Abstract
Background: Rabies is fatal in nearly 100% of cases, making post-exposure prophylaxis (PEP) a required measure for preventing mortality. Currently, the rabies vaccination regimen requires at least three to five clinic visits, with vaccination and transportation costs being very high. This study assessed the safety and efficacy of the 2-1 intramuscular (IM) regimen for rabies immunization with the goal of making rabies prophylaxis more economical.
Methods: One-hundred and eighty-one subjects were divided into two groups: 79 subjects in test group A and 102 subjects in control group B. 2-1 IM regimen was chosen for group A and the Essen regimen was adopted for group B. Serum samples were also collected at D0, D7, D14, D45, D180, and D360 to determine the rabies serum neutralizing antibody by rapid luorescent focus inhibition test (RFFIT).
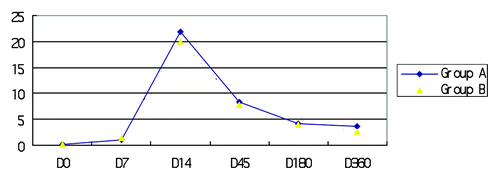
Results: There was no significant difference between groups A and B with respect to the rate of adverse events following each vaccination. Nine-hundred and nineteen blood samples were obtained. At D0 (prior to immunization), all study subjects exhibited a geometric mean titer (GMT) <0.05 IU/ml. On D14, all study subjects exhibited NAb titers >0.5 IU/ml; titers above 0.5 IU/ml were maintained in both groups through D45 and D180 before gradually declining. The percentage of subjects positive for NAbs in group A and group B on D7 were 88.6% and 87.3%, respectively, which was not statistically different (P = 0.545). On D360, the percentage of subjects positive for NAbs in group A and group B were 93.9% and 100% (P < 0.01), respectively. During the study, the GMT was highest for both groups on D14 (21.90 IU/ml, group A; 19.93 IU/ml, group B) (P = 0.045). On D45, the GMTs were 8.28 IU/ml (group A) and 7.89 IU/ml (group B) (P = 0.037). On D7, D180, and D360, there were no statistically significant differences between the two groups with respect to the GMT.
Conclusions: The 2-1 IM regimen demonstrates the same safety and efficacy as the Essen regimen. The use of the 2-1 IM regimen could not only reduce the personal economic burdens of rabies immunization but also improve rabies immunization rates through fewer office visits and compliance with immunization procedures. However, further evaluation is needed before a major recommendation can be made.
Introduction
Rabies is an acute infectious disease with a mortality rate of nearly 100%, which results in almost 55 000 deaths per year with more than 95% of these deaths occurring in Asia and Africa. New rabies cases arise most frequently in India, followed by China.Citation1 In China, over 2000 new cases of rabies have occurred annually in recent years, and rabies has become a serious public health problem.Citation2 Prompt post-exposure prophylaxis (PEP) is the most important measure for preventing and controlling rabies infections. For people exposed to animals carrying the rabies virus, PEP consists of wound cleansing, rabies vaccination, and passive immunization with rabies immune globulin (RIG) if necessary.Citation3 Throughout the world, approximately 150 million individuals receive PEP each year, with India and China accounting for the largest numbers of these immunizations.Citation1 In China, approximately 12–15 million people receive immunization after exposure to rabies each year, which causes enormous economic and social burdens, including direct economic costs of $45–50 million and indirect economic costs that cannot be readily estimated.Citation4
Since the development of the human diploid cell vaccine (HDCV) for rabies in the 1960s,Citation5 countries around the world have engaged in research and development initiatives aimed at developing vaccines with lower production costs than the HDCV but with similar safety and efficacy profiles. Currently, the World Health Organization (WHO) has recommended the use of cell culture vaccines for rabies immunization. Given the continuing development of modern vaccine production techniques and ongoing improvements in vaccine quality control standards, researchers from various countries continue to investigate new methods for simplifying rabies vaccine immunization regimens. In 2010, the WHO recommended the following four types of PEP regimensCitation1: (1) The Essen regimen of five intramuscular (IM) injections; (2) the 2-1-1 regimen; (3) the four-dose regimen; and (4) the four-site regimen of intradermal injections. The 2-1-1 regimen was first examined by Vodopija in 1986 and was recommended by the WHO in 1992Citation6,Citation7; many clinical studies have demonstrated that this regimen is safe and generates robust antibody responses.Citation8-Citation11 The Essen regimen for PEP was the only regimen used in China until 2010 when the State Food and Drug