Abstract
To determine if newer influenza vaccines can safely improve seroprotection rates of older adults, we compared three licensed trivalent inactivated vaccines (TIVs) in a randomized, controlled trial with evaluator blinding. Participants were non-frail adults ≥ 65 y old, annually TIV-immunized. Study vaccines included intradermal (IDV), MF59-adjuvanted (ADV) and subunit (TIV) formulations of equal potency and strain composition. Blood was obtained before vaccination (V1) and 21 (V2) and 180 d (V3) afterward and tested by hemagglutination inhibition (HAI) assay. Safety diaries were completed daily by participants and specific tolerability questions were posed regarding injections and symptoms. In total, 911 participants were immunized and 887 (97.4%) completed V3. Groups had similar demographics. General symptom rates post-vaccination were similar among groups. Rates of injection site redness after IDV/ADV/TIV were 75%/13%/13% and rates of pain were 29%/38%/20%, respectively, but each vaccine was well tolerated, with symptoms causing little bother. Baseline antibody titers did not differ significantly among groups but B/Brisbane titers were too high for meaningful response assessments. At V2, seroprotection rates (HAI titer ≥ 40) were highest after ADV, the rate advantage over IDV and TIV being significant at 11.8% and 11.4% for H3N2 and 10.2% and 12.5% for H1N1, respectively. At day 180, seroprotection rates had declined ~25% and no longer differed significantly among groups. While IDV and TIV were also well tolerated, ADV induced modestly higher antibody titers in seniors to influenza A strains at 3 weeks but not 6 months post-vaccination. Immune responses to IDV and TIV were similar in this population.
Introduction
Older adults suffer the greatest morbidity and mortality of any age group from seasonal influenza infections.Citation1 Age ≥ 65 y is itself a risk factor for more severe disease, potentially synergizing with other known risk factors such as chronic medical conditions, frailty and group living situations.Citation2 Severe outcomes in seniors most often follow infection with A/H3N2 viruses.Citation1 Although seniors are recommended to receive trivalent inactivated (TIV) vaccines annually, they respond sub-optimally because of progressive age-related deterioration of immune functions (immunosenescence).Citation2-Citation4 Consequently the international criteriaCitation5 for acceptable immunogenicity of seasonal TIVs are lower for persons > 60 y of age.
Several TIV formulations have been modified in attempts to improve protection of older adults. These include a high potency formulation with 60 µg of each viral hemagglutinin per dose,Citation6 an MF59-adjuvanted vaccineCitation7,Citation8 and an intradermal formulationCitation9,Citation10 that seeks to engage the dermal immune system to advantage. The latter 2 products were recently marketed in Canada for use in seniors, causing public health officials to question whether they offer sufficient added protection over the standard TIV vaccines supplied to seniors in public programs to warrant the higher purchase costs. Additionally, differences in the safety profiles of the new products might influence public acceptance positively or negatively.
The MF59 adjuvanted TIV vaccine (ADV) was first licensed in Italy in 1997 and has been widely used in Europe for annual vaccination of seniors.Citation7,Citation8 The MF59 adjuvant consists of microdroplets of squalene oil suspended in aqueous solution by two emulsifiers.Citation7 A meta-analysis of 13 clinical trialsCitation8 involving 3,600 seniors immunized with adjuvanted or conventional TIV indicated that antibody geometric mean titers (GMT) were modestly higher after ADV than TIV (GMT ratios 1.10 to 1.18 depending upon the viral antigen) but this effect was not evident in every trial. However, the effectiveness of ADV in seniors has not yet been determined in a controlled trial. A meta-analysisCitation11 of safety data from studies involving elderly vaccinees showed a 5% increase in general symptoms and 10% increase in local reactions (mainly pain) after ADV compared with TIV. Most symptoms were mild and short-lived.Citation11
The intradermal TIV vaccine (IDV) intended for seniors delivers 15 µg of hemagglutinin from each vaccine strain in a volume of ~100 µL into the upper dermal layer of skin.Citation9 This is accomplished using a 30 gauge micro-needle attached to a pre-filled syringe, designed to allow highly reproducible vaccine delivery.Citation12 Comparisons of HAI titers following vaccination of seniors with IDV or TIV have generally shown superior responses to IDV,Citation9,Citation10 with one study demonstrating non-inferiority of IDV to ADV.Citation13 Intradermal vaccination is reportedly less painful than intramuscular vaccination,Citation14 an appealing feature for the needle-averse. However, IDV causes prominent local inflammation,Citation9,Citation10,Citation13 with erythema, swelling and pain reaching peak rates one day after vaccination. Most of these local effects are mild and short-lived.
The purpose of this study was to compare 3 licensed, trivalent, inactivated influenza vaccines (TIV, subunit; ADV, adjuvanted; and IDV, intradermal) for adults 65 y of age and older for differences that could be relevant in public programs, including the nature and frequency of adverse events, tolerability of the injection itself and any subsequent adverse events, and immunogenicity both in the short (21 d) and longer term (6 mo). Seniors who receive annual influenza vaccination often develop progressively higher antibody titers,Citation15,Citation16 a situation that can complicate assessment of vaccine responses and potentially blunt differences between vaccine formulations. Nevertheless, we chose to recruit seniors with a history of annual influenza vaccination because most (~70%) of this age group receives TIV vaccine in CanadaCitation17 and any advantages of a new formulation would need to be evident in this context to support program policy decisions. Other noteworthy features of the study design included a focus on non-frail participants, age- and sex-stratified central randomization, evaluator blinding and use of 3 assays to assess immune responses to immunization, including hemagglutination inhibition (HAI), single radial hemolysis (SRH) and microneutralization (MN) assays.
Results
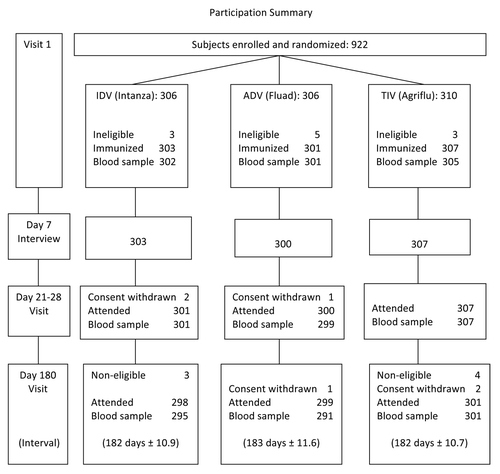
Enrollment and immunization were completed as planned in September and October, 2011, and 180 d follow-up visits were completed by May, 2012. As detailed in , 911 participants were immunized, 908 attended the second visit and 887 (97.4%) provided a blood sample at the final visit.
