Abstract
Potent vaccines require the ability to effectively induce immune responses. Especially for the control of infectious diseases with intracellular pathogens, like viruses or bacteria, potent T-cell responses are indispensable. Several delivery systems such as nanoparticles have been considered to boost the immunogenicity of pathogen derived peptides or subunits for the induction of potent T-cell responses. Since they can be further functionalized with immunostimulants, like Toll-like receptor (TLR) agonists, they improve the response by enhanced activation of the innate immune system. Currently, TLR agonists like unmethylated CpG oligonucleotides and the synthetic dsRNA derivate polyriboinosinic acid-polyribocytidylic acid (poly[I:C]) are widely used as vaccine adjuvants. CpG and poly(I:C) trigger different TLRs and therefore show differential signal transduction. Recently, we established biodegradable calcium phosphate (CaP) nanoparticles as potent T cell inducing vaccination vehicles. In this commentary we discuss the role of CpG and poly(I:C) for the effective induction of virus-specific T cells during immunization with CaP nanoparticles. The presented results underline the importance of the right formulation of vaccines for specific immunization purpose.
Introduction
Nanoparticles are widely used in modern medicine. The ability to reliably transport various types of molecules across cell barriers make nanoparticle-based systems very attractive for the use in drug delivery.Citation1 Since they mimic invading pathogens by their particulate structure they are successfully taken up by several cell types of the immune system. The possibility to combine nanoparticulate structure and immune stimulating molecules such as Toll-like receptor (TLR) agonists provides the opportunity to induce potent immune responses by adequate activation of innate and adaptive immunity. It was also observed, that the combination of both antigen and adjuvant within a particulate carrier can significantly reduce the dose of antigen and adjuvant which attenuates side effects.Citation2,Citation3
Antigen-presenting cells (APCs) such as dendritic cells (DCs) or macrophages are potential targets of functionalized nanoparticles due to their phagocytotic abilities. Especially DCs are in the focus of vaccine developments because of their unique antigen-presenting potential and the ability to induce cellular immune responses.Citation4 As many other APCs, DCs express a set of specialized receptors to recognize pathogen-associated molecular patterns (PAMP). These pattern recognition receptors (PRR) include the TLR family. Unmethylated CpG oligonucleotides (CpG) and the synthetic dsRNA derivate polyriboinosinic acid-polyribocytidylic acid (poly[I:C]) recognized by the endosomal TLRs 9 and 3, respectively, are two prominent TLR agonists frequently considered in the formulation of vaccines.Citation5-Citation7 While both of them are known to boost cellular and humoral immune responses, they induce different signal transduction pathways, and hence can lead to a differential maturation status of the TLR expressing cell.Citation8,Citation9 Due to this effect, it seems to be essential to choose the right agonist for individual purposes. Recently, we established biodegradable calcium phosphate (CaP) nanoparticles as potent vaccination vehicle which efficiently activates DCs by the delivery of a viral antigen in combination with CpG or poly(I:C).Citation10 We also demonstrated that immunization of mice with CaP nanoparticles functionalized with CpG and MHCI/MHCII restricted T-cell peptides from the hemagglutinin (HA) protein of the influenza virus induce potent CD4+ and CD8+ T-cell responses which mediated protection against influenza virus challenge.Citation11 However, from unpublished data we noticed that immunization with poly(I:C) functionalized nanoparticles encapsulating HA peptides was less efficient and resulted in lower numbers of virus-specific T cells. This data seems to underline the importance of an individual vaccine formulation to achieve the most efficient results. In this commentary we discuss our findings and point out the advantage of TLR 9 agonist CpG over TLR3 agonist poly(I:C) as boosting adjuvant using CaP nanoparticles.
