Abstract
Despite a WHO recommendation in 2009, reaffirmed in 2013, that all countries should consider introducing rotavirus vaccines into their National Immunization Programs, as of June 2013 only 45 have done so. One major consideration appears to have been the costs of the vaccine to countries. Of concern, is that Asian countries have been slow to introduce rotavirus vaccines despite having robust data that could inform the decision-making process. Although decisions on new vaccine introduction are very complex and vary by country and region, economic evaluations are often pivotal once vaccine efficacy and safety has been established, and disease burden documented and communicated. Unfortunately, with private sector list prices of vaccines often used in economic evaluations, rather than a potential public health sector pricing structure, policy-makers may defer decisions on rotavirus vaccine introduction based on the belief that “the vaccine price is too high,” even though this might be based on erroneous data. The Pan American Health Organization’s Revolving Fund provides one example of how vaccine price can be made more competitive and transparent through a regional tendering process. Other mechanisms, such as tiered pricing and UNICEF procurement, also exist that could help Asian and other countries move forward more quickly with rotavirus vaccine introduction.
Overview
The first rotavirus vaccine, Rotashield®, was licensed by the United States Food and Drug Administration and recommended for universal use in the United States in 1998. Post-marketing surveillance identified that the vaccine was associated with an increased risk of intussusception of approximately 1 in 10 000 vaccinated infants and it was voluntarily withdrawn from the United States market by the manufacturer in 1999. In January 2006, results of two large clinical trials of two new rotavirus vaccines, Rotarix® and RotaTeq®, each with over 60 000 infants for safety, reported good safety and high efficacy against severe rotavirus gastroenteritis during the first two years of life.Citation1,Citation2 These vaccines showed no increased risk of intussusception in the infants, although cases were identified in both vaccine and placebo recipients. Following the 2007 World Health Organization (WHO) recommendation to introduce rotavirus vaccines in the regions where vaccine efficacy had been demonstrated in initial clinical trials (the Americas and Europe),Citation3 many countries in these regions implemented rotavirus vaccination in their national programs over the next few years (). After clinical trials in Africa and Asia were completed, WHO expanded its initial recommendation in 2009 and recommended introduction of rotavirus vaccination in all countries in the world.Citation4
Table 1. Countries by regions that have introduced rotavirus vaccine in their National Immunization Programs (NIPs) a,Citation47 as of June 2013 and subsequent reductions and rotavirus and all-cause gastroenteritisCitation18
The early adopter countries have witnessed substantial reductions in rotavirus-associated hospitalization rates in children under two-years of age in high income countries (Australia,Citation5,Citation6 Austria,Citation7 Belgium,Citation8,Citation9 United StatesCitation10) and middle- or low-income countries (Brazil,Citation11,Citation12 El Salvador,Citation13,Citation14 Mexico,Citation15 Nicaragua,Citation16 Panama,Citation17). Overall, where reported, rotavirus hospitalizations have fallen by around 70% and all-cause gastroenteritis hospitalizations by around 35% ().Citation18 In addition universal rotavirus vaccination may have reduced nosocomial infections,Citation19 and has been documented to provide significant indirect protection to unvaccinated older children and adults in the United States.Citation20 Finally, it is most exciting to note that substantial 30–40% declines in diarrhea mortality, an outcome not assessed in rotavirus vaccine clinical trials, have been noted in two large middle-income countries in Latin America (Mexico and Brazil) that have examined national data on diarrhea deaths in children.Citation12,Citation21
In January 2013, the WHO updated its previous position papers on rotavirus vaccines and reaffirmed its 2009 recommendation that rotavirus vaccines should be included in all National Immunization Programs (NIPs) globally.Citation22 Yet as of June 2013, only 45 countries have followed this advice, with an additional 3 countries recommending the vaccine for sub-national introduction (). Why did some countries recommend vaccine introduction within months of licensure, while others have not?