Participants
Participant demographics are summarized in . As randomization was stratified by age and sex, study groups were nearly identical in these respects. Other potential influences on responses were present at similar frequencies in each study group, with no statistically significant differences. By selection, all participants had received TIV within the previous 2 y (86.5% in both years) and were not frail. Almost all were White/Caucasian and few were morbidly obese (BMI ≥ 40, 2.2%) or smoked cigarettes (4.4%). Nearly half reported having regular strenuous exercise and one-quarter ingested probiotics daily. Chronic health conditions were commonly present, averaging 2.9 conditions per participant. Conditions predisposing to an increased risk of influenza complications (other than age ≥ 65) were present in 55% of subjects and were similarly distributed among the study groups (). Risk conditions for influenza were mainly cardiac (present in 11.3%), pulmonary (12.1%), diabetes mellitus (13.0%) and other endocrine disorders (15.4%). Other common conditions expected in this age group included musculoskeletal (in 48.2%), vascular (46.2%), elevated cholesterol (27.1%) and gastrointestinal (23.5%) disorders, also similarly distributed among study groups (data not shown).
Table 1. Study participant demographics.
Adverse events, systemic
On the day prior to vaccination, about one-third of participants recalled having one or more of the solicited symptoms (), mainly arthralgia (in 20.4%), myalgia (13.2%), sleep disturbance (12.2%) and tiredness (11.5%). Baseline, peak (days 0–2) and cumulative (days 0–6) rates did not differ significantly among the vaccine groups for any specific systemic symptom. Reported rates of arthralgia and sleep disturbance did not increase significantly over baseline following vaccination, but rates of myalgia, malaise, tiredness and headache increased in each vaccine group (). Following vaccination, rates were low in all groups for nausea (2.9–4.0% across groups, days 0–6), vomiting (0.7–1.6%), diarrhea (2.3–4.6%), shivering (2.0–3.3%) and sweating (3.7–7.6%). Fever was detected in only 2–5 subjects per group (0.7–1.7%) with only two participants reporting fever ≥ 39.5°C, both within two days after IDV.
Table 2. Reported systemic adverse events prior to and following Influenza immunization
Among symptoms graded for severity by participants, most were mild or moderate. Severe symptoms were uncommonly reported by all groups during the week after vaccination: myalgia 1.0–2.6% of participants, arthralgia 0.3–1.7%, malaise 1.7–2.0%, and tiredness 1.7–2.7%. None of these events led to withdrawal of participation. Eight SAEs occurred between visits 1 and 2 (IDV- 4, ADV- 3, TIV- 1), each considered unrelated to immunization. They included trauma (1), cardiorespiratory (2) and vascular (2) conditions and unplanned surgery (3). Three of these events led to withdrawal of participation before visit 2. Between days 21 and 180, 37 SAEs were reported (IDV- 13, ADV- 12, TIV- 12), none of which related to prior immunization. Three events resulted in death (trauma-1, cardiopulmonary disease-2). SAEs included musculoskeletal (5), cardiopulmonary (14), vascular (2), gastrointestinal (9), neoplastic (5) and other (2) conditions expected among seniors.
Adverse events, injection site
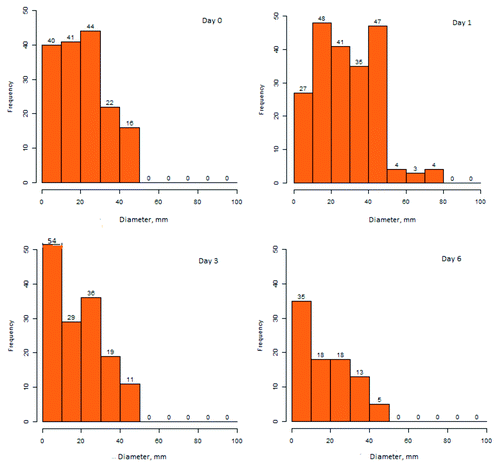
As anticipated, injection site redness more often followed administration of IDV than ADV or TIV (). Onset was typically later in the day after IDV immunization with the peak rate reached next day. Most instances were ≤ 50 mm diameter (). Resolution after IDV was relatively slow, with about half of those affected still reporting some redness 6 d after vaccination (). IDV recipients were also most likely to report injection site swelling, induration/lump and itchiness. IDV accounted for most reports of itchiness, with a peak rate of 12.9% reached on day 2 after vaccination. Of those affected, 10% still reported itchiness on day 6 after IDV vaccination.
Table 3. Injection site reactions following Influenza vaccines.
Figure 2. Frequency and diameter of erythema at IDV injection sites at intervals following immunization.
ADV was the most frequent cause of injection site pain (), followed by IDV then TIV. Only a single instance of severe pain was reported, following IDV. Pain severity ratings on day 1 (peak) after vaccination (mild/moderate/severe, as percentage of subjects affected) were: ADV (25.9/4/0), IDV (15.5/1.3/0.3) and TIV (13.4/1.0/0). Most instances of pain resolved within 2 d after vaccination. ADV and TIV were alike as infrequent causes of redness, induration or itchiness ().
Vaccine tolerability assessment
Tolerability questionnaires were completed by 911 participants shortly after vaccination and by 908 a week later. Ninety percent of participants said they were “not at all anxious” about the procedure and 9% were “a little anxious” about it, with no differences among the study groups. Pain or discomfort during the actual injection was acknowledged after all 3 vaccines (IDV 31.4%, ADV 27.9%, TIV 22.5%, p = 0.05) but almost all affected individuals said they were “not at all” or only “a little” bothered by injection-related pain (IDV group 92.6%, ADV group 94%, TIV group 97.1%, p = 0.32). Given the same experience next year, 91–93% said they would definitely accept vaccination again. Likewise, almost 90% stated no preference between the study vaccine they received and past TIV vaccines.
