Abstract
The integrated US Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) has made great strides in strategic preparedness and response capabilities. There have been numerous advances in planning, biothreat countermeasure development, licensure, manufacturing, stockpiling and deployment. Increased biodefense surveillance capability has dramatically improved, while new tools and increased awareness have fostered rapid identification of new potential public health pathogens. Unfortunately, structural delays in vaccine design, development, manufacture, clinical testing and licensure processes remain significant obstacles to an effective national biodefense rapid response capability. This is particularly true for the very real threat of “novel pathogens” such as the avian-origin influenzas H7N9 and H5N1, and new coronaviruses such as hCoV-EMC. Conventional approaches to vaccine development, production, clinical testing and licensure are incompatible with the prompt deployment needed for an effective public health response. An alternative approach, proposed here, is to apply computational vaccine design tools and rapid production technologies that now make it possible to engineer vaccines for novel emerging pathogen and WMD biowarfare agent countermeasures in record time. These new tools have the potential to significantly reduce the time needed to design string-of-epitope vaccines for previously unknown pathogens. The design process—from genome to gene sequence, ready to insert in a DNA plasmid—can now be accomplished in less than 24 h. While these vaccines are by no means “standard,” the need for innovation in the vaccine design and production process is great. Should such vaccines be developed, their 60-d start-to-finish timeline would represent a 2-fold faster response than the current standard.
The Problem: Delayed Response to Emerging Infections and Biowarfare Attacks
According to the Commission on the Prevention of Weapons of Mass Destruction (WMD) Proliferation and Terrorism, medical counter-measures such as vaccines are critically important for protecting first-responders and non-combatant (civilian) populations from the consequences of a bioterror attack. In 2008, Bob Graham (D-FL) and Jim Talent (R-MO), chairs of the WMD commission and authors of World at Risk, reported that the United States was “seriously lacking” in this vital capability.Citation1 The 2009 H1N1 influenza pandemic highlighted continued weaknesses in the national preparedness system; as a consequence, Graham and Talent gave US bio-defense preparedness an “F” in their follow-up report, published in 2010.Citation2 The Governmental Accounting Office (GAO) also reported poor inter-agency coordination on biodefense.Citation3,Citation4 As a result of renewed emphasis on biodefense, the United States government has expended substantial resources on protecting the nation against a potential bioterror attack, creating specialized units for planning and preparedness within the Departments of Health and Human Services, Defense, Homeland Security, Agriculture, Commerce and State.
Vaccine production infrastructure has also improved due to significant investments by the Federal government. For example, there are now several federally subsidized “Advanced Development and Manufacturing” production facilities distributed in different regions of the country that are capable of producing millions of doses of protein-based vaccines.Citation5 Unfortunately, despite these important advances in the strategic preparedness of US agencies for biodefense, vaccine design remains a significant obstacle to national biodefense. This is particularly true for the very real threat of as-yet-undetermined pathogens for which little is known about their critical antigenic determinants and correlates of immunity, the key parameters used in vaccine design for conventional pathogens.
A Proposed Solution: Design and Delivery of “Vaccines on Demand”
Recent reportsCitation6 of a novel H7N9 avian influenza virus emerging in China have led to even greater scrutiny of methods used to respond to infectious disease public health threats and have, in turn, provided for a “live fire” assessment of novel approaches. In 2009–2010, the FastVax group began to discuss whether existing tools and vaccine production platforms could be used to accelerate the development of vaccines for emerging infectious diseases, as illustrated in . Traditional vaccine development for previously unknown pathogens takes place on the time scale of years. The accelerated process, as proposed by our group, would begin with analysis of the genomic sequence of an emerging pathogen with immunoinformatics tools, followed by rapid design of an epitope-based vaccine containing the most immunogenic components, using an integrated in silico approach illustrated in . Once the vaccine is designed, production and testing would involve a four-step process undertaken by the FastVax consortium arrangement, as described below.
Figure 1. Two vaccine development timelines. Top: Traditional Vaccine Development. Bottom: Proposed “FastVax” timeline for development of “Vaccines on Demand.”
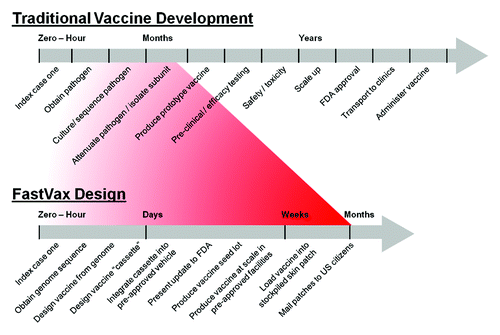
Figure 2. We applied the genome-to-vaccine approach to developing an epitope-based vaccine for avian H7N9 influenza. The design project started on April 5, 2013 and was completed 20 h later. For vaccine production, the genome-derived vaccine sequences would be sent by secure email to a plasmid DNA production facility to manufacture a DNA vaccine (Step 2); following scale-up and production, the vaccine would be distributed in a microneedle patch or another easy-to-distribute formulation (Step 3/4). *ICS = immunogenic consensus sequences.Citation7
Several constraints affecting the proposed approach bear mentioning; each of these is addressed in turn.
