Abstract
Malaria results in over 650 000 deaths each year; thus, there is an urgent need for an effective vaccine. Pre-clinical studies and recently reported human trials suggest that pre-erythrocytic stage vaccines can provide protection against infection. A Phase 1, randomized, placebo-controlled, dose-escalation study was conducted with a vaccine composed of a replication-deficient adenovirus-35 backbone with P. falciparum circumsporozoite (CS) surface antigen (Ad35.CS.01). Healthy adult subjects received three doses of 108, 109, 1010, or 1011 vp/mL Ad35.CS.01 vaccine or saline placebo intramuscularly at 0, 1, and 6-mo intervals. Adverse events were assessed and anti-CS antibody responses were determined by ELISA. Seventy-two individuals were enrolled, with age, gender, and ethnicity similar across each study arm. While the vaccine was generally well tolerated, adverse events were more frequent in the highest dose groups (1010 and 1011 vp/mL). More robust humoral responses were also noted at the highest doses, with 73% developing a positive ELISA response after the three dose series of 1011 vp/mL. The Ad35.CS.01 vaccine was most immunogenic at the highest dosages (1010 and 1011 vp/mL). Reactogenicity findings were more common after the 1011 vp/mL dose, although most were mild or moderate in nature and resolved without therapy.
Keywords: :
Introduction
Malaria is one of the most prevalent global infectious diseases with more than 200 million infections and over 650 000 deaths annually.Citation1–Citation3 As a result of drug-resistant parasites and insecticide-resistant mosquitoes, malaria continues to pose a public health threat.Citation1 This threat is worsened by environmental and climatic changes, civil disturbances, and increased mobility of the human population.
Malaria is caused by mosquito-borne hematoprotozoan parasites belonging to the genus Plasmodium.Citation4 Five species of Plasmodium protozoa (P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi) are responsible for human disease, with P. falciparum accounting for the majority.Citation4 The parasite life cycle has multiple stages, with each stage inducing specific immune responses against specific expressed antigens that may modify disease risk. Initially, infected mosquitoes inject sporozoite stage parasites into the mammalian bloodstream, where the parasites are exposed to host antibodies (in those who are malaria-experienced) primarily directed to the circumsporozoite (CS) protein, a major surface component of the sporozoite. Field studies have correlated antibodies to CS protein and other pre-erythrocytic antigens with protection from P. falciparum infection.Citation5–Citation7 Additionally, the recently reported modest efficacy of a CS-based malaria vaccine candidate (RTS, S) suggests that CS vaccines afford some protection in malaria hyper- and holoendemic areas.Citation8–Citation15 However, a more highly protective malaria vaccine is needed.
Immunity to malaria is decidedly complicated, with a combination of humoral and cellular immunity working together to reduce mortality and morbidity and to decrease the overall parasite burden in the human host.Citation1 The vaccine candidate Ad35.CS.01 has a codon optimized nucleotide sequence representing the P. falciparum circumsporozoite (CS) surface antigen inserted into a replication deficient adenovirus-35 (Ad35) vector.Citation16–Citation18 As a pre-erythrocytic vaccine candidate, Ad35.CS.01 would be expected to work by increasing neutralizing antibodies that could inhibit Plasmodia sporozoites from entering the hepatocyte. In addition, a successful vaccine candidate might be able to destroy infected hepatocytes, harnessing CD4+, CD8+, natural killer T, and γδT-cells to inhibit intrahepatic parasites. Preclinical data are certainly encouraging, given the high CSP-specific cellular and humoral responses observed in mice vaccinated with a combination of Ad35 and Ad5 vectored CSP vaccines and a significant reduction in hepatic infection upon malaria challenge.Citation16,Citation18–Citation20
Adenoviral vectors are attractive for vaccines because the genome is well characterized, easy to manipulate, and capable of being rendered replication-defective. Adenovirus-vectored constructs are also exciting vaccine candidates due to their ability to induce potent T-cell and B-cell memory responsesCitation21 and to boost the response to other CS antigen vaccines. Several adenovirus-vectored CS constructs exist, each with potential advantages.Citation22,Citation23 Recombinant Ad5 expressing P. yoelii derived CS protein (Ad5PyCS) provided potent CD8+ T-cell responses and protection against subsequent challenge in mice models.Citation24 Similarly, Ad26 and Ad35 vectored vaccines are known to stimulate both humoral and cellular responses,Citation25 though the protection afforded by these constructs alone is uncertain. Using a prime-boost strategy, Chuang et al. tested a DNA prime/adenovirus boost in a Phase I malaria challenge study. In this strategy, Ad5-CS and Ad5-AMA1 (apical membrane antigen-1) were given after DNA plasmid prime. This regimen provided modest protection against controlled challenge, but not in those with high pre-existing antibodies to Ad5.Citation26–Citation28 A different prime-boost strategy of Ad35.CS followed by RTS, S in primates resulted in cell-mediated immune responses that were both higher and more durable than those seen following either vaccine alone.Citation17 While CD8+, IFNγ secreting T-cells in pre-clinical animal models have been implicated in protection against malaria challenge,Citation29 primate studies show a predominance of CD4+ T cells after prime-boost vaccination with Ad35.CS followed by RTS, S. The reported success of 30–60% of RTS, S in field efficacy studiesCitation11,Citation30 leaves room for strategies to further increase efficacy.
The primary objective of this trial was to assess the safety of the Ad35.CS.01 malaria vaccine among healthy subjects. A secondary objective was to assess the immunogenicity of the vaccine, with assessment of cellular immune responses an exploratory endpoint.