A week after vaccination, over 70% continued to state no preference between the study vaccine they received and past TIV vaccines. Overall, TIV recipients had the least discomfort during vaccination and the highest preference rate to receive the same vaccine again next year. Most participants who had a local reaction indicated that they were “not at all bothered” or “a little bothered” regardless of the vaccine administered (IDV 95.9%, ADV 92.5%, TIV 100%, p = 0.11). High tolerability rates were also reported by those who experienced systemic symptoms (IDV 78.0%, ADV 88.9%, TIV 77.1%, p = 0.016). Given the same experience, 87–88% of participants said they would definitely get vaccinated again next year and 88–95% would definitely or probably recommend the vaccine they had received to a close friend or relative.
In response to attitudinal questions asked during the day 7 interview, over 80% of participants said they would rather endure more injection-related pain (85.5%) or more frequent, painful local reactions (82.3%) if that led to superior protection against influenza. Responses did not differ among the study groups.
Immunogenicity assessments
As indicates, paired sera obtained pre-vaccination and 21 d later were available from 905 of 911 vaccinees (99.3%) and were of sufficient volume for almost all of the intended HAI and SRH assays. At visit 3 (day 180) sera for HAI testing were obtained from 887 participants (97.4% of those immunized), of which all but 10 (2–5 per group) were obtained per protocol. As delays in obtaining samples were < 14 d beyond window, all available samples were included in the modified intention-to-treat analysis.
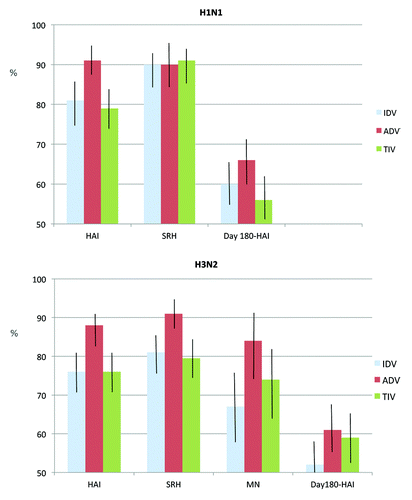
Baseline antibody measures did not differ significantly among the study groups for any of the viral strains, whether by HAI, SRH or MN assay (, and ). Baseline titers against B/Brisbane were very high in each study group, precluding meaningful response assessments. Seroprotection rates 21 d after immunization are shown in . For H1N1, seroprotection rates were significantly higher after ADV than the other vaccines when measured by HAI but not by SRH. Specifically, HAI-determined H1N1 seroprotection rate differences for ADV-IDV were 10.2 (95%CI 4.7,15.8) and for ADV-TIV were 12.5 (95%CI 6.9, 18.2). For H3N2, seroprotection rates were significantly higher after ADV than the other vaccines by both assays, while rates did not differ significantly between IDV and TIV. Specifically, HAI-determined H3N2 seroprotection rate differences for ADV-IDV were 11.8 (95% CI 5.7,17.9) and for ADV-TIV were 11.4 (95% CI 5.5, 17.4). In the MN assay, titers ≥ 40 to H3N2 were achieved more frequently after ADV than the other vaccines
Table 4. Comparison of immune responses to study vaccines at day 21 post-vaccination: HAI Assay.
Table 5. Comparison of immune responses to study vaccines at day 21 post-vaccination: SRH assay.
Table 6. Microneutralization titers to H3N2 virus at day 21–28 following vaccination: Groups of 100.
Figure 3. Seroprotection rates and 95% confidence intervals measured by HAI and SRH assays 21 and 180 d following immunization, for H1N1 and H3N2 antigens.
GMTs following immunization were highest after ADV for both A viruses. GMT ratios for ADV/IDV determined by HAI were 1.34 (95% CI 1.08, 1.66) for H1N1 and 1.46 (95% CI 1.16, 1.83) for H3N2 while ratios for ADV/TIV were 1.44 (95% CI 1.16, 1.79) and 1.45 (95% CI 1.25, 2.81), respectively. GMT ratios for ADV/IDV determined by SRH assay were 1.11 (95% CI 0.99, 1.25) for H1N1 and 1.21 (95% CI 1.07, 1.38) for H3N2 responses. Corresponding ratios for ADV/TIV were 1.12 (95% CI 1.00, 1.26) for H1N1 and 1.26 (95%CI 1.11, 1.43) for H3N2 responses. IDV/TIV ratios did not differ significantly for the A viruses by either assay.
Seroprotection rates 21 d after immunization were higher for women than men after all 3 vaccines for H3N2 virus but only after ADV for H1N1 virus (data not shown). Seroprotection rates did not differ by age group, presence or number of health conditions (0–2 vs ≥ 3), BMI above or below the group mean of 27.2, with regular strenuous exercise or daily probiotic use, with any of the vaccines (data not shown). When observed responses were compared with international criteria for seniors, all 3 vaccines met the seroprotection rate criterion (> 60%) for each virus, by both assays. By HAI, none met the seroconversion or GM fold increase criteria for B/Brisbane because of the high baseline values whereas all 3 vaccines met these criteria for the A viruses (except TIV, which fell short of the seroconversion criterion for H3N2). By SRH assay, the GM fold increase criterion (> 2) was not met for any virus after IDV or TIV vaccines but it was met for the A viruses after ADV.
Residual titers measured at Day 180 following immunization are shown in , when seroprotection rates by HAI differed minimally among the vaccine groups and had declined by 21.3- 25.6% vs. H1N1 and 17.4–26.7% vs. H3N2 from the values observed at Day 21. Rate declines for B/Brisbane were < 3% (data not shown). GMTs against the A viruses declined by about half to day 180, with the residual values ranging from 39.0 (TIV)-47.4 (ADV) for H1N1 and from 35.1 (IDV)-44.3 (ADV) for H3N2. GMTs against B/Brisbane declined 30% on average, with residual values between 158 (TIV) and 196 (IDV).