Factors Influencing Decision-Making Regarding New Vaccine Introduction
In 2002, McKinsey and Co. reported to the GAVI Alliance (GAVI) on factors that decision-makers indicated could accelerate the decision for introduction of new vaccines in developing countries. These included (1) proof of local disease burden, (2) proof of an available safe and effective vaccine, and (3) evidence of value for money. Other factors influencing the decision-making process included the potential impact on existing immunisation programmes and support for the new vaccine from clinical opinion leaders, medical practitioners and the general public. In 2007 McKinsey and Co, undertook a pro-bono “Network Analysis” liaising with the Bill and Melinda Gates Foundation, John Hopkins Bloomberg School of Public Health, the PneumoADIP and PATH.Citation23 This analysis focused on four countries (Egypt, Mauritania, Mexico, Zambia) and mapped the complex relationships between the influencers of the decision making process. Although international organizations were shown to play a key role in the introduction process, there was seen to be a failure to utilize global experts and little cross-country sharing of information. Improving connectivity at an early stage, with involvements of Ministries of Finance and community groups, were suggested as key influencing factors. In addition a perceived lack of data on local disease burden was highlighted as an important barrier to introduction decisions. However, a perception of lack data on disease burden may reflect a failure to communicate these data to both national decision-makers and the general public, rather than a deficiency of scientific evidence. More recently, a study looking at new vaccine introductions in lower-middle-income countries has highlighted that disease burden, cost and WHO recommendations remain key influencers to the decision making process.Citation24
We examine the availability of evidence for these key factors to support decision making for countries in Asia, and look at how financial barriers may play a major role in the lack of uptake of rotavirus vaccines. Additional factors play a role in the decision-making process, such as percentage of national budget allocated to health, real and perceived health priorities, arguments that new vaccine introductions should be sequential and competitive, and public knowledge of and demand for a vaccine are not directly considered.
Disease Burden of Rotavirus in Asia
To accelerate the introduction of rotavirus vaccines WHO and GAVI recognized early the importance of collecting local disease burden data for policy makers and recommended that simple generic protocols be developed and that regional rotavirus surveillance networks be established.Citation25 The first such network to be established was the Asian Rotavirus Surveillance Network (ARSN) which used a WHO active surveillance protocol.Citation26 The initial phase conducted standardized surveillance in China, Hong Kong, Indonesia, Malaysia, Myanmar, South Korea, Taiwan, Thailand, and Vietnam between 2001–2004, followed by a second phase between 2005–2007 in predominantly GAVI-eligible countries ().Citation25 The extensive data collected by the ARSN were widely published and made available for decision makers in the region. The ARSN data included disease burden estimates from some of the world’s most populous countries (China, India, Indonesia, Pakistan, and Bangladesh) and shows that for a number of countries over 50% of gastroenteritis-related hospital admissions were due to rotavirus ().Citation26,Citation27 Yet as of June 2013 only two countries in the Asian region, Philippines and Thailand, had partially introduced rotavirus vaccines into their countries. This experience suggests that having an extensive database of local disease burden alone has been insufficient to drive rapid introduction of rotavirus vaccine in the Asian region.
Table 2. Rotavirus disease burden reported from sites participating in the Asian Rotavirus Surveillance Network
Rotavirus Vaccine Efficacy in Asia
Rotavirus vaccine efficacy trials in Asia lagged behind those in the Americas and Europe by several years and initially involved only high income countries in region (Hong Kong, Singapore, and Taiwan).Citation28 This study demonstrated vaccine efficacy of 96.1% (95% CI: 85.1%; 99.5%) against severe rotavirus gastroenteritis during the first two years of life, with sustained efficacy of 100% (95% CI: 67.5%; 100%) in the third-year.Citation29 Efficacy studies in middle- and low- income countries in Asia followed several years later, demonstrating good efficacy in Vietnam and only modest efficacy in Bangladesh.Citation30 Thus, local evidence of vaccine performance from a variety of socioeconomic settings in Asia is also available, including the significant public health impact of vaccine in Bangladesh, even with a modest vaccine efficacy.Citation3 The perception of a lack of “good” efficacy data in low income settings in the region, despite the WHO Strategic Advisory Group of Experts (SAGE) recommendation of significant public health impact in these populations,Citation3 may be factor in delaying policy decisions for rotavirus vaccine introduction in the region, and have certainly impacted cost effectiveness analyses.
Economic Evaluations and Vaccine Price
Economic evaluations should typically be performed prior to a decision being made to introduce a new vaccine. The typical economic evaluation will take a number factors into consideration including: direct and indirect costs; averted illnesses, averted deaths, DALYs and QALYS averted/gained, avoided medical and indirect costs.Citation31 The main drivers of the model are typically the price of the vaccine and number of deaths and hospitalizations averted.