Results
Study completion
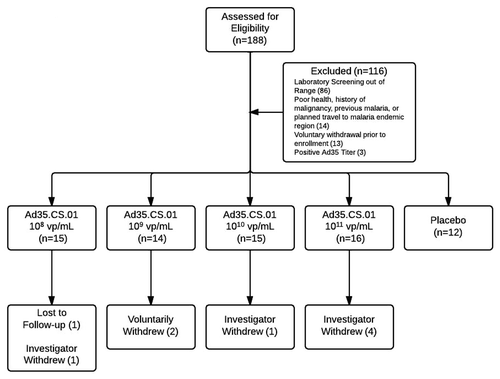
Seventy-two subjects were recruited into the study with an equal number of each gender and a mean age of 28 y (). All placebo recipients completed the three dose series. In the 108 viral particles (vp)/mL group, two subjects discontinued the trial, both after the 2nd dose: one was lost to follow up, and one was terminated due to a dispensing error. In the 109 vp/mL group, two subjects withdrew their consent for personal reasons unrelated to the trial and completed only the first dose. In the 1010 vp/mL group, 1 subject was withdrawn by the investigators due to noncompliance (participation in another clinical trial). Finally, in the 1011 vp/mL group, four subjects were discontinued due to adverse events that are discussed below. All adverse events in these subjects resolved without treatment or sequelae.
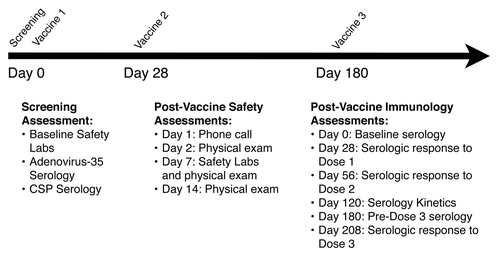
Figure 1. Schedule of events and outcome measures. Baseline screening assessments included complete blood counts; serum chemistries; hepatic enzymes; urinalysis; serologies for HIV, HCV, and HBV; and pregnancy testing. During post-vaccine safety visits, each subject’s memory aid was reviewed for adverse events. The primary outcome measure was the frequency and severity of local and systemic adverse events. Secondary outcome measures included anti-CS antibody titers (ELISA) and neutralizing antibody titers to adenovirus type 35.
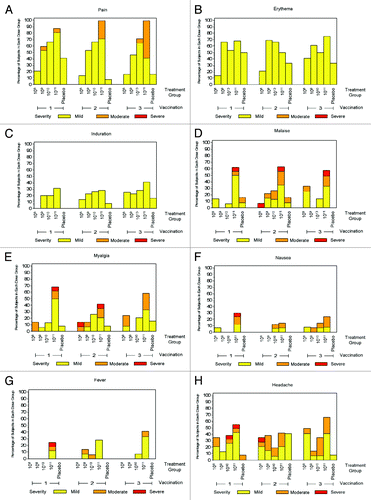
Solicited local reactions
Local injection site pain was generally mild in nature but increased in frequency with increasing dosages. As illustrated in , while the reactions seen after the dosage of 108 vp/mL were not distinguishable from placebo, the frequency of subjects reporting pain increased with increasing dosages. The 1011 vp/mL dosage was associated with more reports moderate pain, particularly after dose 3. Pain was reported only on the day of injection, being either resolved or decreased to mild pain the following day. No subject reported severe pain. Mild erythema was common in both placebo and study product recipients.
Figure 2. CONSORT Flow Diagram of Study Participants. In total, 62/72 subjects completed all vaccinations and follow-up visits. Reasons for voluntary withdrawal include loss to follow-up, concern about risks and study procedures, and insufficient time to complete study visits. Reasons for investigator withdrawal include non-compliance due to participation in another trial (1), dosing error (1), and adverse reactions to vaccine (4). Adverse events leading to withdrawal occurred exclusively in the Ad35.CS.01 1011 vp/mL dosage group.
Solicited systemic reactions
Fever was uncommon in placebo recipients and in the lower dose cohorts (), but more than 20% of subjects in the 1011 vp/mL dose cohort experienced fever (> 100.0 °F). The average duration of reported fever was ≤ 1 d. The numbers of subjects experiencing headache, malaise, myalgia, and nausea are presented in –, respectively. All systemic symptoms other than headache were more common among the highest relative to lower dose groups (p < 0.05 for each).
summarizes the number and proportion of subjects experiencing any solicited adverse event (AE) by maximal severity. Systemic reactogenicity following the first administration of the three lower doses was similar to that observed after placebo, with only a slightly higher occurrence observed after receipt of 1010 vp/mL. However, subjects in the 1011 vp/mL dose group were more likely to experience reactions when compared with placebo (82% of subjects vs. 25%; p = 0.01). 13% of subjects in the highest dose group experienced events that were rated as severe based on interruption of activities of daily living. Tolerability of the 1010 vp/mL dose was only slightly less favorable than placebo with 41% of subjects experiencing solicited systemic reactions including one severe headache. Following the second and third vaccinations, the overall frequency of systemic reactions was similar across the three lowest dosages relative to placebo; however 1011 vp/mL induced more frequent reactions of any grade (p = 0.06) and of moderate or severe grade (p = 0.01). In total, more than 90% of subjects receiving the 1011 dose experienced at least 1 solicited systemic AE.