Discussion
This study is notable for directly comparing 3 influenza vaccines in older adults, whereas previous studies typically compared a new vaccine to TIVCitation8,Citation10,Citation11,Citation18 or new vaccines to each other.Citation13 This study benefitted from a high degree of similarity among the study groups, effectively controlling for a range of potential influences on responses to vaccination including age,Citation19 sex, prior influenza vaccination,Citation15,Citation16 frailty,Citation20 chronic health conditions, obesity,Citation21 moderate exerciseCitation22 and probiotic use.Citation23 Similarity of baseline antibody titers among the study groups avoided the need for adjustment of observed responses.Citation24
Baseline antibody titers to B/Brisbane were much higher in this study (e.g., GMT 157.4) than in recent studies in seniors by Chi et al.Citation25(GMT 10.8) and Falsey et al.Citation6 (GMT 19.0). Although most participants in the latter studies had received influenza vaccination in the previous season, only those studied by Chi et al. had received the same B strain later used to measure baseline titers whereas those studied by Falsey et al. experienced a lineage change between years, limiting cross-protection. In contrast, most subjects in the present study had received the study vaccine strain in both pre-study years and, as annual vaccine recipients, could have received B/Victoria lineage strains in 4 of 5 pre-study years. As others have reported,Citation15,Citation16,Citation24 repeated vaccination of seniors with the same or similar antigens causes titers to approach maximal levels. Our observations of high baseline B/Brisbane titers and limited further responses to vaccination are consistent with this phenomenon, although subjects had also received A/H1N1/California/2009 antigen in both pre-study years without a similar increase in baseline titers. Use of ether-treated B virus in the HAI assay may have contributed to the high baseline titers as ether treatment increases the sensitivity of the assay for B viruses but baseline titers were also elevated by SRH assay that used inactivated B virus. A definitive explanation for the high baseline titers will require further study; they precluded meaningful comparison of responses to the study vaccines.
The results of this study confirm previous reports (reviewed inCitation8) that the adjuvanted vaccine (ADV) is more immunogenic against the influenza A vaccine strains than the non-adjuvanted TIV on which it is based.Citation26 The apparent advantage of ADV over TIV was evident in terms of higher rates of seroprotection (HAI titers ≥ 40) and higher GMTs, measured 21 d after vaccination. Titers ≥ 160 were also seen more often after ADV than TIV and are reportedly more predictive of protection than the standard threshold.Citation27-Citation29 While statistically significant, the differences in seroprotection rates and GMT ratios after ADV or TIV were of modest magnitude. Baseline titers of the annually immunized study participants were moderately high, which might have reduced potential differences in responses to both vaccines. In published comparisons of ADV and TIV in seniors,Citation8 the seroprotection rate advantages after ADV were of similar magnitude to our observations but were variably present and differed among vaccine strains, likely reflecting differences among the studies in participant selection, annual vaccine strains, TIV formulations and antibody assays. In the present study, the seroprotection rate advantage after ADV was evident with both HAI and SRH assay data for H3N2 antigen but only with HAI data for the H1N1 antigen. Microneutralization titers vs. H3N2 were highest after ADV but the differences between vaccines did not reach statistical significance possibly because of the smaller populations tested. The 3 assays generally yield titers of similar magnitude.Citation30-Citation32 Whether the modestly greater seroprotection rates after ADV result in greater protection against infection is not yet certain. A modeling exercise using Canadian data for influenza infections projected that even small increases in vaccine effectiveness with ADV over TIV would be cost-effective.Citation33 An observational study in elderly adults in Northern ItalyCitation34 reported a lower rate of influenza-like illness or pneumonia after ADV relative to TIV but only after multiple data adjustments, which were open to criticism.Citation35 A recent comparison of TIV vaccinesCitation36 with and without AS03 adjuvant (also squalene-based, like ADV) in elderly people indicated modestly greater relative efficacy (17%) of the adjuvanted vaccine vs. laboratory confirmed influenza A in one of two seasons studied.
In the present study, intradermal vaccine (IDV) and TIV were alike in terms of seroprotection rates and GMTs to H1N1 and H3N2 antigens. To B/Brisbane, IDV recipients had a higher post-immunization GMT compared with TIV recipients but this observation was confounded by the high pre-immunization titers in both groups. A recent meta-analysisCitation37 of 4 trials that compared immunogenicity of IDV15 and TIV in seniors concluded that no substantial differences were evident in seroprotection rate ratios or GMT ratios with A or B virus antigens. Van Damme et al.Citation13 reported non-inferior immune responses in adults ≥ 65 y of age after IDV compared with ADV with all 3 vaccine antigens based on SRH assay data. Using the same arbitrary definition of non-inferiority (upper bound of the 95% confidence interval for the GMT ratio of ADV:IDV < 1.5), our findings are similar for responses to all 3 vaccine antigens as assessed by SRH. However, HAI responses to the A virus antigens did not meet the non-inferiority criteria for IDV relative to ADV. Non-inferiority means that selected response measures for two products fall within a pre-set range of acceptable difference, even though the actual response measures may be significantly different by another statistical test.
Six months after vaccination, seroprotective titers to the A viruses were still present in over half of the participants and to B/Brisbane in over 95%. Residual seroprotection rates to the A viruses did not differ significantly among the study groups but only ADV recipients had rates > 60% for each virus, meeting international immunogenicity criteria.Citation5 Few other studies of ADV have included extended follow-up serology but Ruf et al.Citation38 also reported no advantage in seroprotection rates for ADV over TIV, 4 and 8 mo after vaccination of German seniors. The tendency for protective titers to persist for 6 mo and longer after TIV in elderly adults was highlighted in a recent literature reviewCitation39 and is important for season-long protection against influenza infection.
Safety of the study vaccines was thoroughly assessed and took into consideration pre-existing symptoms common among seniors such as tiredness and joint aches, the rates of which were similar among the groups. Bias was minimized by ensuring that vaccine identity was masked from participants and subsequent interviewers. Participants did not witness the injection, the sensation of which turned out not to differ appreciably among the vaccines (see below). Compliance of subjects with requested symptom recording and reporting approached 100%. Reported rates of general solicited symptoms following vaccination did not differ significantly among the study groups and were typical of this TIV vaccine.Citation26 Published meta-analyses indicated the potential for 5% higher general symptom rates with ADVCitation11 and equivalent rates with IDV,Citation37 compared with TIV. Group sizes in this study were not large enough to detect differences of small magnitude (< 12%).
The nature and rate of injection site reactions differed substantially among vaccine groups. Among IDV recipients, local inflammation was prominent, as previously reported.Citation9,Citation10,Citation13 Most IDV vaccinees (76%) developed local erythema shortly after vaccination, which reached up to 80 mm peak size next day. Resolution was relatively slow, with half of the affected subjects (one-third of vaccinees) still reporting some redness at the day 7 interview. Swelling, pain and itching were common accompaniments to erythema, as previously reported.Citation9,Citation10,Citation13 Among ADV recipients, pain was the most frequent injection site complaint (in 38%), with lesser rates of redness or swelling, consistent with other reports (reviewedCitation11). Injection site reactions were least frequently reported by TIV recipients ().
