuvant action of single-stranded viral RNAs that activate toll-like receptor 7. Viral RNA is present in the inactivated virus particles, but is absent in split-product vaccine formulations.Citation35-Citation38 Together, these reports suggest that inactivated whole virus vaccines can induce more effective immune responses than split-virus vaccines after intranasal vaccination in healthy adults.
In the present study HI and neutralizing antibody responses were examined in serum and nasal mucus samples from 50 healthy adults after two intranasal vaccinations with an inactivated whole virus vaccine derived from A/Victoria/210/2009 (H3N2) virus (A/Victoria vaccine; 45 μg HA per dose) with a 3-week interval. Antibody responses in nasal mucus were assayed using concentrated nasal wash (containing 1 mg/ml total protein).Citation31,Citation32 HA-specific IgA and IgG antibodies were also examined to characterize the immune response. It was found that two doses of the nasal vaccine induced high levels of HI and neutralizing antibody responses in both serum and nasal mucus. These responses were accompanied by major changes in HA-specific serum IgG and nasal IgA antibody responses, respectively. In addition, the vaccination with the A/Victoria vaccine resulted in a slight increase in HI and NT titers, which were cross-reactive to the A/Sydney/05/1997 (H3N2) virus.
Results
HI and neutralizing antibody responses in serum
HI antibody responses, currently in use as a correlate of protection for the evaluation of vaccine efficacy, were examined in serum samples from the volunteers aged between 22 and 69 y old who received two intranasal vaccinations with an inactivated whole virus vaccine derived from A/Victoria virus strain (containing 45 μg HA) with a 3-week interval. Serum samples were obtained both before vaccination (week 0) and 3 weeks after primary (week 3) and secondary vaccination (week 6). HI titers against A/Victoria virus were increasing after the first vaccination and again after the second vaccination (). The serum HI titers were evaluated using the mean geometric increase between week 0 and 6, the conversion rate as well as the protection rate, which are also used by the EMA and the FDA to review the efficacy of seasonal influenza vaccines (). After two doses of the intranasal vaccination (week 6), serum HI titers fulfilled the criteria of the mean geometric increase (4.25-fold) and the conversion rate (43.5%), as defined by the EMA for the vaccine efficacy in people aged 18–60 y. In addition, serum samples also reached protective levels (76.1%), which were defined as the protection rate by the EMA ( and ). The subjects over 60 y old produced only low levels of HI titers. These results show that serum HI titers induced by administration of two doses of the nasal A/Victoria vaccine exceeded three of the EMA criteria in people aged 18–60 y.
Figure 1. HI antibody responses in serum. (A) HI antibody responses before and after primary and secondary vaccination are shown for serum. A paired t test was performed to compare data from week 0 (pre) and 6 (post). Correlation coefficient (r) and p value were calculated. *; p < 0.05. (B) The relationship of serum HI antibody responses before and after secondary vaccination. The abscissa and ordinate show the pre- and post-vaccination HI titers, respectively. Further, it is shown how these relate to conversion rate and protection rate, which are on the border or within the area marked by the bold line and by the light gray background, respectively. Each circle represents an individual and shows the relation between the pre- and post-vaccination titers. Gray circles indicate subjects between 60 and 69 y-of-age.
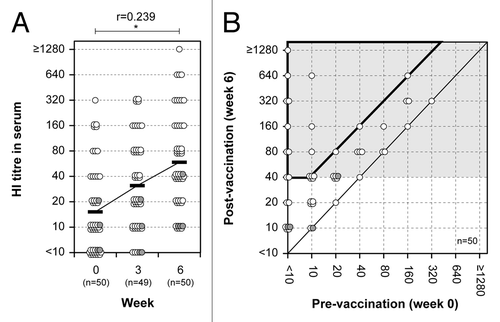
Table 1. Tools currently in use to evaluate vaccine-induced changes in serum HI titer
Table 2. Serum HI antibody responses after two doses of the nasal A/Victoria vaccine
In addition, neutralizing antibody responses were also examined in the serum samples, since those responses are considered to be more functional in the protection against influenza viruses than HI antibody responses. NT titers against A/Victoria virus were increasing after the first vaccination and again after the second vaccination (). Serum HI titers correlated strongly with serum NT titers (r = 0.925, p < 0.0001). Among 46 volunteers below 60 y of age, the ratio of GMTs between week 0 and 6 (the mean geometric increase after two doses of the nasal A/Victoria vaccine) in serum NT titers was 8.00 ( and ), whereas this ratio in serum HI titers was 4.25 (). These results show that the increase in NT titers is about 2-fold higher than the increase in HI titers ().
Figure 2. Neutralizing antibody responses and correlation between HI and NT titers in serum. (A) Neutralizing antibody responses before and after primary and secondary vaccination are shown. A paired t test was performed to compare data from week 0 (pre) and 6 (post). The correlation coefficient (r) and p value were calculated. **; p < 0.01. (B) Correlation between HI and NT titers in serum 3 weeks after the secondary nasal vaccination. The abscissa and ordinate show HI and NT titers, respectively. Pearson r value and p value were calculated. Each individual is represented by a circle showing corresponding HI and NT titers. Gray circles indicate subjects between 60 and 69 y-of-age.