Table 1. Number (%) of subjects with solicited systemic reactions, by maximal severity, after each injection
Overview of serious adverse events and laboratory adverse events
One serious adverse event (SAE), a small bowel obstruction requiring hospitalization and surgery that was deemed unrelated to study product, was reported 74 d after the 3rd injection. After final study unblinding, it was learned that the subject had received placebo. In addition, transient mild increases in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) within 14 d of vaccination were seen in 12 subjects; however, while 8/60 vaccine recipients had elevated ALT, 4/12 of the placebo recipients also had elevated ALT (p > 0.10, Table S1).
Unsolicited adverse events
In total, 241 unsolicited AEs were reported through Day 208; 66 (58 mild; 7 moderate; 1 severe) were considered associated with the study product. The frequencies of subjects experiencing associated AEs in the placebo, 108, 109, 1010, and 1011 vp/mL groups were 17%, 14%, 47%, 33%, and 31%, respectively. All events were transient and resolved spontaneously without sequelae. These events are characterized further by dosage cohort below.
108 vp/mL cohort
Seven unexpected, unsolicited adverse events were considered related to study product. One subject experienced paresthesias in the vaccinated arm after the 2nd and 3rd doses of the vaccine. The events were not associated with motor weakness or sensory loss, and symptoms resolved within 12 h of vaccine administration. The subject had a history of motor weakness and paresthesias in the right arm following childbirth 13 y earlier. Magnetic resonance imaging (MRI) of the spine obtained 18 mo prior to the study (due to musculoskeletal injury following an automobile accident) showed no anatomic abnormalities or nerve root compressions. One subject had a decreased neutrophil count, and another was reported to have anemia. Each of these resolved without sequelae.
109 vp/mL cohort
Twenty events were considered related to study product. Each of these were mild, transient, laboratory abnormalities, including mild elevations of AST/ALT in 4 subjects, hyperglycemia and hypoglycemia (in 3 subjects each), elevated creatinine (1); mild neutropenia (1); and proteinuria (3). All AE’s were followed to resolution and none experienced sequelae.
1010 vp/mL cohort
Ten mild and one moderate event were identified as potentially related to the vaccine. One subject reported a pruritic tongue lesion of moderate severity 4 d after vaccination. No other symptoms were noted and the tongue lesion resolved spontaneously after one week. Other AE’s included mild eosinophilia (1 subject); pharyngitis and cervical adenopathy in a subject whose viral cultures and adenoviral PCR were negative; an erythematous arm lesion adjacent to the injection site (1); headache (1); herpes simplex infection (1); and proteinuria (2 subjects). One subject developed subtle post-immunization weakness of the immunized arm, noticed during weight lifting. Each of these resolved without intervention.
1011 vp/mL cohort
Subjects in the highest dose group experienced 15 mild, 3 moderate, and 1 severe adverse events judged associated with the vaccine. Four subjects were withdrawn by the investigators due to significant reactogenicity or unsolicited adverse events. The first subject developed diaphoresis, tachycardia, and diarrhea that eventually resulted in syncope on the day following vaccination. This subject, who also developed iron-deficiency anemia, was later found to suffer from anorexia nervosa, unknown to study team at time of enrollment. The second subject experienced two transient episodes of weakness in the vaccinated arm, associated with transient loss of balance. Another subject experienced grade 3 fever and chills (and was withdrawn from subsequent vaccinations) and a final participant experienced a brachial plexopathy of moderate severity following dose 2. This latter subject described pain and paresthesias in the anterior aspect of the forearm and hand 2 d after vaccination; however, these symptoms resolved spontaneously in the week following vaccination. Each of these events was considered related to study product and resolved without intervention.
Immunogenicity (humoral response)
Twenty-eight days following the first vaccination, no appreciable anti-CS antibody levels were identified among the 108 or 109 vp/mL dose groups (), but subjects in the 1010 and 1011 groups had significantly higher geometric mean antibody titers (GMTs) than placebo (p < 0.05). Detectable antibody levels were identified in 6/15 (40%) and 10/14 (71%) of subjects in these respective dose groups after the first vaccine dose. Twenty-eight days after the second vaccination, 7/15 (47%; 95% CI 21–72%) in the 1010 and 10/14 (71%; 95% CI 48–95%) in the 1011 group had a ≥ 4 fold increase in antibody from pre-vaccination levels. GMTs remained significantly higher among these dose groups relative to placebo (p < 0.001). The third vaccination yielded minimal increases in antibody titers relative to the previous dose: 28 d after the third vaccination (Day 208) 4/14 (29%; 95% CI 5–52%) in the 1010 and 7/11 (64%; 95% CI 35–92%) in the 1011 group had a ≥ 4 fold rise in anti-CS antibody levels at Day 208 vs. baseline. One month after the third vaccination, the 1011 group demonstrated the highest GMT (61 ELISA units (EU)/mL; 95% CI: 21–176). The frequencies of subjects who had ≥ 4-fold increases in GMT at Day 208 were 7/14 (50%) and 8/11 (73%) in in the 1010 and 1011 vp/mL groups, respectively.
Table 2. Geometric mean titers of CS antibodies at baseline, 1 mo, 2 mo, 6 mo, and 7 mo following first vaccination
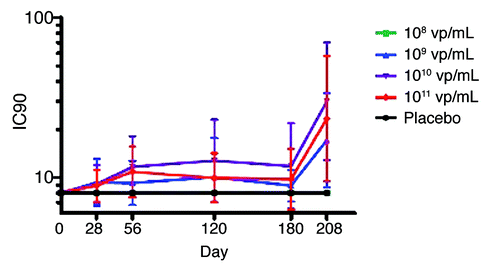
All subjects had undetectable neutralizing antibody titers against Ad35 at the start of vaccination (). In the lowest dosage group of 108 vp/mL, no positive Ad35 neutralizing antibodies were detected at the end of the three-dose series. In all other groups the number of subjects with detectable antibody titers increased modestly at one month after the 3rd vaccination to 5/13 (38%), 9/14 (64%), and 5/11(45%) in groups 109, 1010, and 1011 vp/mL, respectively.