In all groups, adverse events following immunization were generally mild and short-lived. Changes sufficient to limit daily activities or to require medical attention were infrequent (< 3%) in all groups. No serious adverse events were attributed to vaccination. Participants’ responses to the tolerability questionnaires indicated that both the vaccine injection discomfort and any subsequent adverse effects were readily tolerable, posing little or no bother. Pain accompanied vaccine injections at similar rates with all 3 products. This was a surprising observation with IDV as the manufacturer’s promotional materialCitation40 describes it as a “painless injection,” citing data from 3 trials in which 89% of participants ≥ 60 y of age said they had “no or hardly any pain” at the time of the injection. The discrepancy might reflect differences in categorizing minimal pain ratings given by participants in the previous and current studies. Given the same experience with future vaccination, participants indicated little reluctance to receive the same type of vaccine again or preference to receive TIV as in the recent past.
This study had several limitations. The observations may not apply to seniors excluded from participating, such as those who are frail, immunocompromised or living in care facilities. However, the study population was chosen to reflect the majority of the Canadian population age ≥ 65, over 70% of whom receive annual influenza vaccination.Citation17 The new vaccines might elicit stronger immune responses in seniors who are more vaccine-naïve than participants in this trial as repeated annual vaccination is known to limit response capacity as measured by routine serologic assays.Citation15,Citation16 We cannot be certain that the identity of assigned vaccines was not observed or surmised by some participants, introducing ascertainment bias, but injection-related discomfort was reported as often after intradermal as intramuscular injections. Individuals with less experience with TIV might be less accepting of minor adverse events than were study participants. The TIV vaccine used in this study is a subunit formulation that might be less immunogenic than split vaccines. If so, ADV may offer less advantage when compared with split vaccines. The somewhat greater antibody responses to ADV might not translate into greater protection as subunit vaccines potentially induce lesser cellular immune responses.Citation41 Neutralizing antibody assays would ideally have been performed with all 3 viruses and might have shed light on the elevated baseline B titers. Finally, we recognize that the seroprotection threshold (HAI titers ≥ 40) conventionally used to evaluate responses may not accurately predict protection in elderly adults, with greater antibody titers and cellular immune responses being required for protection.Citation2-Citation4,Citation29
Participants and Methods/Materials
Study design
This was a prospective, randomized, controlled, evaluator-blinded, parallel groups study of 3 licensed seasonal influenza vaccines for older adults. Eight Canadian centers enrolled and followed participants, between September 2011 and May 2012. The institutional research review board at each center provided ethics approval. The study was registered at clinicaltrials.gov (NCT 01368796) and conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and Good Clinical Practice Guidelines of the International Committee on Harmonization.
Study participants
Eligible participants were ambulatory seniors ≥ 65 y of age, in good health or with stable health conditions. Volunteers could be living in the community or in facilities providing minimal living assistance. Participants had to have received TIV in one or both of the previous two seasons. Excluded were individuals who met a definition of frailty,Citation42 specifically those needing help with activities of daily living or wholly dependent on others for such activities or terminally ill. Also excluded were those who had a contraindication to receiving influenza vaccine, were unable to provide informed consent or be attentive to required symptom documentation, were immunocompromised for any reason, had an unstable condition likely to require hospitalization during the study, had received blood or blood products within 3 mo, were unable to attend study clinics or would not be available for the scheduled follow-up visits. Each subject provided written informed consent at the time of enrollment. Volunteers were identified through various community-based activities, centers and organizations.
Information provided by participants upon enrollment included age, ethnicity, body weight and height, previous influenza immunizations, current medical conditions and prescribed medications, smoking history, probiotic use and weekly exercise pattern.
Study vaccines and immunizations
Single commercial lots of three TIVs for 2011–2012 were obtained: an intradermal formulation (Intanza 15, Sanofi Pasteur, lot #H8187–1) (IDV), a subunit vaccine (Agriflu, Novartis Vaccines, lot # 112104)(TIV) and a formulation of the subunit vaccine with MF59 (squalene) adjuvant (Fluad, Novartis Vaccines, lot #117703)(ADV). Each formulation delivered 15 µg of hemagglutinin of each component strain per dose. Each vaccine was approved for use in adults ≥ 65 y of age. The constituent strains as recommended by WHO for 2011–2012 were A/California/7/2009 (H1N1)-like, A/Perth/16/2009 (H3N2)-like and B/Brisbane/60/2008.Citation43 Each product was supplied in pre-filled syringes. Vaccines were stored and transported at 2–8°C, avoiding freezing.
All injections were given in the deltoid area. A 1” safety needle was used for IM injections. With IDV, the supplied device included a micro-needle for intradermal injection.
Study procedures
Following enrollment, participants were centrally (electronically) randomized to receive one of TIV, IDV or ADV vaccine, in 1:1:1 ratio. Assignments were arranged in balanced blocks of 6, determined by computer-generated random number lists, with stratification by sex and age (65–69, 70–74, 75+ years). The secure, web-based randomization service was available around the clock. After eligibility was verified and a blood sample was obtained, the assigned vaccine was administered by a non-blinded nurse who had no role in subsequent evaluations. The identity of the administered vaccine was carefully masked from the recipient, who looked away while the vaccine was administered. Investigators and all other study personnel remained blinded to vaccine assignments until the analysis of data to V2 was completed.
Following immunization, participants were observed for 15 min for any immediate adverse events. During this time participants completed Part 1 of an immunization tolerability questionnaire based on the Vaccinees’ Perception of Injection questionnaire (adapted from ref. Citation14) and were instructed regarding subsequent safety monitoring procedures including use of the electronic thermometer and measuring device provided to them. Subsequent visits were scheduled for 21 d (window 20–28 d) and 180 d (window 166–210 d) following immunization, for additional blood samples and safety observations. Exclusion criteria for the Day 180 visit included receipt within 3 mo of a blood product, immunocompromise and inability to attend the study clinic because of deteriorated health or admission to a care facility.