Administration (now known as the China Food and Drug Administration) first approved the use of the 2-1-1 regimen. In 2008, our research group examined the immunogenicity of a Chinese Vero cell-derived rabies vaccine using the 2-1-1 regimen.Citation12 The clinical results indicated that, consistent with findings regarding the 2-1-1 regimen from prior research results in other countries, the induced neutralizing antibody (NAb) concentrations satisfied the standards established by the WHO by 14 d after immunization. In particular, the antibody titers reached the protective level of 0.5 IU/ml, as determined by the rapid fluorescent focus inhibition test (RFFIT) or the fluorescent antibody virus neutralization (FAVN) assay.Citation13 At this time point during the vaccination regimen, the third injection of the immunization had not yet been performed. Therefore, we speculate that if the third injection of the 2-1-1 regimen were eliminated, thereby simplifying this procedure to a 2-1 regimen, the NAb titers may still reach ≥0.5 IU/ml as determined by the RFFIT. Because the 2-1 regimen requires only two clinic visits and three injections, the use of this regimen could significantly reduce rabies vaccination costs and improve compliance. Therefore, in this study, to provide a basis for future investigations into a more economical regimen, we inoculated volunteers with purified Vero cell rabies vaccine (PVRV) manufactured in China to evaluate the immunogenicity and safety of the 2-1 IM regimen. Moreover, the results of this regimen were compared with the Essen regimen.
Results
Basic characteristics of the research subjects
A total of 181 subjects were the veterinary school students and were divided into two groups by using cluster sampling random method. The age distributions of the subjects in the two groups were relatively similar. Males were 34% of group A and 44% of group B (). Most of the research subjects were able to complete the rabies vaccinations and safety observations specified by each immunization regimen.
Table 1. Basic demographic characteristics of the study subjects
Safety observations
No subjects exhibited immediate reactions within the 30 min following their vaccinations. Certain subjects experienced local and systemic reactions at 72 h after inoculation; these reactions were generally minor and primarily consisted of itchiness and pain at the inoculation sites. Several subjects exhibited inflammation, scleroma, and/or lymph node swelling. In addition, after the first inoculation, a small number of subjects experienced transient increases in body temperature (to 37.4–38.1 °C), fatigue, and/or headache. After 72 h of observation, the aforementioned symptoms all spontaneously subsided without requiring medical treatment. During the entire observation period, no abnormal reactions or moderate to severe adverse reactions were observed. There was no significant difference between groups A and B with respect to the rate of adverse events after each vaccination ().
Table 2. Adverse reactions among study subjects
Results of the rabies NAb assays
A total of 1086 blood sampling events were scheduled during the course of the study; 919 blood samples were obtained as planned, whereas 167 blood samples were not obtained. Samples were unable to be obtained primarily because of poor compliance by the control group at D180 and D360 during the follow-up period. The NAb titer was detected using the RFFIT method. At D0 prior to immunization, all study subjects exhibited geometric mean titers (GMTs) <0.05 IU/ml, indicating that none of these subjects had previously been inoculated against rabies. At D14 after the immunization, all study subjects exhibited NAb titers >0.5 IU/ml; titers above 0.5 IU/ml were maintained in all subjects through D45 and D180 and then gradually declined. The percentage of patients positive for NAbs in group A and group B on D7 was 88.6% and 87.3%, respectively; the difference between these percentages was not statistically significant (P = 0.545) (). On D360, the percentages of patients positive for NAbs in group A and group B were 93.9% and 100%, respectively; the difference between these percentages was statistically significant (P < 0.01). During the examined period, the GMT was the highest for both groups on D14 (). Specifically, the GMTs for group A and group B were 21.90 IU/ml and 19.93 IU/ml, respectively; the difference between these GMT values was statistically significant (P = 0.045). On D45, the GMTs in group A and group B were 8.28 IU/ml and 7.89 IU/ml, respectively, and the difference in the GMT values between these groups was again statistically significant (P = 0.037). On D7, D180, and D360, there were no statistically significant differences between the two groups with respect to the GMT (P > 0.05) ().