Discussion
This is the first report on the safety and immunogenicity of a novel, adenovirus-vectored malaria vaccine candidate administered to humans. In this study, we demonstrated that an Ad35.CS.01 malaria vaccine targeting the CS antigen was modestly immunogenic in doses ranging from 109 to 1011 vp/mL, and that local and systemic reactogenicity increased in the highest dosage groups. Common local and systemic reactions included injection site pain, erythema, headache, and fever; these symptoms were self-limited and typically mild in nature. Additional adverse events—such as brachial plexopathy, arm weakness, and severe systemic reactogenicity—required exclusion from subsequent vaccination in four subjects in the highest dosage cohort although subjects recovered quickly without specific therapy or sequelae, These reactions were self-limited, temporally associated with vaccination/injection, and not due to other established causes. However, their relationship to the study product could not be established in all cases. Doses of at least 1010 vp/mL were required to generate a 4-fold rise in antibody titers from baseline and only the 1011 vp/mL dosage generated a sustained humoral response after the 3rd vaccination. Taken together, dosage modifications or heterologous prime-boost strategies will be required for future trials.
One potential disadvantage of an adenoviral vaccine approach is that pre-existing neutralizing antibodies to some adenovirus strains, such as Ad5, can be found in up to 80% of adultsCitation31; in contrast, antibodies to Ad35 are typically found in < 15% of healthy adults globally.Citation31 Indeed, only four subjects in our study were identified with a detectable Ad35 neutralizing antibody titer in our baseline screening of 91 individuals. The importance of pre-existing immunity is unclear, but preliminary data from the STEP trial (Human Immunodeficiency Virus [HIV] vaccine MRKAd5 HIV-1gag/pol/nef in subjects at high-risk for HIV infection) suggested that HIV incidence was higher among Ad5 seropositive vaccines when compared with placebo recipients (5.1% vs. 2.2% per year).Citation32 Among those who were Ad5 seronegative, HIV incidence was similar, implying an independent effect of seropositivity on HIV acquisition. Whether this relationship holds for other adenovirus serotypes, or for other antigen inserts, is unknown but we modified our protocol to exclude volunteers with pre-existing Ad35 antibody.
The primary limitation we observed in this Phase I study was that of increasing reactogenicity with higher doses. Not only did the overall frequency of solicited local and systemic reactions increase in the 1010 and 1011 vp/mL groups, but the intensity of these events was greater, with 4/16 (25%) of individuals in the highest dose group experiencing a severe systemic reaction, defined as interfering with activities of daily living. Typically these symptoms consisted of fever, headache, myalgias, and malaise. Similar reactogenicity has been observed in some other human studies of adenovirus-vectored vaccines.Citation33 In the HIV Vaccine Trials Network (HVTN) 054 study of an Ad5 HIV vaccine, doses of 1011 vp/mL were associated with both a higher frequency and severity of malaise (p = 0.025), myalgia (p = 0.004), headache (p = 0.007), fever (p = 0.005) and chills (p < 0.001) when compared with the 1010 vp/mL dose. However, the higher dosages were required to generate modest immune responses. Future studies will focus on strategies to improve immunogenicity, while reducing reactogenicity, such as modified dose regimens (e.g., 1 × 1010 vs. 5 × 1010 vp/mL) and various prime-boost strategies with other malaria vaccine candidates. In fact, the approach of heterologous prime boost is currently being evaluated with Ad35.CS.01 as priming vector and Ad26.CS.01 as booster in human challenge study as well as RTS, S as booster in human challenge study (NCT01397227 and 01366534, http://www.clincial trials.gov).
As the first administration of the Ad35.CS.01 vaccine in humans, this study has clear implications in the development of a successful immunization strategy against malaria in at-risk hosts. There are certain limitations that should be discussed, however. While 72 subjects is robust for a Phase I study, the sample size is not large enough to evaluate small differences in immunogenicity or reactogenicity that may be influenced by age, gender, race/ethnicity, or other variables. Further, our inability to interpret epitope-specific T-cell responses due to high baseline ELISpot values limits our ability to evaluate this important aspect of the immune response; nevertheless, the antibody responses are reassuring and provide guidance on the dosing that will be required in subsequent studies.
In this population of malaria naïve adults, Ad35.CS.01 was immunogenic and induced antibody responses against the CS protein in a dose dependent manner. Priming was successfully achieved in most of the subjects receiving the dose of 1010 and 1011 vp/mL, with the highest titers observed in the 1011 vp/mL dose group. Our study demonstrates the overall safety of a novel vaccine candidate for the potential prevention of malaria that induces humoral immune responses in the higher dose groups. Ongoing challenge studies of Ad35.CS.01 seek to assess its efficacy.
Materials and Methods
Trial design
The study was a randomized, placebo-controlled clinical trial to assess the safety of four ascending dosages of the Ad35 circumsporozoite-protein containing malaria vaccine administered in 3 intramuscular doses given on a 0, 1, and 6 mo schedule. Subjects were allocated to vaccine or placebo in a 5:1 ratio within each sequentially enrolled dosage group. Dose escalation proceeded following Safety Monitoring Committee (SMC) safety review 14 d after the first vaccination in the prior dosage group. The study, conducted at Vanderbilt University Medical Center and Stanford University Hospital, was approved by the Institutional Review Boards of each institution and registered as clinical trial identifier NCT00371189.