The outcome of this type of analysis can be shown on a cost-effectiveness plane which has 4 quadrants (). Typically most analyses fall in the top right quadrant where the intervention (vaccination) is more costly than the existing intervention (no vaccination and hospitalizations/deaths from rotavirus) but more effective (prevents hospitalizations/deaths from rotavirus). If the intervention is a lot more costly, but only a little bit more effective, it will be deemed not cost-effective i.e., to left of the incremental cost-effective ratio (ICER) line (Price A, ). Conversely if it is a little more costly but a lot more effective it will be deemed cost-effective i.e., to the right of the ICER line (Price B, ). Very occasionally decision makers may opt for intervention that falls in the bottom left quadrant i.e., the intervention is less costly than the existing intervention but also less effective. This would be a rational decision if the intervention is a lot less costly but only a little bit less effective since it might be possible to cover a much greater proportion of the population with the intervention i.e., falls to the right of the ICER line. Every decision maker should hope for the analysis to fall in the bottom right quadrant. Here the intervention is less costly than the existing intervention but more effective i.e., Government would save money and make its population more healthy (Price C, ). It would be hoped that a decision-maker who fails to adopt such an intervention has clear and evidenced-based reasons why the intervention cannot be introduced.
Figure 1. Cost-effectiveness plane showing how vaccine price typically drives the economic model and can easily change the conclusion that introducing a new vaccine will be cost-ineffective (price A), cost-effective (price B) or cost-saving (price C). Often the private sector list or catalog price lies in the region of Price A, whereas an eventual tender or agreed price for use of a vaccine in a National Immunization Program lies between Price B and C. Rotavirus vaccine introductions in early-adopter countries have resulted in a 35% decrease in all-cause gastroenteritis hospitalizations in children below 2 y of age.Citation18
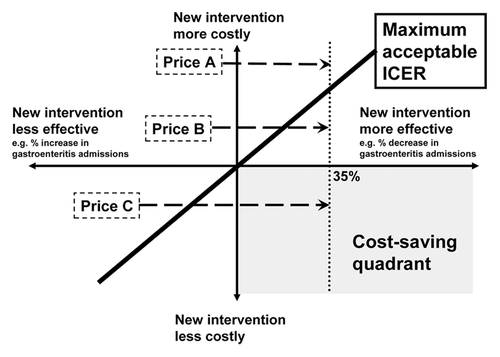
However for this type of analysis to work optimally the cost of the intervention (primarily vaccine price) needs to be very precise since this is a key driver of the model. There are a number of publications of economic evaluations conducted on rotavirus vaccines that have used the catalog or private sector list price, with or without an assumed discount, for the analysis and then conclude that rotavirus immunization is likely to be not cost-effective at this price.Citation32 Although sensitivity analyses are undertaken as part of economic evaluations to show the impact of decreasing or increasing the vaccine price, the concluding statement of the article i.e., the vaccine will not be cost-effective at current market price, is likely to be an important influencer for decision makers. If economic modelers use the private sector list price, or a closely related price, decision-makers are likely to conclude that rotavirus vaccine is not cost-effective (Price A, ). Although the likely tender price for public health use of a vaccine in a National Immunization Program (NIP) (i.e., bulk purchase contract for several years) will typically be very much less than the private sector price (Price B and C, ), this lower price cannot be known until after the vaccine has been recommended for use and tendering process completed. This resultant “catch-22” pushes decision-makers to defer vaccine introduction decisions. One way to solve this problem is to ensure that the tender price or purchase price is known prior to conducting the economic analysis and before an informed decision is made whether or not to introduce the vaccine into the NIP.