Table 3. A comparison of the neutralizing antibody levels between the two groups
Discussion
In 2010, the following four PEP regimens were recommended by the WHO: the Essen IM regimen, a five-dose regimen that involves the administration of one dose of the vaccine on D0, D3, D7, D14, and D28; the 2-1-1 regimen, a four-dose regimen in which two doses of vaccine are administered on D0 (one dose at each of the two deltoids or the two thighs) followed by one dose of vaccine on D7 and D21; a new four-dose regimen in which four doses of vaccine are administered intramuscularly on D0, D3, D7, and D14; and a two-site intradermal (ID) regimen in which injections of 0.1 ml at two sites (deltoid and thigh) are prescribed on D0, D3, D7, and D28. These recommendations reflect certain adjustments to the 4 immunization regimens recommended by the WHO in 2007. In particular, a new four-dose regimen was added in 2010. In 2009, experts organized by the American Advisory Committee on Immunization Practices demonstrated that a four-dose regimen, which removed the D28 injection of the Essen regimen, was sufficient for NAb titers to reach effective levels of protection.Citation14 This four-dose regimen is currently used in the US and results in an annual savings of $1.66 million compared with the Essen regimen. In addition, in 2010, the WHO removed the existing eight-site ID injection regimen from its list of recommended regimens. This eight-site regimen involved more injections and clinic visits than the two-site regimen.Citation15,Citation16 The aforementioned adjustments in immunization recommendations by the WHO suggested that rabies immunization regimens will gradually be simplified as rabies vaccine production processes and vaccine efficacy continues to be improved in various countries. Nevertheless, the current immunization regimens recommended by the WHO require at least 3 to 5 clinic visits and 4 to 8 injections that must be completed within 14–28 d, which is relatively costly (). To a certain degree, high costs impact vaccination rates and compliance following exposure to rabies, particularly in economically underdeveloped areas that lack convenient methods of transportation.Citation17,Citation18
Table 4. A comparison of several different rabies vaccination regimens
In recent years, the Thai Red Cross Society has conducted new investigations addressing rabies immunization regimens.Citation19 In particular, this organization has examined an immunization regimen involving 0.1-ml ID vaccine injections near the left and right deltoid and the right and left anterolateral thigh muscle. These injections are completed within one week (the one-week ID schedule) on D0, D3, and D7. This approach is also referred to as the “4-4-4 schedule.” The results from investigations of this method have indicated that, similarly to other WHO-recommended PEP regimens, the one-week ID schedule produces sufficient immunological responses, with NAb titers >0.5 IU/ml at 14 and 28 d after immunization. The advantage of the one-week ID schedule is that it can be completed within seven days; however, the disadvantages of this approach are that four sites are injected during each treatment session for a total of 12 injections. However, this one-week ID schedule should be an excellent complement to previously established ID regimens.
Currently, China only uses the Essen and 2-1-1 intramuscular injection regimens, perhaps because intradermal injections require higher technical skills to perform and production of the Chinese rabies vaccine is sufficient to meet the demands. Therefore, manufacturers and clinicians generally are not interested in intradermal injections.
In this study, the RFFIT method was used to test serum samples. Samples that tested positive according to the WHO criteria had serum NAb titers ≥0.5 IU/ml. No significant difference was observed between group A and group B on D7 in terms of seroconversion, indicating that both the 2-1 and Essen regimens may rapidly induce NAb production. Age may have biased these findings because all study subjects were young adults between 18 and 26 y of age. At D14, all subjects exhibited NAb titers that were greater than 0.5 IU/ml, and the GMTs for group A and group B reached 21.90 IU/ml and 19.93 IU/ml, respectively; the difference between these two groups was statistically significant. The results suggest that the injection of a double dose on D0 and a single dose of vaccine on D7 induces equal or better early immunological efficacy than one dose of vaccine on D0, D3, and D7. These results are similar to the findings of other studies.Citation12 Moreover, a significant difference in NAb titers was observed between group A and group B on D14 and D45, perhaps indicating that it is provide additional doses on D14 and D28, respectively, under ESSEN regimen. High NAb levels persist in the 2-1 treatment group until D45 and D180 (100% of subjects had NAb titers >0.5 IU/ml). These immunogenicity data are similar to those observed in the control group, which received a five-injection regimen. On D360, the differences between the two groups with respect to rates of positive NAb results were statistically significant. This may be due to insufficient D360 samples from subjects who received the five-injection regimen; alternatively, this result may indicate that the long-term persistence of immunity was slightly reduced following the 2-1 regimen compared with the five-injection regimen. Therefore, clinical research is needed to compare these regimens.