Study population
Subjects were included in the study if they were between the ages of 18 and 45 y; were in good health, as determined by screening medical history, physical examination, and laboratory assessments; and were able to comply with protocol requirements. Subjects were excluded for the following conditions: currently receiving corticosteroids or other immunosuppressive agents; abnormal screening laboratory values as outlined in Study Schedule below; detectable neutralizing antibody titers against Ad35 prior to enrollment; history of intravenous drug or alcohol abuse; recent receipt of live vaccines (30 d), subunit vaccines (14 d) or blood products (6 mo); history of malignancy; or positive serology for HIV, hepatitis C virus (HCV), or hepatitis B virus (HBV). Subjects were also excluded for a past history of malaria infection or vaccination, travel to a malaria endemic area within the previous 12 mo, or anticipated travel to a malaria-endemic area prior to conclusion of the trial (Day 208).
Vaccine
Ad35.CS.01 is a non-adjuvanted malaria vaccine with a codon optimized nucleotide sequence representing the P. falciparum circumsporozoite (CS) surface antigen inserted into a replication deficient Adenovirus 35 backbone. It was produced in complementing PER.C6© cells by Crucell Holland BV, Leiden, The Netherlands, under National Institutes of Health (NIH), Division of Microbiology and Infectious Diseases (DMID) supported contract NOI-AIO5421. The P. falciparum CS gene insert is a synthetic, mammalian-codon-optimized insert encoding a CS protein as previously described.Citation34 Normal saline was used for placebo administration. The vaccine doses were 108 vp/mL, 109 vp/mL, 1010 vp/mL, and 1011 vp/mL. The concentration of the vaccine was determined by optical density (OD260). The absorbance at OD260, after formulation buffer background subtraction, was multiplied by the Maizel factor to attain the number of virus particles.Citation35 Each measurement is calibrated against the reference, which should fall within pre-determined control limits. The strength of the product (i.e., the ratio of virus particles to infectious units) was determined by the vp/IU ratio obtained from the OD260 and the tissue culture infective dose (TCID50) assay. The vp/IU ratio was below 30:1, confirming release criteria.
Three doses of either vaccine or placebo were administered on a 0, 1, and 6-mo schedule. Each 1.0 mL dose of the assigned dose of Ad35.CS.01 or placebo was administered using a sterile, disposable syringe and needle by intramuscular injection into the deltoid muscle by an unblinded vaccinator with no further communication with the subject regarding adverse events. Subsequent injections were administered in alternating arms.
Study schedule
Subjects were evaluated prior to vaccination with a medical history, determination of concomitant medications, physical examination and screening laboratory studies that included: hematology (hemoglobin, white blood cell count [WBC], absolute neutrophil count [ANC], and platelet count); chemistries (glucose, sodium, potassium, alanine aminotransferase [ALT], aspartate aminotransferase [AST], and creatinine); serology (HIV, HCV, Hepatitis B Surface Antigen [HBSAg]); and pregnancy testing (if applicable). In addition, a urine sample was obtained for blood and protein detection and for adenovirus culture. Due to preliminary results from the HIV-STEP Trial,Citation32,Citation36 subjects in dose cohorts 3 and 4 (1010 and 1011 vp/mL, respectively) were screened for Ad35 serum antibodies prior to enrollmentCitation21 and excluded from enrollment if seropositive. HIV antibody testing was performed at screening and at one year after the first vaccination.
Outcomes
The primary objective of the study was to assess the safety and reactogenicity of ascending dosages of the vaccine through laboratory studies and daily reactogenicity diaries (). Subjects were asked to rate both local symptoms (arm pain, bruising, presence of erythema, and presence of induration) and systemic symptoms (fever, malaise, myalgia, headache, chills, nausea, and vomiting) for 14 d after each vaccination. Severity grades were mild (symptoms present), moderate (symptoms interfere with some activities), or severe (symptoms interfere with activities of daily living). These symptoms were reviewed on Day 1 after vaccination (telephone call), and on Days 2, 7, and 14 after vaccination with clinic visits. Study personnel verified the severity rating and study physicians assigned association with the study product. In addition, one week after each vaccine dose, identical safety laboratory testing was performed as at enrollment. Unsolicited adverse events (AEs) were collected through 28 d after the last dose and serious adverse events (SAEs) were collected until trial completion. Secondary objectives included humoral immunogenicity at various time points after each vaccination. Assessment of cellular immunity was included as an exploratory endpoint.
Figure 4. Geometric mean Ad35 Neutralizing antibody titers at baseline, 1 mo, 2 mo, 6 mo, and 7 mo following first vaccination. Negative responses are set at 8 IC90, calculated as half the lower limit of quantification, whiskers indicate 95% CI.