In addition it should be noted that although immunization are widely recognized as one of the most cost-effective of all health interventions,Citation33 it is also likely that their economic benefits have been underestimated since wider economic benefits are not considered in traditional economic evaluations.Citation34
National Immunization Technical Advisory Groups
Ideally decisions on public health policy should be transparent, be based on the best available evidence and be free from external interests—political, commercial, special interest. WHO SAGE recommended in 2009 that, as part of the process of ensuring evidence-based decision-making at country level, it was a priority for countries to establish and/or strengthen their national immunization technical advisory committees (NITAGs), given the increasing complexity of immunization programs and the high cost of new vaccines. NITAGs help health authorities formulate immunization policies according to the specific needs of their country, while taking into account the regional and international context.Citation35 In 2008 the Bill and Melinda Gates Foundation funded an initiative to establish or strengthen independent NITAGs. Detailed descriptions of the experiences and processes of 15 well established NITAGs from all regions of the world highlighted considerable differences between committees including their legal basis, size and scope of committee membership, scope of work, role of the Ministry of Health on the committee, existence of conflict of interest policies, and ultimate role in the decision-making process.Citation36 Nevertheless, NITAGs are recognized as a crucial component for national decision-making for new vaccines, and should be developed nationally as a priority.
Decision-Making and NITAGs in Some Early-Adopter Countries
The United States and Australia were both early adopters of rotavirus vaccines and both have well established NITAGs.Citation37,Citation38 The United States was the first country to recommend the universal use of both Rotashield® (in 1998) and RotaTeq® (in 2006). The Advisory Committee on Immunization Practices (ACIP) provides advice and guidance to the Secretary of the United States Department of Health and Human Services, the Director of the United States Centers for Disease Control and Prevention and other decision makers regarding the most appropriate selection of vaccines and related agents for effective prevention and control of vaccine-preventable diseases in the civilian population.Citation39 ACIP’s recommendations include consideration of population-based studies such as efficacy, cost benefit, and risk benefit analyses. ACIP also establishes a list of vaccines for administration to children and adolescents eligible to receive vaccines through the Vaccines for Children Program and this list will be used for the purchase, delivery, and administration of pediatric vaccines under this program. Currently the private sector price for RotaTeq® (3 dose) and Rotarix® (2 dose) per course is approximately $226 and $213 respectively, which is somewhat higher that the $192 and $184 paid for the same vaccines by the Vaccines for Children Program ().Citation40
Table 3. Examples of prices per course in US$ for Rotarix ® and RotaTeq ®
The Australian Technical Advisory Group on Immunisation (ATAGI) is tasked to provide technical advice to the Minister for Health and Aging on the medical administration of vaccines available in Australia, including those in the NIP.Citation37 The group also advises the Pharmaceutical Benefits Advisory Committee (PBAC) on the strength of evidence relating to existing, new and emerging vaccines in relation to their effectiveness and use in Australian populations. The ATAGI provides advice to both PBAC and to the submitting company about the public health and technical factors considered to be important to the public interest. However cost-effectiveness assessments are the sole responsibility of PBAC. Once the vaccine is supported by ATAGI, the vaccine manufacturer will then submit an application to PBAC and request that the vaccine be considered either for an NIP listing (free to eligible people) or a listing that requires a co-payment under the Pharmaceutical Benefits Scheme. PBAC’s Economic Sub-committee will review and interpret the company’s submission and economic analyses. PBAC will then provide a recommendation to Government on whether or not to fund the vaccine and on what basis. In contrast to the United States, the price that the Australian government pays for a vaccine included in the NIP is not in the public domain.
Apart from some small island states with strong historical links to Australia and the United States, the Philippines was the first Asian country to announce plans to introduce rotavirus vaccine (). This announcement was possible following a recommendation by the Ministry of Health and a decision by the Ministry of Finance to identify a specific sum of money to support the program. At the time of the announcement it was unknown which vaccine (or how many doses) would be used since the tendering process had not occurred - the lower the tender price, the more children could be vaccinated. In July 2012, six months after the announcement, the program was started with the Minister of Health reporting that during the first year 700 000 infants from the poorest communities would receive rotavirus vaccine i.e., approximately 28% of the 2.5 million birth cohort.