T cell epitope-based vaccines provide the minimal, essential information required for protective immunity T cell epitopes are critical mediators of cellular immunity. They are derived from a pathogen’s proteins via two pathways: (1) intracellular proteins are processed, and their constituent peptides are loaded onto major histocompatability complex (MHC) class I molecules; and (2) exogenous proteins are processed in the proteolytic compartment, and their constituent peptides are loaded onto MHC class II molecules. MHC class I and class II-peptide complexes are then transported to the surface of an APC, where they are exposed to interrogation by passing T cells (CD8+ and CD4+ T cells, respectively). From these different antigen processing and presentation pathways, two distinct T cell responses are generated: (1) a CD8+ cytotoxic T lymphocyte immune response that is critical for pathogen clearance, and (2) a CD4+ T helper immune response that is essential for robust and sustained antibody and cytotoxic T lymphocyte responses. After initial exposure to pathogen, memory T cells are established that respond more rapidly and efficiently upon subsequent exposure.
Because epitopes provide the essential information needed to trigger a protective immune response, epitope-based vaccines can be developed to recreate this response. Given the lengthy process that is usually associated with the development of killed, live-attenuated and whole-subunit vaccine approaches, an epitope-based strategy is one rational alternative, particularly when no vaccine exists and an emerging pathogen threatens human health on a global scale.
T cell epitopes do not protect against infection; however, they may protect against disease
There is published evidence demonstrating that epitope-based vaccines can be protective. Vaccination with peptide epitopes stimulates protective immune responses in a range of animal models, including complete protection of BALB/c mice against RSV challenge,Citation8 partial protection of BALB/c mice against Plasmodium yoelii sporozoite challenge,Citation9 partial protection of BALB/c and CBA mice against encephalitis following intracerebral challenge with a lethal dose of measles virus,Citation10 complete protection of BALB/c mice from intraperitoneal HSV challenge,Citation11 high degree of protection of BALB/c mice against infection with malaria or influenza A virus,Citation12 full protection of sheep against BLV,Citation13 and full protection of horses against West Nile Virus.Citation14 Furthermore, experts are generally in agreement that cross-reactive T cell epitopes were responsible for the limited morbidity and mortality associated with pandemic H1N1 in 2009.Citation15-Citation17 The absence of T cell epitopes may be contributing to the rapid spread and significant mortality rate of H7N9 in China.Citation18 T cell epitope-related immune responses appear to be critically important for reducing morbidity and mortality in human infectious disease.Citation19
No “Fast Track” to vaccine-on-demand approval is currently possible under existing FDA regulations
Epitope-driven vaccines offer distinct advantages that should contribute to a reconsideration of the current vaccine approval process for emergency use. Multiple epitopes derived from more than one antigen can be packaged together in a single cassette. In this way, a broad-based immune response directed against multiple antigenic proteins associated with the pathogen can be elicited without the need to manufacture and administer large quantities of protein, much of which will be immunologically irrelevant or potentially even reactogenic. This is likely to reduce formulation challenges, decrease cost and accelerate the development process. The use of epitopes also helps to mitigate potential safety concerns stemming from the use of intact recombinant proteins that may have undesired biological activity (e.g., enzymes, immunomodulators, cross-reactivity, toxins, etc.). For example, the NP protein of Lassa has been associated with immune-suppressive activity.Citation20 Genome sequencing, immunoinformatics tools and the epitope-driven approach now make it possible to develop vaccines on demand in response to emerging pathogens.
A Four-Step Process to Design and Deliver “Vaccines On Demand”
Step one: Genome-derived, epitope-driven vaccine strategy (GD-EDV)
The first step to making “faster vaccines” is to design vaccine immunogens directly from pathogen genomes.Citation21 For example, for emerging influenza strains, the vaccine “payload” is constructed in silico using the pathogen genome sequence provided by the World Health Organization (WHO) or posted on GISAID (http://platform.gisaid.org/). T cell epitope-mapping algorithms that are integrated in a “vaccine design toolkit” developed by Martin and De Groot are applied to the genome sequences.Citation22 These tools derive and concatenate those epitopes that have a high likelihood of driving an effective T cell response into a “string-of-beads” format for insertion into a vaccine delivery vehicle. The process can be performed in less than 24 h; the exact length of time required for the analysis depends on whether comparisons have to be performed to other existing genomes and epitopes. Tools for carrying out the task have been applied to the development of vaccine candidates for SARS,Citation23 2009 H1N1 pandemic influenza,Citation24 smallpox,Citation25 and a number of other emergent and biowarfare agents, such as West Nile Virus, H. pylori and Burkholderia.Citation7,Citation26-Citation28 Most recently, the tools were applied in May 2013 to the design of a vaccine for H7N9, an emerging avian-origin influenza ().Citation29 The integration of epitope mapping into a step-by-step vaccine design process makes it possible to design vaccines in the shortest time possible once the DNA sequence from the emerging infectious disease or biowarfare pathogen is available. Should errors later be found in the sequence, they may impact one or two epitopes. For an epitope-based string of beads vaccine, the overall impact would be minimal, since T cell epitopes are linear; in contrast, sequence variations may compromise the structural integrity of a whole protein vaccine with negative effects on immunogenicity.
How many epitopes?
Available evidence from animal studies suggests that the number of vaccine components (epitopes) required for full protection against disease is a small and definable subset that can be discovered using state-of-the-art computer programs such as the ones described and validated by EpiVax.Citation30,Citation31 We have proposed that any FastVax vaccine would include a minimum of 100 broadly reactive T cell epitopes in several strings, designed to induce multi-functional immune responses that are essential for protective immunity.Citation32 Careful selection of the vaccine components, comprising epitopes covering most common HLA, can provide greater than 99% coverage of diverse human populations.Citation33
Need for adjuvants?