Maturation of Dendritic Cells Induced by CpG- or poly(I:C)-Functionalized Nanoparticles
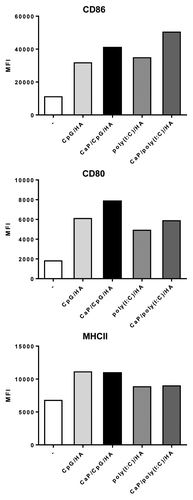
Several delivery systems for the application as new vaccine carriers are currently under investigation. The common purpose of these carriers is to enhance the adaptive immune response against pathogenic peptides or subunits. Vehicles such as nanoparticles reduce the degradation of these proteins and increase the uptake by APCs which is promoted by the pathogen-like nanoparticulate structure.Citation12 Immunization with pathogenic proteins or peptides alone is poorly immunogenic and often ends up in weak immune responses.Citation13 For the maturation of APCs additional signals are needed. TLR agonists have been shown to be powerful tools to induce effective maturation, thus boosting the adaptive immune response. Our group recently established biodegradable CaP nanoparticles as potent delivery vehicles for vaccination.Citation10,Citation11,Citation14-Citation16 These nanoparticles can be functionalized with oligonucleotide sequences such as TLR agonists. So far we tested the functionalization with TLR9 agonist CpG or TLR3 agonist poly(I:C), in combination with an MHCI/MHCII-restricted HA peptide. Previous studies demonstrated that for an efficient uptake by professional APCs such as DCs the optimal size of a particulate carrier should be between 200 and 300 nm.Citation17,Citation18 In fact, microscopic analysis after the preparation of CaP nanoparticles revealed a homogenous particle size of approximately 250 nm and a highly efficient uptake by DCs within a few hours.Citation10,Citation11,Citation14 TLR agonist-functionalized CaP nanoparticles efficiently induced the maturation of splenic DCs in vitro which was indicated by increased expression of costimulatory surface molecules, e.g., CD80, CD86, and MHCII molecules (). Interestingly, DCs treated with functionalized nanoparticles induced a higher expression of these molecules compared with the treatment with soluble agonists which indicates an enhanced uptake. Studies by Kerkman et al. and others also reported an increased uptake and enhanced immune activation when immunostimulatory molecules are given in a nanoparticulated form.Citation19,Citation20 As an effective transport across cell barriers enhances the maturation effect and at the same time reduces the risk of unwanted side effects these results underlined the ideal abilities of CaP nanoparticles as delivery vehicles. Importantly, we found no striking differences between the treatment with CpG- or poly(I:C)-functionalized nanoparticles regarding the activation and maturation of DCs. Thus, the fast and efficient uptake of functionalized CaP nanoparticles by DCs assures a quick response against delivered molecules.
Figure 1. CpG and poly(I:C)-dependent maturation of DCs in vitro. Primary DCs were incubated with CpG- or poly(I:C)-functionalized CaP nanoparticles or the indicated soluble factors. After 24 h cells were harvested and analyzed for the expressions of CD80, CD86, and MHCII by flow cytometry and demonstrated as mean fluorescence intensity (MFI).
CpG- or poly(I:C)-Functionalized CaP Nanoparticles Induce Differential Cytokine Expression
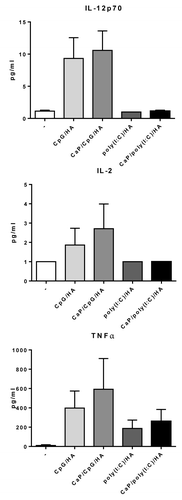
For an efficient activation of adaptive immune responses not only costimulatory signals but also the environmental settings which are mainly affected by secreted cytokines are important. Activation of TLR3 and TLR9 is associated with enhanced secretion of proinflammatory cytokines, whereas TLR3 mainly induces IFN type I and IFN β upon activation and TLR9 enhances TNF-α production. However, both induce IL-12 and IL-6 expression.Citation8,Citation9 To analyze whether CaP nanoparticles functionalized with CpG or poly(I:C) do induce similar cytokine profiles DCs were stimulated in vitro with functionalized nanoparticles or soluble factors.Citation10 Stimulation with CpG/HA peptide-functionalized CaP nanoparticles led to increased expression of IL-12, IL-2, and TNF-α (). In comparison, these cytokines were secreted in a lower extend when DCs were stimulated with poly(I:C)/HA peptide-functionalized CaP nanoparticles. Studies suggested that particle size can have an influence on cytokine production and therefore on the induction of TH1 or TH2 driven responses.Citation21 They found that particles <100 nm induce TH1 responses and particles >100 nm are advantageous for the induction of a TH2 response in vivo. Interestingly, CaP nanoparticles with a size of 250 nm mainly induce IL-12, IL-2, and TNF-α, classical TH1 cytokines which promote cytotoxic T lymphocyte (CTL) immunity. These results suggest that the combination of the nanoparticulate carrier and adjuvant orchestrate the type of immune response during vaccination.