In contrast to Asia, the Americas were quick to use rotavirus vaccines, with 8 of the 12 early adopters (2006/2007) of rotavirus vaccines being situated in the Pan American Health Organization (PAHO) region (). Haiti, the last remaining GAVI-eligible country in the PAHO region yet to introduce rotavirus vaccine into its NIP, did so in May 2013. The early introduction of rotavirus vaccines into NIPs in the Americas can be explained in part by several factors, including good burden of disease data, and the fact that the initial rotavirus vaccine studies were predominantly conducted in the Americas as highlighted in WHO’s 2007 position paper.Citation3 In addition, PAHO’s Revolving Fund played an important role in decision-making and expediting rotavirus vaccine introduction in the region.Citation41–Citation43
Pricing Options for Asia
PAHO's Revolving Fund for Vaccine Procurement
PAHO countries pay per rotavirus vaccine course (2 or 3 doses) $13 for Rotarix® and $15.45 for RotaTeq®.Citation44 This price is in the public domain and provides the decision making process a precise estimate of vaccine cost as a result of PAHO’s Revolving Fund for Vaccine Procurement. The Revolving Fund is a mechanism to facilitate the bulk purchase of vaccines, syringes, cold chain equipment and related supplies.Citation41,Citation43 Taking advantage of economies of scale, the Revolving Fund secures vaccines—prequalified under WHO standards of safety and effectiveness—for member states at affordable prices. By purchasing through the Revolving Fund instead of directly from producers, countries can make significant savings on the purchase price. Founded on the principle of equity, PAHO’s Revolving Fund enables all participating member states to have access to the same products, offered at the lowest price, which is the same regardless of the country’s size or economic situation. Member states all contribute three percent of each net purchase price to a common fund which is used as working capital. Member states in need can take out lines of credit to purchase their vaccines, repaying within 60 d of vaccine receipt. The Revolving Fund also handles key processes like planning, demand estimates, price negotiations, purchase orders, supply coordination, shipment monitoring and billing. As a result, Latin American countries have had continuous access to safe and effective vaccines at low, stable prices for over 30 y. This assists national governments with budget planning, and fosters sustainable immunization programs. Today, the vast majority of vaccines being used in Latin America for some 44 million people were acquired through the Revolving Fund. However PAHO’s Revolving Fund does not have a mechanism to tier the pricing of vaccine between its more or less affluent members.
Tiered pricing
Industry, in particular GlaxoSmithKline Biologicals, indicates that it is willing to enter into tiered pricing agreements with individual governments.Citation45 However the prices agreed between the company and individual countries are generally not in the public domain to guide decision makers in other countries. Although an attractive concept for some countries, such one-to-one negotiations may be in conflict the legal requirements of the tendering process of other countries.
Separating technical decisions from economic evaluations
The ATAGI/PBAC mechanism as described above was introduced in 2005 by the Australian government to bring vaccine funding applications into the same transparent and predictable mechanism that had been used successfully for drugs.Citation37 However in contrast to PAHO’s Revolving Fund the price paid by Australia for rotavirus vaccines is hidden from public scrutiny. This mechanism allows Australia to enter into a process with industry, to obtain a price that is acceptable to both. However smaller countries with less capacity may have difficulty managing such a process and are likely to prefer a regional tendering process like PAHO’s Revolving Fund.
UNICEF hybrid procurement strategy
Recently UNICEF presented a strategy for vaccine procurement for middle-income countries that would include providing industry with demand forecasts, providing countries with information on products and availability; pooling procurement and establishing reference pricing.Citation46
Conclusions
Although decisions on vaccine introduction are complex and impossible to fully dissect for each country,Citation24 it is clear that current understanding of “local data on disease burden” and “cost benefit of the vaccine” may not reliably inform decision-makers on new vaccine introduction. In particular, the cry that “the vaccine price is too high” is often erroneously derived by the scientific community and may not reflect potential tender or negotiated prices. This is directly impacting an informed decision-making process for countries, and removes a sense of urgency from decision-makers tasked with the responsibility for new vaccine introduction. The ARSN does pre-empt a claim of “lack of local data on disease burden” for many countries in the Asian Region, but it is hard to counter the claim that the vaccine price is too high when the vaccine price cannot be known ahead of a tendering process, and the prices in the private market are very high.
What pricing solutions could help Asian policy-makers decide whether rotavirus vaccine is a “good buy” for them? An Asian or ASEAN revolving fund is clearly one option. However to set up such a fund would require considerable high level political will and international coordination. Even getting this topic onto the agendas of ASEAN Health and Finance Minister meetings seems a challenge. Regional WHO offices could take the lead to encourage the establishment of such a fund, but a SAGE recommendation to this effect would probably be required. In addition preliminary seed funding would be needed. Individual countries could try to establish funding mechanisms similar to Australia’s ATAGI/PBAC mechanism, i.e., a recommendation on vaccine use is separated from a decision as to whether the government will pay for the vaccines. However for some countries this is not feasible where a government recommendation for vaccine use is considered synonymous to the government agreeing to pay for the vaccine prior to the tendering process. If all else fails, a one-to-one discussion with industry may be needed to try to gain an advantageous tiered pricing agreement. But for those governments facing difficult trade-off decisions, or that wish to delay the decision for a little bit longer, the lack of good local economic evaluations based on corrected pricing estimates, remains a cry of despair “the vaccine price is too high!”