Currently, MF59 and AS03, both oil-in-water emulsions, and virosome, a liposome formulation, are three adjuvants licensed for use in seasonal, pre-pandemic and pandemic influenza vaccines. No influenza vaccines containing adjuvant are FDA approved. T cell epitope vaccine responses may be enhanced through genetic immunization.Citation34 DNA vaccines are self-adjuvanting through co-encoded sequences, and thus many such vaccines do not incorporate traditional adjuvants in their final formulation. A number of strategies that are currently being evaluated may improve DNA vaccine potency for humans, including use of more efficient promoters and codon optimization, addition of traditional or genetic adjuvants, electroporation and intradermal delivery.Citation35
Step two: Manufacturing and production
Reliable, reproducible methods for producing vaccines are currently available. The FastVax consortium favors DNA vaccines because production is scalable, the vaccines are stable at room temperature, manufacturing can be easily distributed to different geographic locations, and the production method is more rapid than many other vaccine manufacturing technologies. Alternative scalable and rapid production methods for accelerated vaccine production include plant-derived vaccines, phage-based vaccines and recombinant vaccines produced in cell culture. Proteins produced using each of these systems have been approved by the FDA for use in humans.
Rapid production of DNA vaccines
The initial vaccine sequence designed in silico can be electronically provided to a production facility, where a cassette representing the vaccine genetic construct(s) is then synthesized and inserted into a standardized DNA vaccine plasmid. A cGMP seed lot of bacteria containing the vaccine plasmid with cassetted payload can be rapidly produced and vialed using existing SOPs for release and characterization assays. An initial manufacturing lot of plasmid vaccine would be produced from the seed lot and used to initiate safety studies. To reduce time to produce sufficient vaccine product, multiple scale-up facilities could be located in different regions of the US. Using current methods of DNA vaccine development, seed lot production would take one to three weeks. Scale-up for DNA production is much more rapid than traditional vaccine designs; only three to four weeks would be required to produce one million doses per facility. See below for discussion of Biological Agents Research Defense Agency (BARDA) appropriations for the construction of distributed vaccine production facilities.
The DNA vaccine delivery platform and rational in silico design provide for a strong safety profile. The DNA vaccine manufacturing process, particularly the efficient and stringent release criteria, allow for a highly pure and well-characterized final product. Rational design permits in silico analysis of the vaccine sequence for identification of potential unfavorable immune responses including regulatory sequences or cross-reactive immune responses. A fundamental principle of rapid biodefense vaccine production is that safety and speed are paramount for eliciting a protective immune response prior to the epidemic.
Delivery vehicle
The bulk vaccine product would then be coated onto pre-manufactured micro-needle patches that provide direct delivery to the dermis, or would be delivered using another skin-based method such as “scarification.” A number of self-applied patch delivery systems have already been developed. These would be optimal in bioterror and pandemic scenarios, because patches can be pre-manufactured and stored in bulk and do not require refrigeration for delivery or trained practitioners for administration.Citation36 Vaccination centers would not be required, which would minimize transmission of the biothreat organism between patients and health care providers. Alternatively, previously approved electroporation delivery methodsCitation37 could be used, though this would take more time and increase the need for vaccine administration personnel training, leading to an escalation of the vaccine administration expense and more protracted timelines.
Step three: Clinical trials
While there are no Phase III or FDA-approved DNA vaccines, there are more than 30 Phase II trials listed in clinicaltrials.gov. FDA approval of a DNA vaccine appears to be on the horizon, but until then, the FastVax DNA vaccine may encounter an additional FDA-associated barrier. Implementation of a previously untested vaccine is only possible after rapidly completing initial clinical testing to the point that “emergency use authorization” can be invoked by the Secretary of Health and Human Services (HHS). In some biodefense scenarios, approximate correlates of protection may have been previously identified; such is the case with Lassa Fever, Ebola, the encephaloviruses, and a number of other “Category A, B and C” biodefense pathogens. In some cases, correlates of protection are unknown, and either an antibody-focused or a T cell-driven vaccine may prove effective. Where antibody-mediated immunity is critically important, T cell-driven vaccines still merit attention as potential adjuncts to more traditional whole-antigen (B cell-driven) approaches, since T cell help drives higher titer, higher affinity antibody responses. Especially in settings where challenge studies cannot be performed in advance of use in humans, licensure may be possible by means of the “Two Animal Rule” in lieu of a human correlate. Rapid clinical testing can be achieved using existing commercial clinical research organizations and clinical site networks such as the Medical Countermeasures Clinical Studies Network currently envisioned by ASPR/BARDA. Emergency use authorization approval can be based on achievement of “correlates” such as induction of broadly protective T cell or antibody responses, provided an allowed Investigational New Drug (IND) Application is in hand.
One problem facing T cell-driven vaccines that are designed to stimulate HLA-restricted human immune responses is that testing for correlates of immunity as described in the “Two Animal Rule” may not demonstrate the true efficacy of the product. Thus alternative approaches may need to be considered.
The MIMIC assay, a comprehensive measurement of localized reactogenicity, could be utilized for initial safety studies and to qualify release of the actual vaccine intended for emergency use.Citation38 Additionally, in pandemic response simulations, “mock up” or example vaccines (in a specific DNA plasmid backbone) and patch delivery system could be submitted for approval by the FDA, and this formulation would be evaluated in the clinic for immunogenicity that recapitulates the influenza correlates of protective immunity already defined by CBER and EMA. Correlates of protective immunity for currently approved influenza vaccines will not serve as a basis for regulatory approval of a DNA vaccine. The FDA would require correlates to be determined for a new influenza vaccine and will not rely on related, but different, vaccines already approved. Advance trials will establish correlates of protection for a FastVax influenza vaccine to serve as a basis for regulatory review in an emergency. In a pandemic, a novel FastVax sequence composition might be rapidly tested in a small, swiftly completed safety and immunogenicity trial, much like EMA precedence for annual influenza vaccine updates.