Serologic assays
Sera were collected at baseline, 1 mo after the 1st and 2nd vaccination, 5 mo after the 2nd vaccination (i.e., immediately prior to the 3rd vaccination) and 1 mo after the 3rd vaccination. ELISA methodology was used at Crucell, as previously described, to determine IgG antibody concentrations to CS protein using a (NANP)6 peptide as capture antigen.Citation37 In short, the peptide was obtained from Pepscan (Lelystad, Netherlands) at a purity of 90%. The peptide was coated at a concentration of 2 μg/ml in 0.05M carbonate buffer at 2–8 °C overnight or for a maximum of 3 d. Reference sera, serum samples, and internal controls were diluted in phosphate-buffered saline (PBS), 2% gelatin, and 1% human serum (dilution buffer) and incubated for 1 h at room temperature. Caprine anti-human IgG horseradish peroxidase was added and incubated for 1 h at room temperature. Finally, o-phenylenediamine (OPD; Sigma Aldrich) was added for the colorimetric reaction, which was stopped after 10 min using 5.3% H2SO4 stop solution. Subsequently, the optical density (OD) was measured at 492 nm, using a Bio-Tek microplate spectrophotometer PowerWave 340. The titer is expressed in relative ELISA Units/mL (EU/mL), by assessing the titer of a sample in the linear portion of a standard curve run in parallel with a nominal binding activity. The limit of detection for the anti-CS assay was 18 EU/mL. Values below this threshold were set at 50% of the limit of detection. In addition, representative sera will be available through the International Reference Center for Malaria Serology Laboratory, located at the Walter Reed Army Institute of Research.
Ad35-specific neutralizing antibody titers were assessed by luciferase-based virus neutralization assays as described previouslyCitation21 and recently optimized and validated for human serum. In short, sera were heat inactivated and serially diluted by 2-fold (starting dilution is 1:16). Ad35.Luc solution (108 vp/ml) was added to each well at 500 virus particles per cell. A549 cells were added at 104 cells/well and plates were incubated at 37 °C/10% CO2 for 24 to 26 h. After incubation, medium was discarded, PBS was added, and plates were stored frozen overnight. Plates were allowed to thaw at room temperature, Luciferase Steady-Lite substrate was added and the lysate was transferred into black/white isoplates. Luminescence counts were recorded on a 1450 MicroBeta Trilux. Titers were determined by validated software ANAM (Adenovirus neutralization Assay Macro). The limit of detection for the Ad35 neutralizing antibody assay was 16 IC90, the titer at which 90% neutralization was achieved. Values below this threshold were set at 50% of the limit of detection.
Peripheral blood mononuclear cells were collected for assessment of cellular immune responses prior to each vaccination and one month following the 2nd and 3rd vaccinations. Characterization of antigen-specific T cell responses were evaluated based on the quantity and phenotype of cells responding to CS-antigen using an ELISpot assay as the primary quantitative readout and an IFN-γ ICS assay to assess the CD4+ vs. CD8+ T-cell response. These assays have been described in more detail elsewhere.Citation38–Citation41 Due to unusually high baseline ELISpot values in multiple volunteers and overall sample degradation, these data were uninterpretable and not included in the results.
Sample size
Fifteen subjects per dosage group were proposed to receive the Ad35.CS.01 vaccine to obtain preliminary safety information on a small cohort of subjects before proceeding to subsequent larger trials. The study was powered to detect at least a 10% rate of all adverse events with 80% power but was not powered to compare dosage-related immune responses.
Randomization and blinding
Subjects were randomized through the AdvantageEDCSM Data Entry System (provided by the EMMES Corporation) with a 5:1 vaccine to placebo ratio. Study staff members were blinded to assignment with the exception of the investigational pharmacist and the unblinded vaccinator.
Statistical methods
The study was designed as an exploratory study to estimate event rates and patterns of immune responses rather than to test formal statistical hypotheses. As a result, estimates are presented with their 95% confidence intervals. Adverse events and immune responses were reported as frequencies or means. Fisher’s exact tests for categorical and Kruskal-Wallis tests for continuous variables were used to compare safety and immunogenicity outcomes in the vaccine groups relative to placebo.
Additional material
Download Zip (83.5 KB)Acknowledgments
We would like to acknowledge and thank our study volunteers for their participation in this study. In addition we would like to acknowledge the contributions of our staff at Vanderbilt: Deborah Hunter, RN, BSN, CCRC; Deborah Myers, CCRP; Todd Mathis, LPN; at Stanford-LPCH Vaccine Program staff: Clinical Research Nurses Sue Swope, RN, Cynthia Walsh, RN; clinical research assistants Sri Arunachalam, Alicia Gutiérrez, Kyrsten Spann, Thu Quan, Raquel Fleischmann; and phlebotomist Michele Ugur. The Ad35.CS.01 vaccine was produced under NIAID contract AI-N01-045210 to Science Applications International Corporation (SAIC). The work is supported in part by N01-AI-80007 (Vanderbilt Vaccine and Treatment Evaluation Unit), Vanderbilt CTSA grant 5UL1 RR024975-03 from NCRR/NIH, M01 RR-00070 from NCRR/NIH, and Stanford CTSA award 1UL1 RR025744 from NCRR/NIH.
Disclosure of Potential Conflicts of Interest
MGP, JH, and IV are employees of Crucell, Leiden, The Netherlands. All other authors report no relevant conflicts of interest.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/vaccines/article/26038
References
- Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev 2002; 15:564 - 94; http://dx.doi.org/10.1128/CMR.15.4.564-594.2002; PMID: 12364370
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005; 434:214 - 7; http://dx.doi.org/10.1038/nature03342; PMID: 15759000
- World Health Organization. WHO Facts on Malaria. 2013.
- Mandell GL, Douglas RG, Bennett JE, Dolin R. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005.