| Abbreviations: | ||
| ACIP | = | Advisory Committee on Immunization Practices |
| ARSN | = | Asian Rotavirus Surveillance Network |
| ATAGI | = | Australian Technical Advisory Group on Immunization |
| GAVI | = | GAVI Alliance |
| ICER | = | incremental cost-effective ratio |
| NIP | = | National Immunization Program |
| NITAG | = | national immunization technical advisory committees |
| PAHO | = | Pan American Health Organization |
| PBAC | = | Pharmaceutical Benefits Advisory Committee |
| SAGE | = | Strategic Advisory Group of Experts |
| WHO | = | World Health Organization |
Disclosure of Potential Conflicts of Interest
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention (CDC).
Competing Interests
EASN has participated in a vaccine studies funded by Baxter, GlaxoSmithKline, MedImmune, and Wyeth, has received funding to conduct disease surveillance studies from Merck and Pfizer, and lecture fees and travel support from GlaxoSmithKline, Merck, Intercell, and Pfizer. The other authors have declared that no competing interests exist
Acknowledgments
The authors would thank Candace J Rosen, Senior Policy and Advocacy Officer at PATH for providing details about rotavirus vaccine introductions in different countries.
References
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al, Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23 - 33; http://dx.doi.org/10.1056/NEJMoa052664; PMID: 16394299
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al, Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11 - 22; http://dx.doi.org/10.1056/NEJMoa052434; PMID: 16394298
- World Health Organization. Rotavirus vaccines. Wkly Epidemiol Rec 2007; 82:285 - 95; PMID: 17691162
- World Health Organization. Rotavirus vaccination. Wkly Epidemiol Rec 2009; 84:232 - 6
- Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, Akikusa JD, Kelly JJ, Kirkwood CD. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr Infect Dis J 2011; 30:Suppl S25 - 9; http://dx.doi.org/10.1097/INF.0b013e3181fefdee; PMID: 21183837
- Field EJ, Vally H, Grimwood K, Lambert SB. Pentavalent rotavirus vaccine and prevention of gastroenteritis hospitalizations in Australia. Pediatrics 2010; 126:e506 - 12; http://dx.doi.org/10.1542/peds.2010-0443; PMID: 20732946
- Paulke-Korinek M, Rendi-Wagner P, Kundi M, Kronik R, Kollaritsch H. Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in austrian children. Pediatr Infect Dis J 2010; 29:319 - 23; PMID: 19935446
- Hanquet G, Ducoffre G, Vergison A, Neels P, Sabbe M, Van Damme P, Van Herck K. Impact of rotavirus vaccination on laboratory confirmed cases in Belgium. Vaccine 2011; 29:4698 - 703; http://dx.doi.org/10.1016/j.vaccine.2011.04.098; PMID: 21571023
- Raes M, Strens D, Vergison A, Verghote M, Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J 2011; 30:e120 - 5; http://dx.doi.org/10.1097/INF.0b013e318214b811; PMID: 21436757
- Tate JE, Mutuc JD, Panozzo CA, Payne DC, Cortese MM, Cortes JE, Yen C, Esposito DH, Lopman BA, Patel MM, et al. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J 2011; 30:Suppl S30 - 4; http://dx.doi.org/10.1097/INF.0b013e3181ffe3eb; PMID: 21183838
- Gurgel RQ, Ilozue C, Correia JB, Centenari C, Oliveira SMT, Cuevas LE. Impact of rotavirus vaccination on diarrhoea mortality and hospital admissions in Brazil. Trop Med Int Health 2011; 16:1180 - 4; http://dx.doi.org/10.1111/j.1365-3156.2011.02844.x; PMID: 21749584
- do Carmo GMI, Yen C, Cortes J, Siqueira AA, de Oliveira WK, Cortez-Escalante JJ, Lopman B, Flannery B, de Oliveira LH, Carmo EH, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med 2011; 8:e1001024; http://dx.doi.org/10.1371/journal.pmed.1001024; PMID: 21526228
- Yen C, Armero Guardado JA, Alberto P, Rodriguez Araujo DS, Mena C, Cuellar E, Nolasco JB, De Oliveira LH, Pastor D, Tate JE, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J 2011; 30:Suppl S6 - 10; http://dx.doi.org/10.1097/INF.0b013e3181fefa05; PMID: 21048524
- de Palma O, Cruz L, Ramos H, de Baires A, Villatoro N, Pastor D, de Oliveira LH, Kerin T, Bowen M, Gentsch J, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ 2010; 340:c2825; http://dx.doi.org/10.1136/bmj.c2825; PMID: 20551120