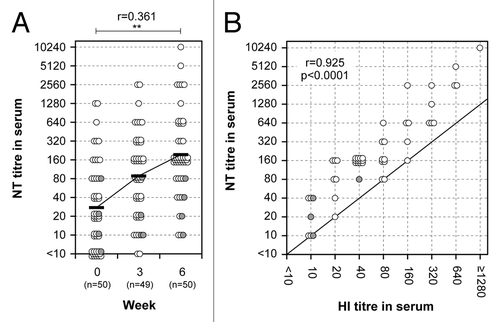
Table 3. Serum neutralizing antibody responses after two doses of the nasal vaccine
These data suggest that a titer of 1:80 for neutralizing antibodies would correspond to a titer of 1:40 for HI antibodies, which is defined as the minimal HI titer providing protection. Using an NT titer of 1:80 temporarily as the lower limit of protection, the enhanced sensitivity of neutralization assay could be corrected so as to tentatively estimate the vaccine efficacy. As shown in , serum NT titers at week 6 showed a 63.0% conversion rate, and an 87.0% protection rate. These results roughly correspond to those obtained in the serum HI titers (), suggesting that NT titers after correcting for the enhanced sensitivity of the NT assay could be used as an indicator to evaluate protective efficacy of the vaccines.
HI and neutralizing antibody responses in nasal mucus
HI and neutralizing antibody responses were also examined in nasal mucus samples from the volunteers who received two intranasal vaccinations with the inactivated vaccine. Nasal mucus samples (containing 1 mg/ml total protein), prepared by concentrating nasal wash samples so that the total amount of IgA was equivalent to about 1/10 of that in natural nasal mucus, were obtained both before vaccination (week 0) and 3 weeks after primary (week 3) and secondary vaccination (week 6). HI and NT titers against A/Victoria virus were increasing after the first vaccination and again after the second vaccination (). Among 46 volunteers below 60 y of age, the ratio of GMTs between week 0 and 6 were 3.13 and 5.88 for HI ( and ) and NT ( and ) titers, respectively. These results show that NT titers in nasal mucus samples are about 2-fold higher than the HI titers (), similar to the relationship between HI and NT titers in serum samples (). Thus, the volunteers who received two doses of intranasal inactivated vaccine induced high levels of HI and neutralizing antibody responses both in nasal mucus and serum.
Figure 3. HI and neutralizing antibody responses in nasal mucus. HI (A) and NT (B) titers before and after primary and secondary vaccination. A paired t test was performed to compare data from week 0 (pre) and 6 (post). The correlation coefficient (r) and p value were calculated. *; p < 0.05, ***; p < 0.001. (C) Correlation between HI and NT titers in nasal wash 3 weeks after the secondary nasal vaccination. The abscissa and ordinate show HI and NT titers, respectively. Pearson r value and p value were calculated. Each circle represents an individual showing corresponding HI and NT titers. Gray circles indicate subjects between 60 and 69 y-of-age.
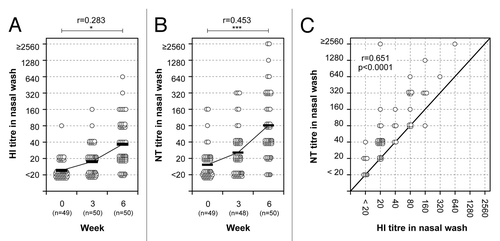
Table 4. Nasal HI and neutralizing antibody responses after two doses of the nasal vaccine
HA-specific IgA and IgG ELISA antibody responses in serum and nasal mucus
The HA-specific IgA and IgG responses in serum from subjects before and after two intranasal vaccinations with the A/Victoria vaccine were determined by ELISA (). Serum HA-specific IgG and IgA titers were obtained using serum samples starting from a 1:10 dilution. The mean geometric increase of IgG and IgA antibodies after two doses of nasal vaccine (week 6) was estimated to be 2.96-fold and 2.47-fold, respectively (Figs. S1A and B and ). The GMTs of IgG responses in serum samples were higher than those of IgA responses at each time point. This indicates that HA-specific IgG, rather than IgA, is likely to be the major isotype responsible for haemagglutination inhibiting and neutralizing activity in serum samples.
Table 5. Serum and nasal HA-specific antibody responses after two doses of the nasal vaccine
The HA-specific IgA and IgG responses from subjects before and after the two intranasal vaccinations were also determined in nasal mucus (). HA-specific IgG and IgA titers in nasal mucus were assayed using concentrated nasal wash samples (containing 1 mg/ml total protein) which were measured by ELISA starting with a 1:160 dilution. The GMTs of HA-specific IgA responses were higher than those of HA-specific IgG responses at each time point. The mean geometric increase of IgA and IgG antibody after two doses of nasal vaccine (week 6) was estimated to be 3.88-fold and 1.37-fold, respectively (Figs. S1C and D; ). This indicates that HA-specific IgA antibody is most likely the predominant antibody isotype responsible for the haemagglutination inhibiting and neutralizing activity in the nasal mucus. Thus, predominant changes in HA-specific IgA and IgG titers were found in the nasal mucus and serum, respectively.
In addition, relationships between NT titer and HA-specific antibody titer in serum or nasal wash were evaluated. In serum, NT titers correlated well with HA-specific IgG titers (r = 0.778, p < 0.0001), but not with IgA titers (Figs. S2A and B). Nasal NT titers show a weak correlation with HA-specific IgA titers (r = 0.473, p < 0.001), but not IgG titers (Figs. S2C and D).