- John CC, Moormann AM, Pregibon DC, Sumba PO, McHugh MM, Narum DL, Lanar DE, Schluchter MD, Kazura JW. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg 2005; 73:222 - 8; PMID: 16014863
- John CC, Tande AJ, Moormann AM, Sumba PO, Lanar DE, Min XM, Kazura JW. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis 2008; 197:519 - 26; http://dx.doi.org/10.1086/526787; PMID: 18275273
- John CC, Zickafoose JS, Sumba PO, King CL, Kazura JW. Antibodies to the Plasmodium falciparum antigens circumsporozoite protein, thrombospondin-related adhesive protein, and liver-stage antigen 1 vary by ages of subjects and by season in a highland area of Kenya. Infect Immun 2003; 71:4320 - 5; http://dx.doi.org/10.1128/IAI.71.8.4320-4325.2003; PMID: 12874308
- Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmüller B, et al, RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863 - 75; http://dx.doi.org/10.1056/NEJMoa1102287; PMID: 22007715
- Bejon P, Cook J, Bergmann-Leitner E, Olotu A, Lusingu J, Mwacharo J, Vekemans J, Njuguna P, Leach A, Lievens M, et al. Effect of the pre-erythrocytic candidate malaria vaccine RTS,S/AS01E on blood stage immunity in young children. J Infect Dis 2011; 204:9 - 18; http://dx.doi.org/10.1093/infdis/jir222; PMID: 21628653
- Olotu A, Moris P, Mwacharo J, Vekemans J, Kimani D, Janssens M, Kai O, Jongert E, Lievens M, Leach A, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P falciparum clinical malaria. PLoS One 2011; 6:e25786; http://dx.doi.org/10.1371/journal.pone.0025786; PMID: 21998698
- Olotu A, Lusingu J, Leach A, Lievens M, Vekemans J, Msham S, Lang T, Gould J, Dubois MC, Jongert E, et al. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect Dis 2011; 11:102 - 9; http://dx.doi.org/10.1016/S1473-3099(10)70262-0; PMID: 21237715
- Aide P, Dobaño C, Sacarlal J, Aponte JJ, Mandomando I, Guinovart C, Bassat Q, Renom M, Puyol L, Macete E, et al. Four year immunogenicity of the RTS,S/AS02(A) malaria vaccine in Mozambican children during a phase IIb trial. Vaccine 2011; 29:6059 - 67; http://dx.doi.org/10.1016/j.vaccine.2011.03.041; PMID: 21443960
- Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, Ballou WR, Conway DJ, Reece WH, Gothard P, et al, RTS, S Malaria Vaccine Trial Team. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 2001; 358:1927 - 34; http://dx.doi.org/10.1016/S0140-6736(01)06957-4; PMID: 11747915
- Stoute JA, Kester KE, Krzych U, Wellde BT, Hall T, White K, Glenn G, Ockenhouse CF, Garcon N, Schwenk R, et al. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J Infect Dis 1998; 178:1139 - 44; http://dx.doi.org/10.1086/515657; PMID: 9806046
- Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Mshamu S, Lang T, Gould J, Dubois MC, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med 2008; 359:2521 - 32; http://dx.doi.org/10.1056/NEJMoa0807381; PMID: 19064627
- Ophorst OJ, Radosević K, Havenga MJ, Pau MG, Holterman L, Berkhout B, Goudsmit J, Tsuji M. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect Immun 2006; 74:313 - 20; http://dx.doi.org/10.1128/IAI.74.1.313-320.2006; PMID: 16368986
- Stewart VA, McGrath SM, Dubois PM, Pau MG, Mettens P, Shott J, Cobb M, Burge JR, Larson D, Ware LA, et al. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect Immun 2007; 75:2283 - 90; http://dx.doi.org/10.1128/IAI.01879-06; PMID: 17307942
- Shott JP, McGrath SM, Pau MG, Custers JH, Ophorst O, Demoitié MA, Dubois MC, Komisar J, Cobb M, Kester KE, et al. Adenovirus 5 and 35 vectors expressing Plasmodium falciparum circumsporozoite surface protein elicit potent antigen-specific cellular IFN-gamma and antibody responses in mice. Vaccine 2008; 26:2818 - 23; http://dx.doi.org/10.1016/j.vaccine.2008.03.080; PMID: 18455276
- Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Béthune MP, Kostense S, Penders G, Helmus N, Koudstaal W, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 2003; 77:8263 - 71; http://dx.doi.org/10.1128/JVI.77.15.8263-8271.2003; PMID: 12857895
- Ophorst OJ, Kostense S, Goudsmit J, De Swart RL, Verhaagh S, Zakhartchouk A, Van Meijer M, Sprangers M, Van Amerongen G, Yüksel S, et al. An adenoviral type 5 vector carrying a type 35 fiber as a vaccine vehicle: DC targeting, cross neutralization, and immunogenicity. Vaccine 2004; 22:3035 - 44; http://dx.doi.org/10.1016/j.vaccine.2004.02.011; PMID: 15297053
- Peiperl L, Morgan C, Moodie Z, Li H, Russell N, Graham BS, Tomaras GD, De Rosa SC, McElrath MJ, NIAID HIV Vaccine Trials Network. Safety and immunogenicity of a replication-defective adenovirus type 5 HIV vaccine in Ad5-seronegative persons: a randomized clinical trial (HVTN 054). PLoS One 2010; 5:e13579; http://dx.doi.org/10.1371/journal.pone.0013579; PMID: 21048953
- Schuldt NJ, Aldhamen YA, Godbehere-Roosa S, Seregin SS, Kousa YA, Amalfitano A. Immunogenicity when utilizing adenovirus serotype 4 and 5 vaccines expressing circumsporozoite protein in naïve and adenovirus (Ad5) immune mice. Malar J 2012; 11:209; http://dx.doi.org/10.1186/1475-2875-11-209; PMID: 22720732