- Quintanar-Solares M, Yen C, Richardson V, Esparza-Aguilar M, Parashar UD, Patel MM. Impact of rotavirus vaccination on diarrhea-related hospitalizations among children < 5 years of age in Mexico. Pediatr Infect Dis J 2011; 30:Suppl S11 - 5; http://dx.doi.org/10.1097/INF.0b013e3181fefb32; PMID: 21183834
- Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, Gonzalez A, Malespin O, Amador JJ, Umaña J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 2009; 301:2243 - 51; http://dx.doi.org/10.1001/jama.2009.756; PMID: 19491186
- Molto Y, Cortes JE, De Oliveira LH, Mike A, Solis I, Suman O, Coronado L, Patel MM, Parashar UD, Cortese MM. Reduction of diarrhea-associated hospitalizations among children aged < 5 Years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J 2011; 30:Suppl S16 - 20; http://dx.doi.org/10.1097/INF.0b013e3181fefc68; PMID: 21183835
- Patel MM, Glass R, Desai R, Tate JE, Parashar UD. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure?. Lancet Infect Dis 2012; 12:561 - 70; http://dx.doi.org/10.1016/S1473-3099(12)70029-4; PMID: 22742639
- Macartney KK, Porwal M, Dalton D, Cripps T, Maldigri T, Isaacs D, Kesson A. Decline in rotavirus hospitalisations following introduction of Australia’s national rotavirus immunisation programme. J Paediatr Child Health 2011; 47:266 - 70; http://dx.doi.org/10.1111/j.1440-1754.2010.01953.x; PMID: 21244557
- Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011; 204:980 - 6; http://dx.doi.org/10.1093/infdis/jir492; PMID: 21878425
- Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, Parashar U, Patel M. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med 2010; 362:299 - 305; http://dx.doi.org/10.1056/NEJMoa0905211; PMID: 20107215
- World Health Organization. Rotavirus vaccines. WHO position paper – January 2013. Wkly Epidemiol Rec 2013; 88:49 - 64; PMID: 23424730
- McKinsey & Co. Mapping influencers in the vaccine introduction decision-making process in developing countries. http://www.who.int/immunization/stakeholders/mapping_vaccine_decision_making_networks.pdf (accessed 9 july 2013). 22-3-2007.
- Makinen M, Kaddar M, Molldrem V, Wilson L. New vaccine adoption in lower-middle-income countries. Health Policy Plan 2012; 27:Suppl 2 ii39 - 49; http://dx.doi.org/10.1093/heapol/czs036; PMID: 22513731
- Nelson EA, Bresee JS, Parashar UD, Widdowson MA, Glass RI, Asian Rotavirus Surveillance Network. Rotavirus epidemiology: the Asian Rotavirus Surveillance Network. Vaccine 2008; 26:3192 - 6; http://dx.doi.org/10.1016/j.vaccine.2008.03.073; PMID: 18485546
- Bresee JS, Hummelman E, Nelson EA, Glass RI. Rotavirus in Asia: the value of surveillance for informing decisions about the introduction of new vaccines. J Infect Dis 2005; 192:Suppl 1 S1 - 5; http://dx.doi.org/10.1086/431515; PMID: 16088790
- Nelson EA, Widdowson MA, Kilgore PE, Steele D, Parashar UD. A decade of the Asian Rotavirus Surveillance Network: achievements and future directions. Vaccine 2009; 27:Suppl 5 F1 - 3; http://dx.doi.org/10.1016/j.vaccine.2009.09.001; PMID: 19931705
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, Teoh YL, Tang H, Boudville I, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 2009; 27:5936 - 41; http://dx.doi.org/10.1016/j.vaccine.2009.07.098; PMID: 19679216
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, van Doorn LJ, Teoh YL, Tang H, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: a randomized clinical trial in an Asian population. Vaccine 2012; 30:4552 - 7; http://dx.doi.org/10.1016/j.vaccine.2012.03.030; PMID: 22497874
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615 - 23; http://dx.doi.org/10.1016/S0140-6736(10)60755-6; PMID: 20692031
- Nelson EA, Sack D, Wolfson L, Walker DG, Seng LF, Steele D. Financing children’s vaccines. Vaccine 2009; 27:Suppl 5 F12 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.08.072; PMID: 19931710
- Jit M, Edmunds WJ. Evaluating rotavirus vaccination in England and Wales. Part II. The potential cost-effectiveness of vaccination. Vaccine 2007; 25:3971 - 9; http://dx.doi.org/10.1016/j.vaccine.2007.02.070; PMID: 17400341
- World Health Organization. Global Vaccine Action Plan. http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/index.html (accessed 1 May 2013).