Step four: Approval and emergency use authorization
One means of obtaining initial FDA review, experience and oversight for the FastVax vaccine-on-demand system would be to firmly establish the immunogenicity of an existing, clinical-trial-ready DNA influenza virus vaccine in a patch or scarification delivery system. Demonstration that the vaccine candidate meets influenza correlates of protection criteria with an acceptable profile in human trials would inform regulatory review for products of similar composition, much as current regulatory policy supports annual marketing re-authorization despite changes in influenza subunit vaccine composition (from trivalent to quadrivalent) to reflect seasonal shifts and drifts.
Timely approval by the FDA to allow distribution of product in response to a rapidly emerging threat would require close cooperation between the vaccine manufacturer and the Agency. The manufacturer can assist by providing clinical safety and efficacy data for a variety of vaccine products based on standardized vaccine platform, manufacturing, specifications, operating procedures and method of delivery. If the manufacturer can establish predictable immunogenicity of epitopes in a demonstrated safe and reproducible vaccine platform and rapidly perform Phase I and Phase II trials establishing safety and immunogenicity in terms of a surrogate endpoint that predicts clinical benefit, the Agency may be able to provide a rapid review and emergency use allowance/authorization; release of the vaccine would then be possible through emergency use authorization by the HHS Secretary.
Scale up
To reduce the time to vaccine production, manufacturing sites could be pre-inspected and maintained at a state of operational readiness. While this would involve redundancy and higher costs, it would allow for the rapid production and scale-up of vaccines at any given moment. Each site would need to utilize the same manufacturing process to ensure consistency across vaccine batches, and entities would need to be willing to share their specific methodologies to harmonize an approach. One site would create the master cell bank (MCB), and then generate the manufacturer's working cell bank (MWCB) for distribution to all other sites. In order to reduce production time by two weeks, this step would be performed “at risk,” meaning MWCBs would be distributed prior to the completion of testing on either the MCB or MWCB. Sequencing on the MCB could likely be completed before the MWCB goes into fermenter starters. Assuming that a dose would constitute 0.2 mg of DNA vaccine and that each site has several 240 L fermenters (either as back-ups or for parallel growth), one million doses (200 g) per site could be produced in a three- to four-week period. BARDA recently invested hundreds of millions of dollars in distributed influenza vaccine production; adapting these facilities for DNA vaccine production would be an added but not insurmountable expense (as compared with the initial investment).Citation39
An in vitro assay like the MIMIC system could serve as a release characteristic of the multi-site lots that would run in parallel with the patch loading, preventing a single problematic DNA vaccine batch from impeding the release of patches generated with other batches. If the backbone-host system is proven to be rugged with virtually any type of insert, a pilot run would no longer be necessary. Conversely, if the system is not shown to be rugged, then pilot runs would be important, as some inserts can greatly influence stability and growth characteristics. Such pilot runs would need to be undertaken at every facility, most likely with different methods tested, to maximize the likelihood of determining the best method for production.
Summary
A number of technological advances are moving T cell-driven vaccines to the foreground with lessons applicable to influenza T cell-driven vaccine development. Perhaps the most prominent example of this new focus is the expanding use of T cell-driven immunotherapy as an adjunct to cancer therapy. Many of the barriers to effective T cell-driven vaccine development are being addressed and surmounted in clinical cancer trials. For example, dendritic-cell pulsing vaccines using tumor antigens have moved into clinical use.Citation40,Citation41 Outcomes of these types of vaccination protocols have improved as MHC class II epitopes (CD4+ T cell help) were includedCitation42 and antibodies against cytotoxic T lymphocyte antigen-4 (anti-CTLA-4; see ref. Citation43) and other anti-T regulatory cell (Treg) agents have been added to the conditioning regimen.
Quite a few T cell-driven vaccines are currently in human clinical trials (reviewed by Gilbert in 2012; see ref. Citation44). While it is true that infectious disease T cell-driven vaccines have lagged behind T cell-driven vaccines for cancer, the regulatory pathway for T cell vaccines is improving, since more than 250 cancer vaccines that are based on T cell-driven immune responses are in clinical trials.a Furthermore, recent challenge studies have shown that humoral immunity is not required for protection against all human pathogens. This was demonstrated in the case of influenza, following vaccination of study participants with a multi-antigen vaccine. Following exposure to live influenza virus, two of 11 vaccinees and five of 11 control subjects developed laboratory-confirmed influenza (symptoms plus virus shedding). Symptoms of influenza were less pronounced in the vaccinees and there was a significant reduction in the number of days of virus shedding in those vaccinees who developed influenza (mean of 1.09 d in controls, 0.45 d in vaccinees, p = 0.036)Citation45,Citation46 for a final efficacy of 60%, which is better than many vaccines currently available.
This is a major milestone for T cell vaccines for infectious disease, as it is one of the first vaccines to reach a Phase 2 clinical trial and none have reached Phase 3. While one cannot directly extrapolate from this trial nor the many cancer T cell-driven immunotherapy trials to state that the approach will work for all types of vaccines against infectious disease, successful implementation of the T cell-driven approach in a range of contexts suggests that it is worth pursuing.
Immunome-mining (computational immunology) tools have played a major role in the design and development of T cell-driven vaccines for infectious diseases. The process was first termed “vaccinomics” by Brusic and Petrovsky in 2002,Citation47 then “reverse vaccinology” by Rappuoli in 2003,Citation48 and more recently, “immunome-derived or genome-derived vaccine design” by Pederson,Citation49 De Groot and Martin,Citation50 and Doytchinova, Taylor, and Flower.Citation51 The concept behind these descriptors is that a minimal set of antigens that induces a competent immune response to a pathogen or neoplasm can be discovered using immunoinformatics, and that administration of these epitopes in the right delivery vehicle and with the correct adjuvant will result in a degree of protection against infection by the pathogen. In short, the T cell-driven approach to developing vaccines is based on these fundamental principles: Payload + Adjuvant + Delivery vehicle = Vaccine.