HI and neutralizing antibody responses show cross-reactivity with the A/Sydney/05/1997 virus
Cross-reactivity of HI and neutralizing antibody responses that were induced upon vaccination with A/Victoria (H3N2) with the A/Sydney/05/1997 (A/Sydney, H3N2) virus was examined in serum (). Before vaccination, moderate HI and very high neutralizing antibody responses, were found to be cross-reactive to A/Sydney virus (week 0, GMTs in ), when compared with responses to the homologous A/Victoria virus ( and ). The mean geometric increase in the cross-reactive HI and NT titers were very similar and showed a 1.44-fold and a 1.46-fold, respectively. This increase was lower than that directed against the homologous A/Victoria virus. (, and ).
Table 6. Cross-reactive HI and neutralizing antibody responses to A/Sydney virus after two doses of the A/Victoria vaccine
The cross-reactive HI and neutralizing antibody responses in nasal mucus samples were also examined using concentrated nasal wash samples. They showed a 1.63-fold and a 2.12 mean geometric increase, respectively. Similar to the responses in serum, this increase was lower than that directed against the homologous A/Victoria virus. ( and ).
Clinical observation for adverse reactions
Before and after each vaccination clinical data were compiled from the health check records and personal interviews. None of the subjects experienced systemic adverse effects after the nasal vaccination. Minor complaints included light local reactions (mainly runny nose and nasal congestion) that resolved spontaneously within a few days (data not shown). Thus, the intranasal vaccine was considered to be well tolerated.
Discussion
The present study examined HI antibodies as well as neutralizing antibodies in serum and nasal mucus of 50 healthy adults who received two intranasal doses of an inactivated whole virus vaccine derived from A/Victoria/210/2009 (H3N2) virus (45 μg HA per dose) with an interval of 3 weeks. The two doses of nasal vaccine induced serum HI titers, demonstrated by a > 4-fold mean geometric increase, a > 40% conversion rate or significant increase in titer, and a > 70% protection rate in subjects aged 18–60 y ( and ). When the efficacy of vaccination was evaluated using the serum HI antibody responses observed in the present experiments, the serum HI titers exceeded three of the criteria used by the EMA, however, they did not meet the FDA criteria. These results suggest that two intranasal administrations of inactivated whole virus vaccine (45 μg HA per dose) could be a candidate treatment regimen. This vaccination procedure is simple, safe and effective, at least for adults with some immunological memory induced by previous infection or vaccination.
The intranasal A/Victoria vaccination also induced high levels of serum neutralizing antibody responses, measured by the microneutralization assay, of which the sensitivity was about 2-fold higher than that of the HI assay ( and ). When the enhanced sensitivity of the NT assays was corrected in such a way that it could be compared with the antibody response obtained by the HI assays, the neutralizing antibody responses at week 6 showed a mean geometric increase, a conversion rate, and a protection rate which corresponded to those obtained in the serum HI antibody responses in subjects aged 18–60 y ( and ). These results suggest that serum NT titers could be used as an indicator to evaluate protective efficacy of the vaccines.
The present study also examined HI, NT and HA-specific antibody titers in nasal mucus samples, prepared by concentrating nasal wash samples resulting in a total amount of IgA that was equivalent to about 1/10 of that in undiluted nasal mucus. In our previous study, the total protein level and the levels of IgA, IgG and IgM and human serum albumin before and after concentration of nasal wash samples from several participants were examined.Citation31 About 70% of the total nasal wash proteins, 67% of IgA and 26% of IgG were lost during the concentration processes. These decreases might be caused by degradation by proteolytic enzymes or the aggregation of immunoglobulin complexes with other materials in the process of concentration. The amount of total IgA and total IgG recovered from each participant varied slightly at each sampling time; however, the average amount was comparable and constant. This means that the concentrated nasal material is sufficiently comparable within different isolations to be used to express relative antibody responses in the nasal wash. Under the described experimental conditions, allowing for small variations in the recovery of total IgA and IgG from the nasal mucus, the specific antibody titers in the nasal wash samples could be considered to be suitable to compare absolute antibody titers in the nasal mucus before and after vaccination.
The two doses of intranasal vaccine induced both nasal HI and NT titers, demonstrated by a 3.13-fold and 5.88-fold mean geometric increase (the ratio of GMTs between week 0 and 6 after the nasal A/Victoria vaccine), respectively ( and ). In addition, the nasal vaccine induced HA-specific IgA and IgG antibodies in nasal mucus, demonstrated by a 3.88-fold and 1.37-fold mean geometric increase, respectively (). Thus, the nasal inactivated vaccine induced high levels of nasal HI and neutralizing antibody responses with dominant HA-specific IgA antibody responses, in parallel with high levels of serum HI and neutralizing antibody responses dominated by HA-specific IgG antibody responses ( and Fig. S2). It is currently unknown how long these antibody responses are maintained in nasal mucus. Nasal HA-specific IgA and IgG antibody responses induced by live attenuated influenza A virus vaccine were shown to persist for at least 12 mo after inoculation in children who had not previously been infected by influenza A virus.Citation2 However, the duration of antibody responses in nasal mucus induced by intranasal vaccination with an inactivated whole virus vaccine in healthy adults remains to be exmamined.