In terms of safety, the study data demonstrated that there were no significant differences between the two groups with respect to the rates of adverse events. The observed local adverse events and systemic reactions were mild, and no drug treatment was required to treat any of these events. Thus, the tested regimens are generally safe.
However, this study did have several limitations. First, no control group included vaccine combined with RIG. However, according to the “4-4-4” research results, there was no significant suppressive effect due to the four-site intradermal regimen when used with RIG. Second, all of the study subjects were young adults between 18 and 26 y of age; thus, no research data from other age groups were obtained.
In summary, the advantages of the 2-1 regimen are evident, and this regimen demonstrates the same safety and immunogenicity profile as the control five-injection regimen. Moreover, the 2-1 regimen is more convenient and simple than the other immunization regimens currently being used in various countries. The complete immunization process requires only two clinic visits and is completed within one week. In addition, relative to the other available treatments, patient distress is minimized by the 2-1 regimen, which utilizes only three injection sites during the entire regimen. Thus, this regimen may also reduce the probability of developing adverse reactions following vaccination. Based on calculations of the direct and indirect costs of rabies immunization in China, the five-injection regimen costs $112 per patient, whereas the 2-1-1 and 2-1 regimens cost $80 and $52 per patient, respectively. Currently, an average of ~14 million patients receive rabies immunizations each year in China. Therefore, adopting the 2-1 procedure could save approximately $316 million plus the cost of clinic visits.Citation20 Otherwise, the 2-1-1 regimen is much simpler than the Essen regimen. Because the RIG cost is high (average $190–320 per patient), no matter whether 2-1-1 or the Essen regimen is administered with RIG, it rarely can be accepted by patients. If vaccination costs can be reduced more, the acceptability of the RIG should be improved. The use of the 2-1 regimen could not only reduce the personal economic burden of rabies immunization but also improve rabies immunization rates and patient compliance with the immunization procedures. However, because we have only described a preliminary study, it is necessary to continue to assess the immunological efficacy of the 2-1 IM regimen before making a recommendation to change the current immunization protocols.
Materials and Methods
This study was conducted in immunization clinics of the Guangzhou Center for Disease Control and Prevention from June 2010 to December 2011. Guangzhou, located in the Tropic of Cancer, is the largest trading city in southern China.Citation21 This research was approved by the medical ethics committee of the Guangzhou Center for Disease Control and Prevention, and each volunteer for this study signed an informed consent form.
Vaccines
Purified Vero cell rabies vaccines were produced in China by Liaoning Cheng Da Biotechnology. These vaccines were produced by inoculating the Pasteur PV-2061-fixed rabies virus into Vero cells in a bioreactor. The vaccine potency was 5.5 IU/dose. The vaccine used in this study originated from normal commercial circulation and were produced in the same lot (20101001-1).
Research subjects
A total of 181 research subjects participated in this study. All subjects were healthy volunteers who provided informed consent and were required to satisfy strict screening criteria. These screening criteria included the absence of fever, alcoholism, pregnancy, diabetes, anemia, kidney disease, liver disease, or other chronic diseases; no blood sampling difficulties; and no history of rabies vaccination. In addition, subjects were excluded from the study if they had been treated with immunization inhibitors (such as various hormones and anti-cancer drugs), had been inoculated with other vaccines, or had received passive immunization within the previous month. Subjects were also required to be capable of complying with a one-year follow-up schedule for inoculation, blood sampling, and safety observations.
Experimental design
Group A, the experimental group, included 79 subjects (27 males and 52 females; subjects were between 19 and 23 y of age). All study subjects were the veterinary school students, who were divided into two groups according to the class by using cluster sampling random method. The 2-1 regimen was used to vaccinate these subjects. On D0, the subjects received two intramuscular injections, with one dose of the vaccine injected into the deltoid muscle of each upper arm; on D7, one intramuscular injection was administered into the deltoid muscle of an upper arm. Thus, these subjects received a total of three doses of the vaccine.