Safety observation procedures
Participants were first asked to record any symptoms recalled from the day prior to vaccination (referred to as baseline) and then any adverse health events they experienced during Days 0–6 after vaccination, using a daily study diary. On the 7th day after vaccination subjects were contacted by telephone and questioned about any local or general adverse events they had recorded. Solicited local adverse effects included pain, redness, swelling, induration/nodule and itching, while solicited general adverse events included, malaise, myalgia, arthralgia and tiredness. The severity of these events was graded by subjects according to a rating scale on the diary, with severe symptoms precluding normal daily activities and/or requiring medical attention. Other general symptoms solicited but not severity graded included sleep disturbance, headache, diarrhea, nausea, vomiting, shivering and sweating. Unsolicited symptoms could also be reported. Part 2 of the tolerability questionnaire was administered, posing questions covering the week-long experience after vaccination. This version repeated some specific questions from Part 1 about tolerability of any adverse experiences.
During Days 7–21 after vaccination, participants were asked to record only health events that required medical attention and/or precluded normal daily activities. Serious adverse events (SAEs) were documented and promptly reported to the study manager throughout the study, with a final check on ascertainment made at the Day 180 visit.
Serologic tests
Blood samples (8–10 mL) were collected on Days 0, 21 and 180 from all available subjects. Samples were processed promptly and sera divided into multiple code-labeled aliquots before storage at or below -20°C. Following completion of all Day 21 visits, paired samples were shipped frozen for concurrent testing at participating laboratories. Samples from Day 180 were shipped later to the same laboratories, where test standardization was confirmed by retesting duplicate serum aliquots from Day 21. All assays were done in duplicate, with the geometric mean sample value used in the analyses. Hemagglutination inhibition (HAI) assays for antibodies to individual vaccine viruses were conducted at separate laboratories (in Winnipeg - B assays, Montreal - H1N1 assays and Halifax - H3N2 assays) using cross-validated methods based on the WHO recommended procedure.Citation44 All samples were tested by HAI, using live A viruses and ether-treated B virus. Test strains were equivalent to those included in the vaccines. Microneutralization (MN) assays were performed for the target H3N2 virus in 100 randomly selected subjects per vaccine group, on samples from days 0 and 21. Microneutralization was determined using serial 2-fold dilutions of heat-inactivated sera according to a modified WHO protocol.Citation45 Briefly, MDCK monolayers were seeded in 96 well plates using MegaVir serum free media (Hyclone—Thermo Fisher Scientific) supplemented with gentamicin/amphotericin B and penicillin (Sigma) and L-glutamine (Gibco). Each serum was mixed with 100 TCID50 units of virus and incubated for 2 h at 37°C in 5% CO2 after which the virus/serum mixture was added to confluent monolayers of MDCK cells and supplemented with MegaVir containing TPCK trypsin. Following 3 h incubation at 37°C, the virus/serum suspension was removed and the cells were supplemented with MegaVir and TPCK trypsin, incubated at 37°C and monitored daily for 3–5 d for cytopathic effect (CPE). The neutralization titer was the inverse of the dilution that completely protected the monolayer from CPE. The lower limit of detection in both HAI and MN assays was a titer of 10; samples with undetectable activity were assigned a titer of 5 for calculation of geometric mean titers (GMTs). Single radial hemolysis (SRH) assays were conducted for all 3 viruses on samples obtained from all subjects at Days 0 and 21, using published methodology.Citation46 Whole, inactivated, vaccine-homologous viruses were used. Results were expressed as area of hemolysis in mm2. MN assays were performed in Halifax and SRH assays in Siena, Italy. All tests were performed on code-labeled samples, without identification of the subjects’ vaccine assignment.
Data analysis and statistical considerations
Data were assembled using a secure, customized, web-based platform (Daciforms, Montreal). Data entry screens contained numerous prompts and logic checks to minimize errors but each participant’s file was assessed for completeness and queried as necessary before the database was locked for analysis. Comparability of the 3 study groups was assessed in terms of each relevant demographic parameter (using chi-square or Fisher’s exact test) and baseline antibody titers (by chi-square and ANOVA). Participation rates were calculated for each scheduled contact with participants. The safety analysis included all vaccinated subjects and the immunogenicity analysis included all available samples of adequate volume, at each time point (modified intention-to-treat analysis). Adverse event rates were determined daily and for days 0–2 (peak rates) and 0–6 (cumulative rates) following immunization and compared among the study groups using the chi-square test. Rates of severe symptoms and those present at baseline were examined and compared among the groups. Unsolicited and serious adverse events were tabulated for each group; the relatedness of such events to the study vaccines was determined by the local investigator.
Serologic responses were analyzed according to standard international criteriaCitation5,Citation46 for adults over 60 y of age given seasonal influenza vaccines, when measured by HAI or SRH assay. Seroprotection was considered the primary serologic outcome measure, defined as an HAI titer ≥ 40 or SRH area of hemolysis ≥ 25 mm2.Citation24,Citation30 Seroprotection rate differences among products following immunization were calculated for each strain, with 95% confidence intervals. GMT ratios and 95% confidence intervals were similarly determined following immunization, using HAI data. Related outcomes were seroconversion, defined as ≥ 4-fold rise in titer or conversion from negative to a seroprotective titer between baseline and day 21 samples, and the mean fold rise in geometric mean titers (GMT) between these samples. Seroprotection and seroconversion rates were compared among groups by chi-square test, GMT titers were compared by ANOVA and GM fold rises by t-test with the Satterthwaite unequal variance assumption. Secondary serologic analyses included evaluation of a higher threshold for protection (HAI titer ≥ 160) among the groups and the proportionate decrease in seroprotection rates and GMT between days 21 and 180. Exploratory analyses included the effects of selected host variables such as age, sex and BMI on HAI seroprotection rates at day 21 post-vaccination, for each vaccine group. All statistical calculations were performed using SAS version 9.2 (SAS Institute). No correction was made for multiple comparisons. Differences with p values ≤ 0.05 for 2-sided tests were considered statistically significant.
The intention of this study was to be able to recognize any programmatically important advantages between new and old products, such as a ≥ 15% superiority margin in seroprotection rates. Based on reported seroprotection rate differences in comparisons of ADV and a different TIV,Citation8,Citation18 we determined that groups of 300 would allow detection of rate differences ≥ 15% with at least 90% probability using one-sided tests, across a range of potential rate pairs with 3 viral strains. Group size of 300 also provided over 90% power to detect GMT ratios in the range of 1.25 to 1.5, as in previous comparisons of ADV and different TIVs.Citation8,Citation18 The selected group size also applied satisfactorily to comparisons of IDV and TIV, as published response rate differences were similar to those above.Citation9,Citation10 Group sizes of 300 provided sufficient power to recognize differences in rates of adverse events of 12% or more, with 80% probability.