Regarding antibody responses, Clements et al. compared the correlation between antibody responses and the degree of protection in adults who received a live attenuated intranasal vaccine followed by a challenge with wild-type influenza virus with those in adults who received an inactivated parenteral vaccine prior to challenge.Citation39,Citation40 They found that serum HI titers correlated with protection against viral replication after parenteral vaccination, but not after intranasal vaccination. In contrast, intranasal vaccination induced nasal HA-specific IgA antibodies that correlated with the degree of protection. It was shown that in the upper respiratory tract the majority of the protective immunity induced by influenza virus infection is mainly due to S-IgA antibodies.Citation2,Citation3 In this study, the nasal inactivated vaccine induced high levels of HI, NT and HA-specific IgA antibody responses in nasal mucus (, and ). Althogh several reports show a good correlation between HA-specific antibody titers and HI or NT titers in serum,Citation41,Citation42 HA-specific antibody responses seem to be more sensitive than microneutralization assay in this study (Figs. S2A and D). In addition, NT titers were well correlated with HI titers in both serum and nasal wash ( and ). Among three antibody measurements (HI, microneutralization and HA-specific antibody ELISA), ELISA may be the best choice for achieving sensitivity, but microneutralization has the advantage of representing not only antibody responses against HA like HI and ELISA, but also those against neuraminidase (NA), since it was already shown that NA-specific antibody can contribute partially to the virus neutralization.Citation43 Therefore, neutralizing antibody might be more useful for the evaluation of protective antibody response.Citation10 Together with the fact that at present there are no guidelines available for the evaluation of antibodies in nasal mucus, we chose to focus on the neutralizing antibodies. In the present study, HI, neutralizing and HA-specific antibody titers in nasal mucus, as well as in serum, showed synchronous changes in many of the subjects following nasal vaccination, although the degree of the respective responses and the type of dominant neutralizing antibody varied slightly from subject to subject (data not shown). In addition, antibody responses decreased with age; subjects aged > 60 y produced only low levels of HI and neutralizing antibodies (, and ). Thus, as reported previously, the magnitude of the antibody response in each subject appeared to change depending on parameters that affect the immune responses of the host, such as pre-vaccination antibody levels, sex and age.Citation34,Citation44
HI and neutralizing antibodies cross-reactive with the A/Sydney virus were detected in pre-vaccination serum and nasal wash, and were enhanced after two doses of the nasal A/Victoria vaccine, although the mean geometric increase in the cross-reactive antibody responses increased only about 1.5 to 2-fold (). These results suggest that cross-protection against infection with different strains of influenza virus could be enhanced by an intranasal inactivated whole virus vaccine. Since this field is relatively unexplored yet, it will be interesting to find to which extent this cross-protection can be enhanced by optimizing intranasal vaccination.
The present study first demonstrated that intranasal vaccination with an inactivated whole virus vaccine alone (45 μg HA per dose) could induce serum HI antibody responses which exceeded the EMA criteria for serum HI antibody titers as well as serum neutralizing antibody responses, and that high levels of nasal HI and neutralizing antibody responses were detected using concentrated nasal wash samples. Thus, at least among adults with some immunological memory induced by previous infection or vaccination, the intranasal inactivated whole virus vaccine could be a promising candidate for a needle-free mucosal vaccine. Antibody responses induced by intranasal vaccination in individuals who are naïve to influenza virus antigens remain to be determined. In addition, further studies which compare the HI and NT antibody responses between groups treated with intranasal and existing non-mucosal vaccines are needed to gain more insight in the possible benefits of intranasal vaccination. Some clinical trials have already shown that trivalent inactivated whole virus vaccines induce significantly higher vaccine-specific IgA antibody responses in intranasally immunized subjects than in intramuscularly-immunized elderly subjects, with no significant differences in serum HI antibody responses between the groups.Citation16 A drawback for analyses of nasal antibody responses is that there are currently no criteria to evaluate these type of responses. In this study it is shown that the measurement of nasal HI and NT titers can provide important data in addition to the serum antibody titers. Improved knowledge on these different types of antibody responses in different types of samples will be useful to increase the understanding of immune responses to both vaccination and infection and will be of help for defining the criteria for evaluation of responses in nasal mucus. In order to optimize the accuracy of the data acquired from nasal mucus, the techniques for recovery of nasal antibodies from nasal wash samples might need further improvement.
In conclusion, high levels of serum HI antibody responses, which exceeded all criteria used by the EMA for the evaluation of vaccine efficacy, could be induced by intranasal administration of an inactivated whole A/Victoria virus vaccine containing 45 μg HA in healthy adults. The serum antibody responses were accompanied by high levels of HI, NT and HA-specific IgA antibodies in nasal mucus. The results show that the intranasal vaccination induces both high levels of serum and nasal antibody responses that may be involved in mounting effective protective responses, including cross-protection, against both upper and lower respiratory tract infection by various influenza viruses. The inactivated whole virus vaccine therefore appears to be a promising candidate for intranasal vaccination.
Materials and Methods
Subjects
The study subjects comprised 50 healthy volunteers aged between 22 and 69 y (average 36.5 ± 12.8 y); 14 (28%) were female and four subjects (8%) were over 60 y of age. None of the subjects had to be excluded due a history of allergy to eggs, past or current neurological conditions, or respiratory illness or fever at the time of vaccination. Based on the answers of the volunteers on questions regarding their history of influenza infection and vaccination during the past 5 y, almost all subjects were considered to have acquired at least some degree of immunity to influenza viruses. Written informed consent was obtained from each subject before the onset of the trial. The protocol and other relevant study documentation were reviewed and approved by the Ethics Committee of the National Institute of Infectious Diseases (Tokyo, Japan).