Figure 2. CpG and poly(I:C)-dependent secretion of cytokines. Primary DCs were incubated with CpG- or poly(I:C)-functionalized CaP nanoparticles or the indicated soluble factors. After 24 h supernatants were analyzed for the concentrations of IL-12p70, IL-2, and TNF-α. Results are shown as mean ± SEM in pg/mL.
Immunization with CpG-functionalized CaP Nanoparticles Induce Higher Virus-Specific T-Cell Numbers
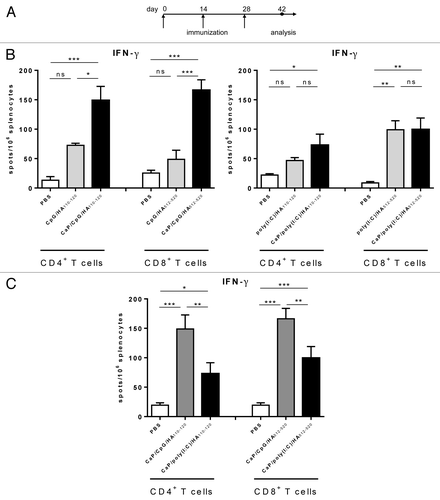
Protection against viral infection is mediated by long lasting virus specific immune responses. Especially for the control of intracellular pathogens a strong TH1 and CTL response is important to avoid viral spreading during infection.Citation22–Citation24 Both, CpG and poly(I:C) have been shown to promote the development of TH1 and CTL responses.Citation25,Citation26 Hafner et al. proposed that due to the different expression patterns of e.g., TLR3 and TLR9 also the choice of TLR agonist as adjuvant can be critical for the induction of an appropriate immune response.Citation27 In a vaccination trial against human papillomavirus poly(I:C) was found to be more potent in the induction of virus-specific TH1 cells.Citation7 Interestingly, in our system we observed differences in the number of virus-specific CD4+ and CD8+ T cells post immunization of mice with CpG/HA- or poly(I:C)-functionalized CaP nanoparticles. Mice were immunized three times via intraperitoneal injection and analyzed two weeks after the final boost immunization (). In general, virus-specific T-cell numbers were significantly elevated after nanoparticle-based immunization compared with the results from mice which were immunized with PBS or soluble CpG and HA peptides which led to a strong protection against influenza virus infection ().Citation11 However, when we compared the efficiency of CpG and poly(I:C)-functionalized nanoparticles for immunization we observed a significant lower frequency of virus-specific CD4+ and CD8+ T cells after immunization with poly(I:C)-functionalized nanoparticles () suggesting CpG as the optimal adjuvant for pathogen-specific T-cell expansion. Although we could not detect any differences in the expression of costimulatory molecules when we stimulated DCs with CpG- and poly(I:C)-functionalized nanoparticles, the differential and higher TH1-proned cytokine expression (IL-12, IL-2, or TNF-α) after CpG exposure could be one reason for the increased expansion of virus-specific T cells underlining the important role of the cytokine environment.