Conclusions
Compared with subunit TIV, adjuvanted TIV elicited 10–12% higher seroprotection rates to influenza A antigens in older adults regularly given TIV. This advantage did not persist 6 mo after vaccination. Intradermal TIV provided no significant advantage over intramuscular TIV using standard serologic criteria. All 3 vaccines were well tolerated by this TIV-experienced population.
| Abbreviations: | ||
| ADV | = | adjuvanted trivalent influenza vaccine |
| ANOVA | = | analysis of variance |
| BMI | = | body mass index |
| CI | = | confidence interval |
| CPE | = | cytopathic effect |
| GMT | = | geometric mean titer |
| HAI | = | hemagglutination inhibition assay |
| IDV | = | intradermal trivalent influenza vaccine |
| IM | = | intramuscular |
| MN | = | microneutralization |
| SAE | = | serious adverse event |
| SRH | = | single radial hemolysis |
| TCID50 | = | tissue culture infectious dose 50 |
| TIV | = | trivalent inactivated influenza vaccine |
| V1 | = | initial study visit for enrollment, baseline blood sample, immunization |
| V2 | = | second study visit, 21 days after immunization |
| V3 | = | final study visit, 6 months after immunization |
| WHO | = | World Health Organization |
Acknowledgments
All named authors participated in the implementation of the study, including contributions toward the study design and gathering and interpretation of the data. All authors were involved in drafting the article and approved the final draft.
Contributing PHAC/CIHR Influenza Research Network Investigators included (by center): Joanne Langley MD, Bruce Smith PhD (Dalhousie University, Halifax); Caroline Quach MD, Jesse Papenburg MD (McGill University, Montreal); André Beauchesne MD, Vladimir Gilca PhD, Gaston De Serres MD (Institut national de santé publique du Quebec, Laval University, Quebec City); Allison McGeer MD (Mt Sinai Hospital, University of Toronto); Greg Hammond MD, Fred Aoki MD (University of Manitoba, Winnipeg); Grant Stiver MD (University of British Columbia, Vancouver), Shu Yu Fan MSc (Vaccine Evaluation Center, Vancouver); Nathalie Bastien PhD (National Microbiology Laboratory, Winnipeg); Giulia Lapini PhD, Sara Sbragi MPharm (University of Siena, Siena, Italy).
We gratefully acknowledge the excellent support provided by the project manager (Carol LaJeunesse), data manager (Kim Marty) and the participating field teams, data center staff, laboratory personnel, administrative center staff and the many volunteers who generously shared their time and experiences.
Presented in brief at the 10th Canadian Immunization Conference, Vancouver, British Columbia, December 3–5, 2012.
Clinical Trial Registration: NCT01368796
Conflicts of Interest
Previous study funding (within 3 y) from Sanofi and/or Novartis: DWS, JAB, SAH, BJW, SM. Advisory board participation (within 3 y) with Sanofi and/or Novartis: DWS, JAB, SAH, BJW, JM, SM. Financial interest in Sanofi and/or Novartis: None. No conflicts to declare: TH, YL, EM, AS, CC, MD, ML, BC, ER.
Financial Disclosure Statement
This study was funded by a grant from the Public Health Agency of Canada and the Canadian Institutes of Health Research to the PCIRN Network. Vaccine supplies and restricted research grants for supplemental laboratory tests were provided by Sanofi Pasteur Vaccines and Novartis Vaccines. These companies had no role in study design, conduct or reporting.
References
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179 - 86; http://dx.doi.org/10.1001/jama.289.2.179; PMID: 12517228
- McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012; 30:2060 - 7; http://dx.doi.org/10.1016/j.vaccine.2012.01.015; PMID: 22289511
- Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. In Vaccines for Pandemic Influenza, RW Compans and WA Orenstein (eds), Current Topics in Microbiology and Immunology 2009; 333:413-429.
- Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine 2007; 25:3066 - 9; http://dx.doi.org/10.1016/j.vaccine.2007.01.025; PMID: 17275144
- Wood JM, Newman RW, Ploss K. The use of correlates of immunity in European Union licensing of influenza vaccines. Dev Biol (Basel) 2003; 115:9 - 16; PMID: 15088770
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172 - 80; http://dx.doi.org/10.1086/599790; PMID: 19508159
- O’Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines 2007; 6:699 - 710; http://dx.doi.org/10.1586/14760584.6.5.699; PMID: 17931151
- Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology 2003; 49:177 - 84; http://dx.doi.org/10.1159/000069172; PMID: 12679609
- Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis 2008; 198:650 - 8; http://dx.doi.org/10.1086/590434; PMID: 18652550
- Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine 2009; 27:7304 - 12; http://dx.doi.org/10.1016/j.vaccine.2009.10.033; PMID: 19849996
- Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 2009; 27:6959 - 65; http://dx.doi.org/10.1016/j.vaccine.2009.08.101; PMID: 19751689
- Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine 2007; 25:8833 - 42; http://dx.doi.org/10.1016/j.vaccine.2007.10.020; PMID: 18023942
- Van Damme P, Arnou R, Kafeja F, Fiquet A, Richard P, Thomas S, et al. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC Infect Dis 2010; 10:134; http://www.biomedcentral.com/1471-2334/10/134 http://dx.doi.org/10.1186/1471-2334-10-134; PMID: 20504306
- Reygrobellet C, Viala-Danten M, Meunier J, Weber F, Nguyen VH. Perception and acceptance of intradermal influenza vaccination: Patient reported outcomes from phase 3 clinical trials. Hum Vaccin 2010; 6:336 - 45; http://dx.doi.org/10.4161/hv.6.4.10753; PMID: 20372083
- de Bruijn IA, Remarque EJ, Jol-van der Zijde CM, van Tol MJD, Westendorp RGJ, Knook DL. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J Infect Dis 1999; 179:31 - 6; http://dx.doi.org/10.1086/314540; PMID: 9841819
- Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One 2008; 3:e2975; www.plosone.org http://dx.doi.org/10.1371/journal.pone.0002975; PMID: 18714352