T cell-driven vaccines also offer some significant advantages over conventional vaccines for infectious diseases. For example, despite strain-to-strain variation at the protein level, immunoinformatics tools can be used to identify highly conserved T cell epitopes that are immunogenic and broadly representative or universal, covering a wide range of variant strains; our group has published results for TB, HIV, smallpox, HCV and H. pylori,Citation16,Citation52-Citation58 and additional evidence can be found in literature published by other gene-to-vaccine researchers (e.g., Sette and Newman, Brusic, Petrovsky, Reche, and He). Concatenation of multiple epitopes, either from a single organism or from multiple pathogens in a single delivery vehicle, has been shown to elicit broad-based immune response directed at the epitopes and is associated with improved efficacy when compared with the whole organism (lysate) in animal challenge studies.Citation59,Citation60 Furthermore, epitope-based vaccines limit the antigenic load, diminishing the need to manufacture and administer large quantities of immunogen, much of which is immunologically irrelevant. In an important advance for T cell-driven vaccines, new tools (e.g., JanusMatrix; see ref. Citation61) may enable vaccine developers to select potent T effector epitopes, and to differentiate these from Treg-activating epitopes and/or self-cross-reactive epitopes that may lead to immunopathogenic responses (Losikoff P, et al. Forthcoming).Citation62-Citation64
Over the past five years, the authors of this report have advanced a number of T cell-driven vaccines described to the point of formulation and delivery studies. Vaccines for many of the high-priority biodefense pathogens and emerging or re-emerging infectious diseases under development are not currently available, and evidence that T cell-mediated immune response is critically important for protection against these pathogens is emerging.Citation43,Citation65-Citation69
Members of the FastVax consortium are well aware that there are many obstacles to overcome before the proposed “rapid response” or FastVax platform for biodefense vaccines can be implemented. Nonetheless, there is a critical national need for an accelerated vaccine design, development and production process that can be accomplished in weeks, not months, in the event of a serious infectious disease outbreak or biowarfare attack. The development of a rapid response to emerging infectious disease threats, using best-in-class technologies to provide a first line of defense, will contribute to greater biodefense preparedness and a significant improvement in the ability of the US to protect its citizens against pandemic infectious diseases. The need for new vaccines for protecting against bioterror pathogens and emerging infectious disease is great, and we would argue that, for the reasons cited above, the time to advance these vaccines to the clinic is now.
Disclosure of potential conflicts of interest
ADG and WDM are senior officers and majority shareholders at EpiVax, Inc., a privately owned immunoinformatics and vaccine design company located in Providence, RI, USA. LM is an employee and holds stock options in EpiVax. LE and RWM have been paid consultants of EpiVax on vaccine development programs. JB and MC are employees and stockholders at Aldevron, Inc. The authors acknowledge that there is a potential conflict of interest related to their employment and attest that the work contained in this research report is free of any bias that might be associated with the commercial goals of the companies.
Acknowledgments
Funding to support the discussions leading to the development of the FastVax consortium can be attributed to the NIH U19 grant AI082642 (to ADG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Endnote
aThere are 276 clinical trials for “T cell vaccines” currently reported at http://www.cancer.gov/clinicaltrials/search.
References
- Graham B, Talent J. World at Risk. 2008 Dec 3. Retrieved from http://www.preventwmd.gov/static/docs/report/worldatrisk_full.pdf
- Graham B, Talent J. Prevention of WMD Proliferation and Terrorism Report Card. 2010. Retrieved from http://www.preventwmd.gov/static/docs/report-card.pdf
- Government Accounting Agency. Report GAO-11-318SP: Homeland security/Law enforcement: Strategic oversight mechanisms could help integrate fragmented interagency efforts to defend against biological threats. 2011 Mar 1. Retrieved from http://www.gao.gov/modules/ereport/handler.php?m=1&p=1&path=/ereport/GAO-11-318SP/data_center/Homeland_security–Law_enforcement/Strategic_oversight_mechanisms_could_help_integrate_fragmented_interagency_efforts_to_defend_against_biological_threats