Figure 3. CpG- and poly(I:C)-dependent induction of pathogen-specific T cells. (A) BALB/c mice were immunized three times i.p. with CaP nanoparticles functionalized with CpG/HA peptides, poly(I:C)/HA peptides, or soluble CpG / poly(I:C) and HA peptides. (B,C) Two weeks after the last immunization mice were sacrificed and splenocytes were restimulated in vitro HA peptides. After 24 h, the numbers of IFN-γ-producing CD4+ and CD8+ T cells were determined by ELISPOT. Spots were counted and expressed as spots per splenocyte. Bars represent mean ± SEM. *P < 0.05; *** P < 0.001; ns, not significant.
Conclusion
The induction of a potent long lasting immune response is the defined goal in vaccine development. Certainly, a variety of vaccine vehicles have been shown to induce pathogen-specific immune responses that differ in their efficiency. Cytokines are thought to be a major player during priming of immune responses but also the effective delivery of adjuvants and antigens is crucial. We demonstrated that biodegradable CaP nanoparticles serve as excellent delivery vehicles for vaccines consisting of pathogenic peptides and adjuvants in kind of TLR agonists CpG and poly(I:C). The immunization with functionalized CaP nanoparticles leads to an activation and maturation of DCs accompanied with the expansion of pathogen-specific CD4+ and CD8+ T cells. However, we demonstrated significant differences in the ability to induce cytokines and expand pathogen-specific T cells when we compared CpG- and poly(I:C)-functionalized CaP nanoparticles. In our experimental setting CpG-functionalized CaP nanoparticles induced a much better T-cell response which is essential for the protection against intracellular pathogens. This leads to the conclusion that not only the adjuvant but also the delivery system dedicates the efficiency of an immunization strategy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine 2008; 3:133 - 49; http://dx.doi.org/10.2147/IJN.S596; PMID: 18686775
- Diwan M, Elamanchili P, Cao M, Samuel J. Dose sparing of CpG oligodeoxynucleotide vaccine adjuvants by nanoparticle delivery. Curr Drug Deliv 2004; 1:405 - 12; http://dx.doi.org/10.2174/1567201043334597; PMID: 16305402
- Wilson KD, de Jong SD, Tam YK. Lipid-based delivery of CpG oligonucleotides enhances immunotherapeutic efficacy. Adv Drug Deliv Rev 2009; 61:233 - 42; http://dx.doi.org/10.1016/j.addr.2008.12.014; PMID: 19232375
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18:767 - 811; http://dx.doi.org/10.1146/annurev.immunol.18.1.767; PMID: 10837075
- Klinman DM, Ishii KJ, Gursel M, Gursel I, Takeshita S, Takeshita F. Immunotherapeutic applications of CpG-containing oligodeoxynucleotides. Drug News Perspect 2000; 13:289 - 96; PMID: 12937643
- Klinman DM, Klaschik S, Sato T, Tross D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv Drug Deliv Rev 2009; 61:248 - 55; http://dx.doi.org/10.1016/j.addr.2008.12.012; PMID: 19272313
- Stahl-Hennig C, Eisenblätter M, Jasny E, Rzehak T, Tenner-Racz K, Trumpfheller C, Salazar AM, Uberla K, Nieto K, Kleinschmidt J, et al. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog 2009; 5:e1000373; http://dx.doi.org/10.1371/journal.ppat.1000373; PMID: 19360120
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature 2006; 442:39 - 44; http://dx.doi.org/10.1038/nature04946; PMID: 16823444
- Robbins MA, Rossi JJ. Sensing the danger in RNA. Nat Med 2005; 11:250 - 1; http://dx.doi.org/10.1038/nm0305-250; PMID: 15746933
- Sokolova V, Knuschke T, Kovtun A, Buer J, Epple M, Westendorf AM. The use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation. Biomaterials 2010; 31:5627 - 33; http://dx.doi.org/10.1016/j.biomaterials.2010.03.067; PMID: 20417963