- Schuldt NJ, Amalfitano A. Malaria vaccines: focus on adenovirus based vectors. Vaccine 2012; 30:5191 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.05.048; PMID: 22683663
- Rodrigues EG, Zavala F, Nussenzweig RS, Wilson JM, Tsuji M. Efficient induction of protective anti-malaria immunity by recombinant adenovirus. Vaccine 1998; 16:1812 - 7; http://dx.doi.org/10.1016/S0264-410X(98)00181-9; PMID: 9795385
- Radosevic K, Rodriguez A, Lemckert AA, van der Meer M, Gillissen G, Warnar C, von Eyben R, Pau MG, Goudsmit J. The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert. Clin Vaccine Immunol 2010; 17:1687 - 94; http://dx.doi.org/10.1128/CVI.00311-10; PMID: 20826614
- Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, Tamminga C, Patterson N, Guerrero M, Bennett JW, McGrath S, et al. DNA prime/Adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS One 2013; 8:e55571; http://dx.doi.org/10.1371/journal.pone.0055571; PMID: 23457473
- Tamminga C, Sedegah M, Regis D, Chuang I, Epstein JE, Spring M, Mendoza-Silveiras J, McGrath S, Maiolatesi S, Reyes S, et al. Adenovirus-5-vectored P. falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component. PLoS One 2011; 6:e25868; http://dx.doi.org/10.1371/journal.pone.0025868; PMID: 22003411
- Sedegah M, Tamminga C, McGrath S, House B, Ganeshan H, Lejano J, Abot E, Banania GJ, Sayo R, Farooq F, et al. Adenovirus 5-vectored P. falciparum vaccine expressing CSP and AMA1. Part A: safety and immunogenicity in seronegative adults. PLoS One 2011; 6:e24586; http://dx.doi.org/10.1371/journal.pone.0024586; PMID: 22003383
- Rodrigues EG, Claassen J, Lee S, Wilson JM, Nussenzweig RS, Tsuji M. Interferon-gamma-independent CD8+ T cell-mediated protective anti-malaria immunity elicited by recombinant adenovirus. Parasite Immunol 2000; 22:157 - 60; http://dx.doi.org/10.1046/j.1365-3024.2000.00289.x; PMID: 10672197
- Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmüller B, Issifou S, Kremsner PG, et al, RTS,S Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 2012; 367:2284 - 95; http://dx.doi.org/10.1056/NEJMoa1208394; PMID: 23136909
- Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Béthune MP, Kostense S, Penders G, Helmus N, Koudstaal W, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 2003; 77:8263 - 71; http://dx.doi.org/10.1128/JVI.77.15.8263-8271.2003; PMID: 12857895
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al, Step Study Protocol Team. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372:1881 - 93; http://dx.doi.org/10.1016/S0140-6736(08)61591-3; PMID: 19012954
- Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, et al, VRC 205 Study Team. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine 2010; 29:304 - 13; http://dx.doi.org/10.1016/j.vaccine.2010.10.037; PMID: 21034824
- Radosevic K, Rodriguez A, Lemckert AA, van der Meer M, Gillissen G, Warnar C, von Eyben R, Pau MG, Goudsmit J. The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert. Clin Vaccine Immunol 2010; 17:1687 - 94; http://dx.doi.org/10.1128/CVI.00311-10; PMID: 20826614
- Maizel JV Jr., White DO, Scharff MD. The polypeptides of adenovirus. II. Soluble proteins, cores, top components and the structure of the virion. Virology 1968; 36:126 - 36; http://dx.doi.org/10.1016/0042-6822(68)90122-0; PMID: 5669983
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, et al, Step Study Protocol Team. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 2008; 372:1894 - 905; http://dx.doi.org/10.1016/S0140-6736(08)61592-5; PMID: 19012957
- Kostense S, Mommaas B, Hendriks J, Verhoeven M, Ter Haak M, Tirion F, Wiesken E, Pau MG, Radosevic K, Goudsmit J. A peptide-based Plasmodium falciparum circumsporozoite assay to test for serum antibody responses to pre-erythrocyte malaria vaccines. Clin Vaccine Immunol 2011; 18:776 - 82; http://dx.doi.org/10.1128/CVI.00547-10; PMID: 21411600
- Rock MT, Yoder SM, Talbot TR, Edwards KM, Crowe JE Jr.. Cellular immune responses to diluted and undiluted aventis pasteur smallpox vaccine. J Infect Dis 2006; 194:435 - 43; http://dx.doi.org/10.1086/505506; PMID: 16845626
- Rock MT, Yoder SM, Wright PF, Talbot TR, Edwards KM, Crowe JE Jr.. Differential regulation of granzyme and perforin in effector and memory T cells following smallpox immunization. J Immunol 2005; 174:3757 - 64; PMID: 15749916
- Rutigliano JA, Rock MT, Johnson AK, Crowe JE Jr., Graham BS. Identification of an H-2D(b)-restricted CD8+ cytotoxic T lymphocyte epitope in the matrix protein of respiratory syncytial virus. Virology 2005; 337:335 - 43; http://dx.doi.org/10.1016/j.virol.2005.04.032; PMID: 15916793
- Rock MT, Crowe JE Jr.. Identification of a novel human leucocyte antigen-A*01-restricted cytotoxic T-lymphocyte epitope in the respiratory syncytial virus fusion protein. Immunology 2003; 108:474 - 80; http://dx.doi.org/10.1046/j.1365-2567.2003.01619.x; PMID: 12667209