Viruses and vaccines
Influenza viruses, A/Victoria/210/2009 (H3N2; A/Victoria) and A/Sydney/05/1997 (H3N2; A/Sydney) strains, were obtained from the National Institute of Infectious Diseases (Tokyo, Japan), propagated in the allantoic cavity of 10-d-old embryonated hen’s eggs, and purified from the allantoic fluid. The TCID50 (50% infectious dose in tissue culture) of the virus was estimated using a previously described method.Citation45,Citation46 In brief, 10-fold serial dilutions of allantoic fluid containing the virus were inoculated into Madin-Darby canine kidney cells (MDCK: ATCC No. CCL-34) in a 96-well culture plate and incubated for 3 d at 37°C in a 5% CO2 humidified atmosphere. The cytopathic effect observed in the virus-containing wells was evaluated using a microscope and the TCID50 was calculated using the Reed-Muench method.Citation47 An inactivated whole virus vaccine derived from A/Victoria/210/2009 (H3N2) virus, containing 45 μg HA per dose, was supplied by the Research Foundation for Microbial Disease of Osaka University (BIKEN, Kanonji, Kagawa, Japan). The vaccine was prepared from the purified viruses, which were sedimented through a linear sucrose gradient and treated with formalin by the method of Davenport et al.Citation48
Vaccinations and adverse effects
Intranasal vaccination with an inactivated whole virus vaccine (45 μg HA/dose) was performed twice, with a 3-week interval (week 0 and 3), by spraying 0.25 ml of vaccine into each nostril (0.5 ml total) using an atomizer (Keytron: Ichikawa, Chiba, Japan). The mean droplet diameter of the mist generated by the atomizer was 56.5 μm (range: 10 μm to 90 μm). Blood and nasal wash samples were taken from each of the subjects prior to vaccination and three weeks after each vaccination (week 0, 3 and 6).
A health check sheet was given to each subject to record any symptoms after the nasal vaccination as well as their answers to questions regarding their medical history. Medical examinations were held every 3 weeks after the primary and secondary vaccinations to assess their medical condition. Adverse effects were evaluated in terms of local reactions (discomfort, pain in the nose, sneezing, stuffiness and/or running nose, throat pain, or cough) along with systemic reactions, such as malaise, headache, fever and abdominal pain, by means of health check sheets and personal interviews.
Nasal wash specimens
About 80 ml of nasal wash was collected by washing the nasal cavity several times with a nose irrigation device (Hananoa: Kobayashi Pharmaceutical, Osaka, Japan) according to the manufacturer’s instructions.Citation31 The collected nasal wash samples were filtered using bottle top filters (Nalgene Nunc International, Chiba, Japan) with membranes covered with a cotton mat to remove mucopolysaccharides and other debris. The pooled cleaned nasal wash samples were then concentrated to a final volume of approximately 1 ml using Vivaspin centrifugal concentrators (Vivaspin 20, MWCO 30.000: Sartorius Stedim Biotech, Aubagne, France). The concentrated nasal wash samples were stored at -80°C until use. The protein concentration in the concentrated nasal wash was measured using a BCA Protein Assay Kit (Thermo Fisher Scientific, Yokohama, Japan) according to the manufacturer’s instructions.
Neutralization and haemagglutination inhibition assays
NT titers were examined using microneutralization assays as previously described with minor modifications.Citation41,Citation46 Briefly, serum samples were treated with a receptor-destroying enzyme (RDE: Denka Seiken, Niigata, Japan) overnight at 37°C and heat-inactivated for 30 min at 56°C, and then diluted 1:10 before the assay. Nasal wash samples were adjusted to 1 mg/ml total protein, and the final samples contained about 1/10 of the total IgA found in nasal mucus (0.22 mg/ml; original concentration in nasal mucus is 2.20 mg/ml).Citation31 These standardized nasal wash samples were treated with RDE and heat-inactivated similar to the serum samples, and then diluted 1:20 for use in the assays.
2-fold serial dilutions of samples were mixed with an equal volume of diluent containing influenza virus equivalent to 100 TCID50 and added to the wells of a 96-well plate containing a monolayer culture of MDCK cells. Four control wells containing virus or diluent alone were included on each plate. The plates were incubated for 3 or 4 d at 37°C in a 5% CO2 humidified atmosphere. All wells were observed for the presence or absence of cytopathic effects and then fixed with 10% formalin phosphate buffer for more than 5 min at room temperature and stained with Naphthol blue black. After washing and drying, cells were solubilized with 0.1 M NaOH and the absorbance (A) was read at 630 nm. The average A630 value was determined from virus-only controls (Avirus) and medium-only controls (Acell). All values above 50% of the specific signal, calculated using the formula X = (Acell –Avirus)/2 + Avirus, were considered positive for neutralization. The titers recorded were the reciprocal of the highest dilution, where A630 was > X.
HI titers were examined using a microtitration method as previously described.Citation49 Serum and nasal wash samples were prepared in the same manner as for the neutralization assay and treated with packed red blood cells to remove non-specific haemagglutination-inhibiting materials. The starting dilutions for the HI assay were 1:10 and 1:20 for the serum and standardized nasal wash samples, respectively.