- Franco C, Sell TK. Federal agency biodefense funding, FY2012-FY2013. Biosecur Bioterror 2012; 10:162 - 81; http://dx.doi.org/10.1089/bsp.2012.0025; PMID: 22691277
- Department of Health and Human Services. HHS creates new centers to develop, manufacture medical countermeasures. 2012. Retrieved from http://www.hhs.gov/news/press/2012pres/06/20120618a.html
- GISAID. EpiFlu Database. 2008-2013. Retrieved from http://platform.gisaid.org/epi3/frontend#36c986
- De Groot AS, Ardito M, Moise L, Gustafson EA, Spero D, Tejada G, et al. Immunogenic consensus sequence T helper epitopes for a pan-Burkholderia biodefense vaccine. Immunome Res 2011; 7:7; PMID: 22130150
- Simmons CP, Hussell T, Sparer T, Walzl G, Openshaw P, Dougan G. Mucosal delivery of a respiratory syncytial virus CTL peptide with enterotoxin-based adjuvants elicits protective, immunopathogenic, and immunoregulatory antiviral CD8+ T cell responses. J Immunol 2001; 166:1106 - 13; PMID: 11145691
- Franke ED, Sette A, Sacci J Jr., Southwood S, Corradin G, Hoffman SL. A subdominant CD8(+) cytotoxic T lymphocyte (CTL) epitope from the Plasmodium yoelii circumsporozoite protein induces CTLs that eliminate infected hepatocytes from culture. Infect Immun 2000; 68:3403 - 11; http://dx.doi.org/10.1128/IAI.68.6.3403-3411.2000; PMID: 10816491
- Schadeck EB, Partidos CD, Fooks AR, Obeid OE, Wilkinson GW, Stephenson JR, et al. CTL epitopes identified with a defective recombinant adenovirus expressing measles virus nucleoprotein and evaluation of their protective capacity in mice. Virus Res 1999; 65:75 - 86; http://dx.doi.org/10.1016/S0168-1702(99)00103-3; PMID: 10564754
- Rosenthal KS, Mao H, Horne WI, Wright C, Zimmerman D. Immunization with a LEAPS heteroconjugate containing a CTL epitope and a peptide from beta-2-microglobulin elicits a protective and DTH response to herpes simplex virus type 1. Vaccine 1999; 17:535 - 42; http://dx.doi.org/10.1016/S0264-410X(98)00231-X; PMID: 10075159
- Tsuji M, Bergmann CC, Takita-Sonoda Y, Murata K, Rodrigues EG, Nussenzweig RS, et al. Recombinant Sindbis viruses expressing a cytotoxic T-lymphocyte epitope of a malaria parasite or of influenza virus elicit protection against the corresponding pathogen in mice. J Virol 1998; 72:6907 - 10; PMID: 9658144
- Hislop AD, Good MF, Mateo L, Gardner J, Gatei MH, Daniel RC, et al. Vaccine-induced cytotoxic T lymphocytes protect against retroviral challenge. Nat Med 1998; 4:1193 - 6; http://dx.doi.org/10.1038/2690; PMID: 9771756
- Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol 2001; 75:4040 - 7; http://dx.doi.org/10.1128/JVI.75.9.4040-4047.2001; PMID: 11287553
- De Groot AS, Ardito M, McClaine EM, Moise L, Martin WD. Immunoinformatic comparison of T-cell epitopes contained in novel swine-origin influenza A (H1N1) virus with epitopes in 2008-2009 conventional influenza vaccine. Vaccine 2009; 27:5740 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.07.040; PMID: 19660593
- Greenbaum JA, Kotturi MF, Kim Y, Oseroff C, Vaughan K, Salimi N, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A 2009; 106:20365 - 70; http://dx.doi.org/10.1073/pnas.0911580106; PMID: 19918065
- Schanen BC, De Groot AS, Moise L, Ardito M, McClaine E, Martin W, et al. Coupling sensitive in vitro and in silico techniques to assess cross-reactive CD4(+) T cells against the swine-origin H1N1 influenza virus. Vaccine 2011; 29:3299 - 309; http://dx.doi.org/10.1016/j.vaccine.2011.02.019; PMID: 21349362
- De Groot AS, Ardito M, Terry F, Levitz L, Ross TM, Moise L, et al. Low immunogenicity predicted for emerging avian-origin H7N9: Implication for influenza vaccine design. Hum Vaccin Immunother 2013; 9:950 - 6; http://dx.doi.org/10.4161/hv.24939; PMID: 23807079
- Gilbert SC. T-cell-inducing vaccines - what’s the future. Immunology 2012; 135:19 - 26; http://dx.doi.org/10.1111/j.1365-2567.2011.03517.x; PMID: 22044118
- Hastie KM, Kimberlin CR, Zandonatti MA, MacRae IJ, Saphire EO. Structure of the Lassa virus nucleoprotein reveals a dsRNA-specific 3′ to 5′ exonuclease activity essential for immune suppression. Proc Natl Acad Sci U S A 2011; 108:2396 - 401; http://dx.doi.org/10.1073/pnas.1016404108; PMID: 21262835
- Cohen T, Moise L, Martin W, DeGroot AS. Immunoinformatics: The next step in vaccine design. In: Sintchenko V, ed. Infectious Disease Informatics. New York: Springer, 2010:223-244.