Group B, the control group, included 102 subjects (45 males and 57 females; subjects were between 18 and 26 y of age). The five-injection Essen regimen was used to vaccinate these subjects. Thus, one dose of the vaccine was injected into a deltoid muscle of an upper arm on D0, D3, D7, D14, and D28. These subjects received a total of five doses of the vaccine.
Neither group of subjects received injections of rabies immunoglobulin.
Safety observation
Prior to each vaccination, the subject’s body temperature was measured. Immediate reactions within 30 min following the vaccination and local or systemic reactions during the first 72 h post-immunization were monitored and evaluated in accordance with the “Clinical Study Guidelines for Adverse Effects Evaluation of Prophylactic Vaccine” (Chinese State Food and Drug Administration, 2005). Fevers were graded as follows: mild (37.1–37.5 °C), moderate (37.6–39 °C), and severe (>39 °C). The severity of the adverse effects was graded as follows: mild, with transient (<48 h) discomfort requiring no medical care; moderate, restricting daily activities but requiring no or minimal medical intervention; severe, seriously restricting daily activities and requiring routine care, medical care or even hospitalization; and life-threatening, extremely restricting daily activities and requiring specific care, medical treatment, and hospitalization.
Blood sampling times and serum NAb assays
Blood sampling occurred prior to immunization on D0 and after the immunizations on D7, D14, D45, D180, and D360. Rabies NAb titers in the sera were measured under masked conditions using the RFFIT,Citation22,Citation23 which was performed at the Institute for Viral Disease Control and Prevention of the Chinese Center for Disease Control and Prevention. According to WHO recommendations (WHO, 1992), subjects were considered to have seroconverted for rabies NAbs if they achieved a RFFIT titer ≥0.5 IU/ml.
Statistical analysis
This research was a randomized, single-center study, and the analyst was single-blinded. The SPSS 11.5 statistical software package was used for all analysis. The GMTs of NAbs for the two groups were compared using t tests with P < 0.05 as the threshold for statistical significance. Based on the WHO standard, a rabies NAb titer of ≥0.5 IU/ml was regarded as a positive immunization result. Chi-square tests were used for comparisons between the two groups with respect to the rates of adverse events and positive NAb results after immunization.
Disclosure of Potential Conflicts of Interest
This work was supported by grants from the Department of Health of Guangdong (A2013540), Guangdong Provincial Department of Science and Technology (2012B091100045) and the Science and Information Technology of Guangzhou (2012J5100005). The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
References
- WHO Publication. Rabies vaccines: WHO position paper--recommendations. Vaccine 2010; 28:7140 - 2; http://dx.doi.org/10.1016/j.vaccine.2010.08.082; PMID: 20831913
- Song M, Tang Q, Wang DM, Mo ZJ, Guo SH, Li H, Tao XY, Rupprecht CE, Feng ZJ, Liang GD. Epidemiological investigations of human rabies in China. BMC Infect Dis 2009; 9:210; http://dx.doi.org/10.1186/1471-2334-9-210; PMID: 20025742
- WHO Expert Consultation on rabies. World Health Organ Tech Rep Ser 2005; 931:1 - 88; PMID: 16485446
- [In Editor.] China Rabies Control Situation. China Animal Husbandry and Veterinary Medicine 2009; 36:144 - 6
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 1961; 25:585 - 621; http://dx.doi.org/10.1016/0014-4827(61)90192-6; PMID: 13905658
- Vodopija I, Sureau P, Lafon M, Baklaic Z, Ljubicić M, Svjetlicić M, Smerdel S. An evaluation of second generation tissue culture rabies vaccines for use in man: a four-vaccine comparative immunogenicity study using a pre-exposure vaccination schedule and an abbreviated 2-1-1 postexposure schedule. Vaccine 1986; 4:245 - 8; http://dx.doi.org/10.1016/0264-410X(86)90138-6; PMID: 3541428