Evaluation of serum HI titers
Serum HI titers were evaluated using the following three parameters: the ratio of the geometric mean titer (GMT) in post-vaccination to that in pre-vaccination (the mean geometric increase); the percentage of subjects showing an increase from a pre-vaccination titer of <1:10 to a post-vaccination titer of ≥1:40 (the conversion rate) or showing a ≥4-fold increase from a pre-vaccination titer of ≥1:10 (significant increase in titer); and the percentage of subjects with a post-vaccination titer of ≥1:40 (the protection rate). Hereafter, the conversion rate or significant increase in titer is indicated as conversion rate. As shown in , the parameters for serum HI titers after vaccination are currently used as criteria to evaluate the vaccine efficacy by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA).Citation22-Citation24
Determination of HA-specific IgA and IgG antibody titers
The titers of IgA and IgG antibodies specific for the HA molecule of the A/Victoria virus (HA-specific IgA titer and HA-specific IgG titer, respectively) in the serum and standardized nasal wash samples were determined by ELISA. The ELISA assay was performed in microtiter plates (Costar, Cambridge, MA) using the following procedure. First, wells of microtiter plates were coated with HA molecules purified from the A/Victoria virus according to the procedure of Phelan et al.Citation50 Second, the HA molecules were incubated with 2-fold serial dilutions of serum or standardized nasal wash samples followed by detection with goat anti-human IgA (α-chain specific) or goat anti-human IgG (γ-chain specific) (BETHYL Laboratories, Montgomery, AL) antibodies conjugated to alkaline phosphatase. Third, the enzymatic reaction was started by adding 1 mg/ml of p-nitrophenyl-phosphate as the substrate. Color development was measured at 405 nm using a microplate reader (Model 680: Bio-Rad Laboratories, Hercules, CA). The antibody titer for a given sample was calculated as the reciprocal of the highest dilution of the test sample that gave an absorbance read at 405 nm (A405) greater than a cut-off value equal to the mean A405 + 2SD of 11 2-fold serial dilutions (starting at 1:10 for serum and at 1:160 for nasal sample due to sample limitation) of the negative control samples (NT titer, <10 × 20; HI titer, <10 × 20; HA-specific antibody titer, <10 × 20 for serum and <10 × 24 for nasal wash) selected from the pre-vaccination serum and nasal wash samples of 50 subjects.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism statistical software package (Version 5.0c: Graph Pad Software Inc., CA, USA). The threshold of statistical significance was set at 5% (p < 0.05).
| Abbreviations: | ||
| S-IgA | = | secretory IgA |
| HA | = | haemagglutinin |
| NT titer | = | neutralization titer |
| HI assay | = | haemagglutination inhibition assay |
| HI titer | = | haemagglutination inhibition titer |
| HI antibody response | = | haemagglutination-inhibiting antibody response |
| GMT | = | geometric mean titer |
| ELISA | = | enzyme-linked immunosorbent assay |
Additional material
Download Zip (540.8 KB)Acknowledgments
The authors wish to express their appreciation to all the study subjects, who were recruited from the National Institute of Infectious Diseases (Tokyo, Japan), Toko Yakuhin Kogyo Co., Ltd., and BIKEN, and to thank the authors whose work is cited in this paper. We would like to thank Mr Takeshi Tanimoto, Dr Yasuhiro Gomi, Dr Sadao Manabe, Mr Toyokazu Ishikawa and Dr Yoshinobu Okuno at BIKEN for supplying the inactivated whole virus vaccine derived from A/Victoria/210/2009 virus strain. We would also like to thank the researchers of BIKEN and Mr Takashi Miyazaki and Mr Taizo Kamishita of Toko Yakuhin Kogyo Co., Ltd. for the helpful discussions. Dr E. van Riet is a recipient of a Postdoctoral Fellowship for Foreign Researchers of the Japan Society for the Promotion of Science. The work described in this report was supported by grants from the Japanese Ministry of Health, Labour, and Welfare. The founders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have declared that no competing interests exist.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Murphy BR, Webster RG. Orthomyxoviruses. In: Fields BN, Knipe DM, M. HP, Chanock RM, Melnick JL, Monath TP, et al., eds. Fields Virology. Philadelphia: Lippincott Williams & Wilkins, 1996:1397-445.
- Murphy BR, Clements ML. The systemic and mucosal immune response of humans to influenza A virus. Curr Top Microbiol Immunol 1989; 146:107 - 16; http://dx.doi.org/10.1007/978-3-642-74529-4_12; PMID: 2659262
- Murphy BR. Mucosal Immunity to Viruses. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGee JR, Bienenstock J, eds. Handbook of Mucosal Immunology. San Diego: Academic Press, 1994:333-43.