- De Groot AS, Martin W. From immunome to vaccine: epitope mapping and vaccine design tools. Novartis Found Symp 2003; 254:57 - 72, discussion 72-6, 98-101, 250-2; http://dx.doi.org/10.1002/0470090766.ch5; PMID: 14712932
- De Groot AS. How the SARS vaccine effort can learn from HIV-speeding towards the future, learning from the past. Vaccine 2003; 21:4095 - 104; http://dx.doi.org/10.1016/S0264-410X(03)00489-4; PMID: 14505885
- De Groot AS, Ardito M, McClaine EM, Moise L, Martin WD. Immunoinformatic comparison of T-cell epitopes contained in novel swine-origin influenza A (H1N1) virus with epitopes in 2008-2009 conventional influenza vaccine. Vaccine 2009; 27:5740 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.07.040; PMID: 19660593
- Moise L, Buller RM, Schriewer J, Lee J, Frey SE, Weiner DB, et al. VennVax, a DNA-prime, peptide-boost multi-T-cell epitope poxvirus vaccine, induces protective immunity against vaccinia infection by T cell response alone. Vaccine 2011; 29:501 - 11; http://dx.doi.org/10.1016/j.vaccine.2010.10.064; PMID: 21055490
- De Groot AS, Saint-Aubin CS, Bosma A, Sbai H, Rayner J, Martin W. Rapid determination of HLA B*07 ligands from the West Nile virus NY99 genome. Emerg Infect Dis 2001; 7:706 - 13; PMID: 11585536
- De Groot AS, Martin W, Moise L, Guirakhoo F, Monath T. Analysis of ChimeriVax Japanese Encephalitis Virus envelope for T-cell epitopes and comparison to circulating strain sequences. Vaccine 2007; 25:8077 - 84; http://dx.doi.org/10.1016/j.vaccine.2007.09.026; PMID: 17942198
- Moss SF, Moise L, Lee DS, Kim W, Zhang S, Lee J, et al. HelicoVax: epitope-based therapeutic Helicobacter pylori vaccination in a mouse model. Vaccine 2011; 29:2085 - 91; http://dx.doi.org/10.1016/j.vaccine.2010.12.130; PMID: 21236233
- De Groot AS, Ardito MA, Terry F, Levitz L, Ross TM, Moise L, et al. Low immunogenicity predicted for emerging avian-origin H7N9: Implication for influenza vaccine design. Hum Vaccin Immunother 2013; 9; http://dx.doi.org/10.4161/hv.24939; PMID: 23807079
- Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, Bui HH, et al. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol 2006; 24:817 - 9; http://dx.doi.org/10.1038/nbt1215; PMID: 16767078
- Moise L, De Groot AS. Putting immunoinformatics to the test. Nat Biotechnol 2006; 24:791 - 2; http://dx.doi.org/10.1038/nbt0706-791; PMID: 16841062
- Wille-Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J Immunol 2005; 174:7676 - 83; PMID: 15944268
- Sette A, Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 1999; 50:201 - 12; http://dx.doi.org/10.1007/s002510050594; PMID: 10602880
- Schirmbeck R, Fissolo N, Chaplin P, Reimann J. Enhanced priming of multispecific, murine CD8+ T cell responses by DNA vaccines expressing stress protein-binding polytope peptides. J Immunol 2003; 171:1240 - 6; PMID: 12874211
- Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines 2012; 11:189 - 209; http://dx.doi.org/10.1586/erv.11.188; PMID: 22309668
- Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol 2009; 333:369 - 93; http://dx.doi.org/10.1007/978-3-540-92165-3_18; PMID: 19768415
- Ramirez LA, Arango T, Boyer J. Therapeutic and prophylactic DNA vaccines for HIV-1. Expert Opin Biol Ther 2013; 13:563 - 73; http://dx.doi.org/10.1517/14712598.2013.758709; PMID: 23477730
- Dhir V, Fort M, Mahmood A, Higbee R, Warren W, Narayanan P, et al. A predictive biomimetic model of cytokine release induced by TGN1412 and other therapeutic monoclonal antibodies. J Immunotoxicol 2012; 9:34 - 42; http://dx.doi.org/10.3109/1547691X.2011.613419; PMID: 22074378
- Perdue ML, Bright RA. United States of America Department of Health and Human Services support for advancing influenza vaccine manufacturing in the developing world. [Review] Vaccine 2011; 29:Suppl 1 A48 - 50; http://dx.doi.org/10.1016/j.vaccine.2011.02.080; PMID: 21684430
- Sonpavde G, Kantoff PW. Immunotherapy for castration-resistant prostate cancer. [Review] Urol Clin North Am 2012; 39:465 - 81; http://dx.doi.org/10.1016/j.ucl.2012.07.004; PMID: 23084524
- Gardner TA, Elzey BD, Hahn NM. Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccin Immunother 2012; 8:534 - 9; http://dx.doi.org/10.4161/hv.19795; PMID: 22832254
- Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res 2013; 73:19 - 29; http://dx.doi.org/10.1158/0008-5472.CAN-12-1127; PMID: 23087058
- Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun 2013; 13:5; PMID: 23390376
- Gilbert SC. T-cell-inducing vaccines - what’s the future. Immunology 2012; 135:19 - 26; http://dx.doi.org/10.1111/j.1365-2567.2011.03517.x; PMID: 22044118
- Powell TJ, Peng Y, Berthoud TK, Blais ME, Lillie PJ, Hill AV, et al. Examination of influenza specific T cell responses after influenza virus challenge in individuals vaccinated with MVA-NP+M1 vaccine. PLoS One 2013; 8:e62778; http://dx.doi.org/10.1371/journal.pone.0062778; PMID: 23658773
- Lillie PJ, Berthoud TK, Powell TJ, Lambe T, Mullarkey C, Spencer AJ, et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis 2012; 55:19 - 25; http://dx.doi.org/10.1093/cid/cis327; PMID: 22441650
- Elliott SL, Suhrbier A, Miles JJ, Lawrence G, Pye SJ, Le TT, et al. Phase I trial of a CD8+ T-cell peptide epitope-based vaccine for infectious mononucleosis. J Virol 2008; 82:1448 - 57; http://dx.doi.org/10.1128/JVI.01409-07; PMID: 18032491