- Quach S, Hamid JS, Pereira JA, Heidebrecht CL, Deeks SL, Crowcroft NS, et al, Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network Vaccine Coverage Theme Group. Influenza vaccination coverage across ethnic groups in Canada. CMAJ 2012; 184:1673 - 81; http://dx.doi.org/10.1503/cmaj.111628; PMID: 22966054
- Banzoff A, Pellegrini M, Del Giudice G, Fragapane E, Groth N, Podda A. MF59-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influenza Other Respir Viruses 2008; 2:243 - 9; http://dx.doi.org/10.1111/j.1750-2659.2008.00059.x
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159 - 69; http://dx.doi.org/10.1016/j.vaccine.2005.08.105; PMID: 16213065
- Yao X, Hamilton RG, Weng NP, Xue QL, Bream JH, Li H, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine 2011; 29:5015 - 21; http://dx.doi.org/10.1016/j.vaccine.2011.04.077; PMID: 21565245
- Talbot HK, Coleman LA, Crimin K, Zhu Y, Rock MT, Meece J, et al. Association between obesity and vulnerability and serologic response to influenza vaccination in older adults. Vaccine 2012; 30:3937 - 43; http://dx.doi.org/10.1016/j.vaccine.2012.03.071; PMID: 22484350
- Kohut ML, Arntson BA, Lee W, Rozeboom K, Yoon KJ, Cunnick JE, et al. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine 2004; 22:2298 - 306; http://dx.doi.org/10.1016/j.vaccine.2003.11.023; PMID: 15149789
- Boge T, Rémigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 2009; 27:5677 - 84; http://dx.doi.org/10.1016/j.vaccine.2009.06.094; PMID: 19615959
- Beyer WEP, Palache AM, Lüchters G, Nauta J, Osterhaus ADME. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination?. Virus Res 2004; 103:125 - 32; http://dx.doi.org/10.1016/j.virusres.2004.02.024; PMID: 15163500
- Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin Infect Dis 2010; 50:1331 - 8; http://dx.doi.org/10.1086/652144; PMID: 20377407
- Tregnaghi MW, Stamboulian D, Vanadía PC, Tregnaghi JP, Calvari M, Fragapane E, et al. Immunogenicity, safety, and tolerability of two trivalent subunit inactivated influenza vaccines: a phase III, observer-blind, randomized, controlled multicenter study. Viral Immunol 2012; 25:216 - 25; PMID: 22691101
- Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767 - 77; http://dx.doi.org/10.1017/S0022172400022610; PMID: 4509641
- Gorse GJ, O’Connor TZ, Newman FK, Mandava MD, Mendelman PM, Wittes J, et al. Immunity to influenza in older adults with chronic obstructive pulmonary disease. J Infect Dis 2004; 190:11 - 9; http://dx.doi.org/10.1086/421121; PMID: 15195238
- Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 2010; 10:18; http://www.biomedcentral.com/1471-2288/10/18 http://dx.doi.org/10.1186/1471-2288-10-18; PMID: 20210985
- Wood JM, Montomoli E, Newman RW, Daas A, Buchheit KH, Terao E. Collaborative study on influenza vaccine clinical trial serology - part 2: reproducibility study. Pharmeur Bio Sci Notes 2011; 2011:36 - 54; PMID: 21619855
- Wood JM, Gaines-Das RE, Taylor J, Chakraverty P. Comparison of influenza serological techniques by international collaborative study. Vaccine 1994; 12:167 - 74; http://dx.doi.org/10.1016/0264-410X(94)90056-6; PMID: 8147099
- Stephenson I, Das RG, Wood JM, Katz JM. Comparison of neutralising antibody assays for detection of antibody to influenza A/H3N2 viruses: an international collaborative study. Vaccine 2007; 25:4056 - 63; http://dx.doi.org/10.1016/j.vaccine.2007.02.039; PMID: 17412461
- Fisman DN, Tuite AR. Estimation of the health impact and cost-effectiveness of influenza vaccination with enhanced effectiveness in Canada. PLoS One 2011; 6:e27420; http://dx.doi.org/10.1371/journal.pone.0027420; PMID: 22110645
- Mannino S, Villa M, Apolone G, Weiss NS, Groth N, Aquino I, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol 2012; 176:527 - 33; http://dx.doi.org/10.1093/aje/kws313; PMID: 22940713
- Skowronski DM, De Serres G, Janjua NZ, Hottes TS. Re: “effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy”. Am J Epidemiol 2013; 177:593 - 4; http://dx.doi.org/10.1093/aje/kwt021; PMID: 23436898
- McElhaney JE, Beran J, Devaster JM, Esen M, Launay O, Leroux-Roels G, et al, Influence65 study group. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis 2013; 13:485 - 96; http://dx.doi.org/10.1016/S1473-3099(13)70046-X; PMID: 23518156
- Marra F, Young F, Richardson R, Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respir Viruses 2012; 1200
- Ruf BR, Colberg K, Frick M, Preusche A. Open, randomized study to compare the immunogenicity and reactogenicity of an influenza split vaccine with an MF59-adjuvanted subunit vaccine and a virosome-based subunit vaccine in elderly. Infection 2004; 32:191 - 8; http://dx.doi.org/10.1007/s15010-004-3204-z; PMID: 15293073
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant?. J Infect Dis 2008; 197:490 - 502; http://dx.doi.org/10.1086/524146; PMID: 18275271
- Sanofi Pasteur “Medical and Scientific Fact Sheet” for IntanzaR, Document CIRC.10/06/COM/375.
- Co MDT, Orphin L, Cruz J, Pazoles P, Green KM, Potts J, et al. In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses. Vaccine 2009; 27:319 - 27; http://dx.doi.org/10.1016/j.vaccine.2008.09.092; PMID: 18977404
- Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489 - 95; http://dx.doi.org/10.1503/cmaj.050051; PMID: 16129869
- World Health Organization. Recommended composition of influenza vaccines for use in the 2011-2012 northern hemisphere influenza season. Wkly Epidemiol Rec 2011; 86:81 - 92
- World Health Organization. Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza. 2011. http://wholibdoc.who.int/publications/2011/9789241548090_eng.pdf. Accessed 23 August 2012.
- World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance. Geneva, 2002.
- Groth N, Montomoli E, Gentile C, Manini I, Bugarini R, Podda A. Safety, tolerability and immunogenicity of a mammalian cell-culture-derived influenza vaccine: a sequential Phase I and Phase II clinical trial. Vaccine 2009; 27:786 - 91; http://dx.doi.org/10.1016/j.vaccine.2008.11.003; PMID: 19027046