- Knuschke T, Sokolova V, Rotan O, Wadwa M, Tenbusch M, Hansen W, Staeheli P, Epple M, Buer J, Westendorf AM. Immunization with biodegradable nanoparticles efficiently induces cellular immunity and protects against influenza virus infection. J Immunol 2013; 190:6221 - 9; http://dx.doi.org/10.4049/jimmunol.1202654; PMID: 23667109
- Schijns VE, Lavelle EC. Trends in vaccine adjuvants. Expert Rev Vaccines 2011; 10:539 - 50; http://dx.doi.org/10.1586/erv.11.21; PMID: 21506650
- Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW. Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int J Pharm 2008; 364:272 - 80; http://dx.doi.org/10.1016/j.ijpharm.2008.04.036; PMID: 18555624
- Kozlova D, Chernousova S, Knuschke T, Buer J, Westendorf AM, Epple M. Cell targeting by antibody-functionalized calcium phosphate nanoparticles. J Mater Chem 2012; 22:396 - 404; http://dx.doi.org/10.1039/c1jm14683a
- Sokolova V, Knuschke T, Buer J, Westendorf AM, Epple M. Quantitative determination of the composition of multi-shell calcium phosphate-oligonucleotide nanoparticles and their application for the activation of dendritic cells. Acta Biomater 2011; 7:4029 - 36; http://dx.doi.org/10.1016/j.actbio.2011.07.010; PMID: 21784177
- Sokolova V, Kozlova D, Knuschke T, Buer J, Westendorf AM, Epple M. Mechanism of the uptake of cationic and anionic calcium phosphate nanoparticles by cells. Acta Biomater 2013; 9:7527 - 35; http://dx.doi.org/10.1016/j.actbio.2013.02.034; PMID: 23454056
- Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm 2005; 298:315 - 22; http://dx.doi.org/10.1016/j.ijpharm.2005.03.035; PMID: 15961266
- Zwiorek K, Bourquin C, Battiany J, Winter G, Endres S, Hartmann G, Coester C. Delivery by cationic gelatin nanoparticles strongly increases the immunostimulatory effects of CpG oligonucleotides. Pharm Res 2008; 25:551 - 62; http://dx.doi.org/10.1007/s11095-007-9410-5; PMID: 17912489
- Kerkmann M, Lochmann D, Weyermann J, Marschner A, Poeck H, Wagner M, Battiany J, Zimmer A, Endres S, Hartmann G. Immunostimulatory properties of CpG-oligonucleotides are enhanced by the use of protamine nanoparticles. Oligonucleotides 2006; 16:313 - 22; http://dx.doi.org/10.1089/oli.2006.16.313; PMID: 17155907
- Zolnik BS, González-Fernández A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology 2010; 151:458 - 65; http://dx.doi.org/10.1210/en.2009-1082; PMID: 20016026
- Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm 2007; 4:73 - 84; http://dx.doi.org/10.1021/mp060096p; PMID: 17274665
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature 1996; 383:787 - 93; http://dx.doi.org/10.1038/383787a0; PMID: 8893001
- Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol 2006; 177:2888 - 98; http://dx.doi.org/10.1038/383787a0; PMID: 8893001
- Papi A, Stanciu LA, Papadopoulos NG, Teran LM, Holgate ST, Johnston SL. Rhinovirus infection induces major histocompatibility complex class I and costimulatory molecule upregulation on respiratory epithelial cells. J Infect Dis 2000; 181:1780 - 4; http://dx.doi.org/10.1086/315463; PMID: 10823784
- de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, Yazdanbakhsh M, Kapsenberg ML. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. J Immunol 2002; 168:1704 - 9; PMID: 11823500
- Roman M, Martin-Orozco E, Goodman JS, Nguyen MD, Sato Y, Ronaghy A, Kornbluth RS, Richman DD, Carson DA, Raz E. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med 1997; 3:849 - 54; http://dx.doi.org/10.1038/nm0897-849; PMID: 9256274
- Hafner AM, Corthésy B, Merkle HP. Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Adv Drug Deliv Rev 2013; http://dx.doi.org/10.1016/j.addr.2013.05.013; PMID: 23751781