- Rappuoli R, Covacci A. Reverse vaccinology and genomics. Science 2003; 302:602; http://dx.doi.org/10.1126/science.1092329; PMID: 14576423
- Pederson T. The immunome. Mol Immunol 1999; 36:1127 - 8; http://dx.doi.org/10.1016/S0161-5890(99)00125-X; PMID: 10698314
- De Groot AS, Martin W. From immunome to vaccine: epitope mapping and vaccine design tools. Novartis Found Symp 2003; 254:57 - 72, discussion 72-6, 98-101, 250-2; http://dx.doi.org/10.1002/0470090766.ch5; PMID: 14712932
- Doytchinova IA, Taylor P, Flower DR. Proteomics in Vaccinology and Immunobiology: An Informatics Perspective of the Immunone. J Biomed Biotechnol 2003; 2003:267 - 90; http://dx.doi.org/10.1155/S1110724303209232; PMID: 14688414
- De Groot AS, McMurry J, Marcon L, Franco J, Rivera D, Kutzler M, et al. Developing an epitope-driven tuberculosis (TB) vaccine. Vaccine 2005; 23:2121 - 31; http://dx.doi.org/10.1016/j.vaccine.2005.01.059; PMID: 15755582
- De Groot AS, Marcon L, Bishop EA, Rivera D, Kutzler M, Weiner DB, et al. HIV vaccine development by computer assisted design: the GAIA vaccine. Vaccine 2005; 23:2136 - 48; http://dx.doi.org/10.1016/j.vaccine.2005.01.097; PMID: 15755584
- Otero M, Calarota SA, Dai A, De Groot AS, Boyer JD, Weiner DB. Efficacy of novel plasmid DNA encoding vaccinia antigens in improving current smallpox vaccination strategy. Vaccine 2005; 24:4461 - 70; http://dx.doi.org/10.1016/j.vaccine.2005.08.010
- De Groot AS, Ardito M, Moise L, Gustafson EA, Spero D, Tejada G, et al. Immunogenic consensus sequence T helper epitopes for a pan-Burkholderia biodefense vaccine. Immunome Res 2011; 7:7; http://immunome-research.net/ PMID: 22130150
- Schanen BC, De Groot AS, Moise L, Ardito M, McClaine E, Martin W, et al. Coupling sensitive in vitro and in silico techniques to assess cross-reactive CD4(+) T cells against the swine-origin H1N1 influenza virus. Vaccine 2011; 29:3299 - 309; http://dx.doi.org/10.1016/j.vaccine.2011.02.019; PMID: 21349362
- Levitz L, Koita OA, Sangare K, Ardito MT, Boyle CM, Rozehnal J, et al. Conservation of HIV-1 T cell epitopes across time and clades: validation of immunogenic HLA-A2 epitopes selected for the GAIA HIV vaccine. Vaccine 2012; 30:7547 - 60; http://dx.doi.org/10.1016/j.vaccine.2012.10.042; PMID: 23102976
- De Groot AS, Levitz L, Ardito MT, Skowron G, Mayer KH, Buus S, et al. Further progress on defining highly conserved immunogenic epitopes for a global HIV vaccine: HLA-A3-restricted GAIA vaccine epitopes. Hum Vaccin Immunother 2012; 8:987 - 1000; http://tinyurl.com/GAIA-HLA-A3 http://dx.doi.org/10.4161/hv.20528; PMID: 22777092
- Moise L, Buller RM, Schriewer J, Lee J, Frey SE, Weiner DB, et al. VennVax, a DNA-prime, peptide-boost multi-T-cell epitope poxvirus vaccine, induces protective immunity against vaccinia infection by T cell response alone. Vaccine 2011; 29:501 - 11; http://dx.doi.org/10.1016/j.vaccine.2010.10.064; PMID: 21055490
- Moss SF, Moise L, Lee DS, Kim W, Zhang S, Lee J, et al. HelicoVax: Epitope-based therapeutic H. pylori vaccination in a mouse model of gastric cancer. Vaccine 2011; 29:2085 - 91; http://dx.doi.org/10.1016/j.vaccine.2010.12.130; PMID: 21236233
- Moise L, Gutierrez AH, Bailey-Kellogg C, Terry F, Leng Q, Abdel Hady KM, et al. The two-faced T cell epitope: Examining the host-microbe interface with JanusMatrix. Hum Vaccin Immunother 2013; 9; http://dx.doi.org/10.4161/hv.24615; PMID: 23584251
- Moise L, Gutierrez AH, Bailey-Kellogg C, Terry FA, Leng Q, Abdel Hady KM, et al. The two-faced T cell epitope: Examining the host-microbe interface with JanusMatrix. Hum Vaccin Immunother 2013; 9; In press http://dx.doi.org/10.4161/hv.24615; PMID: 23584251
- Elfaki ME, Khalil EA, De Groot AS, Musa AM, Gutierrez A, Younis BM, et al. Immunogenicity and immune modulatory effects of in silico predicted L. donovani candidate peptide vaccines. Hum Vaccin Immunother 2012; 8:1769 - 74; http://dx.doi.org/10.4161/hv.21881; PMID: 22922767
- Poland GA. Vaccines against Lyme disease: What happened and what lessons can we learn?. Clin Infect Dis 2011; 52:Suppl 3 s253 - 8; http://dx.doi.org/10.1093/cid/ciq116; PMID: 21217172
- Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med 2005; 11:Suppl S25 - 32; http://dx.doi.org/10.1038/nm1212; PMID: 15812486
- Chen L, Li B, Yang WC, He JL, Li NY, Hu J, et al. A dominant CD4(+) T-cell response to Helicobacter pylori reduces risk for gastric disease in humans. Gastroenterology 2013; 144:591 - 600; http://dx.doi.org/10.1053/j.gastro.2012.12.002; PMID: 23232294
- Kaabinejadian S, Piazza PA, McMurtrey CP, Vernon SR, Cate SJ, Bardet W, et al. Identification of Class I HLA T Cell Control Epitopes for West Nile Virus. PLoS One 2013; 8:e66298; http://dx.doi.org/10.1371/journal.pone.0066298; PMID: 23762485
- Silva EB, Dow SW. Development of Burkholderia mallei and pseudomallei vaccines. Front Cell Infect Microbiol 2013; 3:10; http://dx.doi.org/10.3389/fcimb.2013.00010; PMID: 23508691
- Eneslätt K, Normark M, Björk R, Rietz C, Zingmark C, Wolfraim LA, et al. Signatures of T cells as correlates of immunity to Francisella tularensis. PLoS One 2012; 7:e32367; http://dx.doi.org/10.1371/journal.pone.0032367; PMID: 22412866