- Tamura S, Funato H, Hirabayashi Y, Kikuta K, Suzuki Y, Nagamine T, et al. Functional role of respiratory tract haemagglutinin-specific IgA antibodies in protection against influenza. Vaccine 1990; 8:479 - 85; http://dx.doi.org/10.1016/0264-410X(90)90250-P; PMID: 2251874
- Tamura S, Funato H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, et al. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol 1991; 21:1337 - 44; http://dx.doi.org/10.1002/eji.1830210602; PMID: 1646112
- Asahi Y, Yoshikawa T, Watanabe I, Iwasaki T, Hasegawa H, Sato Y, et al. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J Immunol 2002; 168:2930 - 8; PMID: 11884464
- Asahi-Ozaki Y, Yoshikawa T, Iwakura Y, Suzuki Y, Tamura S, Kurata T, et al. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J Med Virol 2004; 74:328 - 35; http://dx.doi.org/10.1002/jmv.20173; PMID: 15332283
- Ito R, Ozaki YA, Yoshikawa T, Hasegawa H, Sato Y, Suzuki Y, et al. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine 2003; 21:2362 - 71; http://dx.doi.org/10.1016/S0264-410X(03)00078-1; PMID: 12744867
- Renegar KB, Small PA Jr., Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 2004; 173:1978 - 86; PMID: 15265932
- Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis 2004; 57:236 - 47; PMID: 15623947
- Jodar L, Duclos P, Milstien JB, Griffiths E, Aguado MT, Clements CJ. Ensuring vaccine safety in immunization programmes--a WHO perspective. Vaccine 2001; 19:1594 - 605; http://dx.doi.org/10.1016/S0264-410X(00)00358-3; PMID: 11166881
- Kuno-Sakai H, Kimura M, Ohta K, Shimojima R, Oh Y, Fukumi H. Developments in mucosal influenza virus vaccines. Vaccine 1994; 12:1303 - 10; http://dx.doi.org/10.1016/S0264-410X(94)80056-6; PMID: 7856295
- Hashigucci K, Ogawa H, Ishidate T, Yamashita R, Kamiya H, Watanabe K, et al. Antibody responses in volunteers induced by nasal influenza vaccine combined with Escherichia coli heat-labile enterotoxin B subunit containing a trace amount of the holotoxin. Vaccine 1996; 14:113 - 9; http://dx.doi.org/10.1016/0264-410X(95)00174-Y; PMID: 8852406
- Muszkat M, Yehuda AB, Schein MH, Friedlander Y, Naveh P, Greenbaum E, et al. Local and systemic immune response in community-dwelling elderly after intranasal or intramuscular immunization with inactivated influenza vaccine. J Med Virol 2000; 61:100 - 6; http://dx.doi.org/10.1002/(SICI)1096-9071(200005)61:1<100::AID-JMV16>3.0.CO;2-5; PMID: 10745240
- Greenbaum E, Furst A, Kiderman A, Stewart B, Levy R, Schlesinger M, et al. Mucosal [SIgA] and serum [IgG] immunologic responses in the community after a single intra-nasal immunization with a new inactivated trivalent influenza vaccine. Vaccine 2002; 20:1232 - 9; http://dx.doi.org/10.1016/S0264-410X(01)00396-6; PMID: 11803086
- Greenbaum E, Engelhard D, Levy R, Schlezinger M, Morag A, Zakay-Rones Z. Mucosal (SIgA) and serum (IgG) immunologic responses in young adults following intranasal administration of one or two doses of inactivated, trivalent anti-influenza vaccine. Vaccine 2004; 22:2566 - 77; http://dx.doi.org/10.1016/j.vaccine.2003.12.018; PMID: 15193382
- Durrer P, Glück U, Spyr C, Lang AB, Zurbriggen R, Herzog C, et al. Mucosal antibody response induced with a nasal virosome-based influenza vaccine. Vaccine 2003; 21:4328 - 34; http://dx.doi.org/10.1016/S0264-410X(03)00457-2; PMID: 14505915
- Treanor J, Nolan C, O’Brien D, Burt D, Lowell G, Linden J, et al. Intranasal administration of a proteosome-influenza vaccine is well-tolerated and induces serum and nasal secretion influenza antibodies in healthy human subjects. Vaccine 2006; 24:254 - 62; http://dx.doi.org/10.1016/j.vaccine.2005.07.088; PMID: 16129526
- Atmar RL, Keitel WA, Cate TR, Munoz FM, Ruben F, Couch RB. A dose-response evaluation of inactivated influenza vaccine given intranasally and intramuscularly to healthy young adults. Vaccine 2007; 25:5367 - 73; http://dx.doi.org/10.1016/j.vaccine.2007.05.002; PMID: 17559990
- Ichinohe T, Watanabe I, Ito S, Fujii H, Moriyama M, Tamura S, et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J Virol 2005; 79:2910 - 9; http://dx.doi.org/10.1128/JVI.79.5.2910-2919.2005; PMID: 15709010
- Ichinohe T, Tamura S, Kawaguchi A, Ninomiya A, Imai M, Itamura S, et al. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J Infect Dis 2007; 196:1313 - 20; http://dx.doi.org/10.1086/521304; PMID: 17922395
- European Medicines Agency. Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96). In: (CPMP) CfPMP, ed. London: Committee for Proprietary Medical Products (CPMP), 1997.
- U.S. Food and Drug Administration. Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. In: Services USDoHaH, ed., 2007.
- European Medicines Agency. Guideline on dossier structure and content for pandemic influenza vaccine marketing authorization application (CPMP/VFG/4717/03-Rev.1). In: (CHMP) CfMPfHU, ed. London: Committee for Medicinal Products for Human Use (CHMP), 2008.