- Deogaonkar R, Hutubessy R, van der Putten I, Evers S, Jit M. Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public Health 2012; 12:878; http://dx.doi.org/10.1186/1471-2458-12-878; PMID: 23072714
- Duclos P. National Immunization Technical Advisory Groups (NITAGs): guidance for their establishment and strengthening. Vaccine 2010; 28:Suppl 1 A18 - 25; http://dx.doi.org/10.1016/j.vaccine.2010.02.027; PMID: 20412991
- Gessner BD, Duclos P, Deroeck D, Nelson EA. Informing decision makers: experience and process of 15 National Immunization Technical Advisory Groups. Vaccine 2010; 28:Suppl 1 A1 - 5; http://dx.doi.org/10.1016/j.vaccine.2010.02.025; PMID: 20412988
- Nolan TM. The Australian model of immunization advice and vaccine funding. Vaccine 2010; 28:Suppl 1 A76 - 83; http://dx.doi.org/10.1016/j.vaccine.2010.02.038; PMID: 20413003
- Smith JC. The structure, role, and procedures of the U.S. Advisory Committee on Immunization Practices (ACIP). Vaccine 2010; 28:Suppl 1 A68 - 75; http://dx.doi.org/10.1016/j.vaccine.2010.02.037; PMID: 20413002
- CDC. Advisory Committee on Immunization Practices (ACIP). http://www.cdc.gov/vaccines/acip/committee/charter.html (accessed 3 July 2013).
- CDC. Vaccines for Children Program (VFC). http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html (accessed 3 July 2013).
- de Qadros CA, Carrasco P, Umstead W. EPI in the Americas Benefits for Revolving Fund. WHO Chron 1983; 37:8185
- DeRoeck D, Bawazir SA, Carrasco P, Kaddar M, Brooks A, Fitzsimmons J, Andrus J. Regional group purchasing of vaccines: review of the Pan American Health Organization EPI revolving fund and the Gulf Cooperation Council group purchasing program. Int J Health Plann Manage 2006; 21:23 - 43; http://dx.doi.org/10.1002/hpm.822; PMID: 16604847
- Freeman P.. The PAHO Revolving Fund: History Operations and Contributions to Speeding Vaccine Introductions. An Information Paper for the Children's Vaccine Initiative. 1999.
- PAHO. PAHO Revolving Fund. http://new.paho.org/hq/index.php?option=com_content&task=view&id=1864&Itemid=2234&lang=en (accessed 3 July 2013).
- Stéphenne J. Vaccines as a global imperative--a business perspective. Health Aff (Millwood) 2011; 30:1042 - 8; http://dx.doi.org/10.1377/hlthaff.2011.0338; PMID: 21653955
- UNICEF. UNICEF presents its Middle Income Country New Vaccine Procurement Strategy to SAGE. http://www.unicef.org/supply/index_66348.html. (accessed 3 July 2013).
- PATH. Page on Country Introductions of Rotavirus Vaccines. PATH Rotavirus Vaccine Access and Delivery Website. http://sites.path.org/rotavirusvaccine/rotavirus-advocacy-and-communications-toolkit/country-introduction-maps-and-list/ (accessed 11 July 2013).