- Cox RJ. Correlates of protection to influenza virus, where do we go from here?. Hum Vaccin Immunother 2013; 9; In press http://dx.doi.org/10.4161/hv.22908; PMID: 23291930
- Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J Infect Dis 2005; 58:195 - 207; PMID: 16116250
- Tamura S. Studies on the usefulness of intranasal inactivated influenza vaccines. Vaccine 2010; 28:6393 - 7; http://dx.doi.org/10.1016/j.vaccine.2010.05.019; PMID: 20493820
- Okuno Y, Tanaka K, Baba K, Maeda A, Kunita N, Ueda S. Rapid focus reduction neutralization test of influenza A and B viruses in microtiter system. J Clin Microbiol 1990; 28:1308 - 13; PMID: 2380359
- Lu BL, Webster RG, Hinshaw VS. Failure to detect hemagglutination-inhibiting antibodies with intact avian influenza virions. Infect Immun 1982; 38:530 - 5; PMID: 6292104
- Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937 - 43; PMID: 10074505
- Ainai A, Tamura S, Suzuki T, Ito R, Asanuma H, Tanimoto T, et al. Characterization of neutralizing antibodies in adults after intranasal vaccination with an inactivated influenza vaccine. J Med Virol 2012; 84:336 - 44; http://dx.doi.org/10.1002/jmv.22273; PMID: 22170556
- Kurono Y, Mogi G. Secretory IgA and serum type IgA in nasal secretion and antibody activity against the M protein. Ann Otol Rhinol Laryngol 1987; 96:419 - 24; PMID: 3304089
- Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 2003; 21:3212 - 8; http://dx.doi.org/10.1016/S0264-410X(03)00234-2; PMID: 12804850
- Tamura S, Hasegawa H, Kurata T. Estimation of the effective doses of nasal-inactivated influenza vaccine in humans from mouse-model experiments. Jpn J Infect Dis 2010; 63:8 - 15; PMID: 20093755
- Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A 2004; 101:5598 - 603; http://dx.doi.org/10.1073/pnas.0400937101; PMID: 15034168
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004; 303:1529 - 31; http://dx.doi.org/10.1126/science.1093616; PMID: 14976261
- Kang SM, Guo L, Yao Q, Skountzou I, Compans RW. Intranasal immunization with inactivated influenza virus enhances immune responses to coadministered simian-human immunodeficiency virus-like particle antigens. J Virol 2004; 78:9624 - 32; http://dx.doi.org/10.1128/JVI.78.18.9624-9632.2004; PMID: 15331695
- Koyama S, Aoshi T, Tanimoto T, Kumagai Y, Kobiyama K, Tougan T, et al. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci Transl Med 2010; 2:25ra24; http://dx.doi.org/10.1126/scitranslmed.3000759; PMID: 20424013
- Clements ML, Betts RF, Tierney EL, Murphy BR. Resistance of adults to challenge with influenza A wild-type virus after receiving live or inactivated virus vaccine. J Clin Microbiol 1986; 23:73 - 6; PMID: 3700611
- Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol 1986; 24:157 - 60; PMID: 3722363
- Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis 2000; 181:1133 - 7; http://dx.doi.org/10.1086/315323; PMID: 10720541
- Murphy BR, Phelan MA, Nelson DL, Yarchoan R, Tierney EL, Alling DW, et al. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J Clin Microbiol 1981; 13:554 - 60; PMID: 7240388
- Takahashi Y, Hasegawa H, Hara Y, Ato M, Ninomiya A, Takagi H, et al. Protective immunity afforded by inactivated H5N1 (NIBRG-14) vaccine requires antibodies against both hemagglutinin and neuraminidase in mice. J Infect Dis 2009; 199:1629 - 37; http://dx.doi.org/10.1086/598954; PMID: 19385735
- Hirota Y, Kaji M, Ide S, Goto S, Oka T. The hemagglutination inhibition antibody responses to an inactivated influenza vaccine among healthy adults: with special reference to the prevaccination antibody and its interaction with age. Vaccine 1996; 14:1597 - 602; http://dx.doi.org/10.1016/S0264-410X(96)00153-3; PMID: 9032887
- Tobita K, Sugiura A, Enomote C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol 1975; 162:9 - 14; http://dx.doi.org/10.1007/BF02123572; PMID: 1214709
- Kadowaki S, Chen Z, Asanuma H, Aizawa C, Kurata T, Tamura S. Protection against influenza virus infection in mice immunized by administration of hemagglutinin-expressing DNAs with electroporation. Vaccine 2000; 18:2779 - 88; http://dx.doi.org/10.1016/S0264-410X(00)00087-6; PMID: 10812219
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoint. Am J Hyg 1938; 27:493 - 7
- Davenport FM, Hennessy AV, Brandon FM, Webster RG, Barrett CD Jr., Lease GO. Comparisons of Serologic and Febrile Responses in Humans to Vaccination with Influenza a Viruses or Their Hemagglutinins. J Lab Clin Med 1964; 63:5 - 13; PMID: 14102904
- Hierholzer JC, Suggs MT, Hall EC. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol 1969; 18:824 - 33; PMID: 4984203
- Phelan MA, Mayner RE, Bucher DJ, Ennis FA. Purification of influenza virus glycoproteins for the preparation and standardization of immunological potency testing reagents. J Biol Stand 1980; 8:233 - 42; http://dx.doi.org/10.1016/S0092-1157(80)80039-4; PMID: 7410447