- Vodopija I. Current issues in human rabies immunization. Rev Infect Dis 1988; 10:Suppl 4 S758 - 63; http://dx.doi.org/10.1093/clinids/10.Supplement_4.S758; PMID: 3060962
- Lang J, Simanjuntak GH, Soerjosembodo S, Koesharyono C. Suppressant effect of human or equine rabies immunoglobulins on the immunogenicity of post-exposure rabies vaccination under the 2-1-1 regimen: a field trial in Indonesia. MAS054 Clinical Investigator Group. Bull World Health Organ 1998; 76:491 - 5; PMID: 9868840
- Colnot F, Sureau P, Alexandre JL, Arnaudo JP, Hesse JY, Jeanmaire H. [Post-exposure antirabies vaccination. Early serological response to vaccine cultivated on VERO cells using a reduced 2-1-1 schedule]. Presse Med 1994; 23:1609 - 12; PMID: 7831241
- Chutivongse S, Wilde H, Fishbein DB, Baer GM, Hemachudha T. One-year study of the 2-1-1 intramuscular postexposure rabies vaccine regimen in 100 severely exposed Thai patients using rabies immune globulin and Vero cell rabies vaccine. Vaccine 1991; 9:573 - 6; http://dx.doi.org/10.1016/0264-410X(91)90244-Z; PMID: 1771970
- Vodopija R, Lafont M, Baklaić Z, Ljubicić M, Svjetlicić M, Vodopija I. Persistence of humoral immunity to rabies 1100 days after immunization and effect of a single booster dose of rabies vaccine. Vaccine 1997; 15:571 - 4; http://dx.doi.org/10.1016/S0264-410X(97)00207-7; PMID: 9160527
- Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, Cao Q, Liu B, Pan H, Wang M. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin 2011; 7:220 - 4; http://dx.doi.org/10.4161/hv.7.2.14003; PMID: 21311216
- Rabies vaccines. WHO position paper. Wkly Epidemiol Rec 2007; 82:425 - 35; PMID: 18064757
- Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett S, Levis R, Meltzer MI, Schaffner W, et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine 2009; 27:7141 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.09.029; PMID: 19925944
- Warrell MJ, Warrell DA, Suntharasamai P, Viravan C, Sinhaseni A, Udomsakdi D, Phanfung R, Xueref C, Vincent-Falquet JC, Nicholson KG, et al. An economical regimen of human diploid cell strain anti-rabies vaccine for post-exposure prophylaxis. Lancet 1983; 2:301 - 4; http://dx.doi.org/10.1016/S0140-6736(83)90288-X; PMID: 6135830
- Warrell MJ, Suntharasamai P, Nicholson KG, Warrell DA, Chanthavanich P, Viravan C, Sinhaseni A, Phanfung R, Xueref C, Vincent-Falquet JC. Multi-site intradermal and multi-site subcutaneous rabies vaccination: improved economical regimens. Lancet 1984; 1:874 - 6; http://dx.doi.org/10.1016/S0140-6736(84)91340-0; PMID: 6143187
- Dodet B, Asian Rabies Expert Bureau. Preventing the incurable: Asian rabies experts advocate rabies control. Vaccine 2006; 24:3045 - 9; http://dx.doi.org/10.1016/j.vaccine.2005.10.015; PMID: 16652450
- Hampson K, Cleaveland S, Briggs D. Evaluation of cost-effective strategies for rabies post-exposure vaccination in low-income countries. PLoS Negl Trop Dis 2011; 5:e982; http://dx.doi.org/10.1371/journal.pntd.0000982; PMID: 21408121
- Shantavasinkul P, Tantawichien T, Wilde H, Sawangvaree A, Kumchat A, Ruksaket N, Lohsoonthorn V, Khawplod P, Tantawichien T. Postexposure rabies prophylaxis completed in 1 week: preliminary study. Clin Infect Dis 2010; 50:56 - 60; http://dx.doi.org/10.1086/649211; PMID: 19995217
- Chuan-lin W, Xiao-wei Z, Yong-xin Y. Study on the Compliance and Economic Cost of Rabies Vaccination. Chinese Journal of Vaccines and Immunization 2010; 3:254 - 7
- Fu C, He Q, Xu J, Xie H, Ding P, Hu W, Dong Z, Liu X, Wang M. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine 2012; 31:154 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.10.078; PMID: 23127516
- Yu PC, Lv XJ, Shen XX, Cao L, Zheng XX, Shan H, Tang Q. [The establishment of a rapid fluorescent focus inhibition test for testing rabies virus neutralizing antibody]. Zhonghua Liu Xing Bing Xue Za Zhi 2010; 31:438 - 41; PMID: 20513292
- Yu PC, Noguchi A, Inoue S, Tang Q, Rayner S, Liang GD. Comparison of RFFIT tests with different standard sera and testing procedures. Virol Sin 2012; 27:187 - 93; http://dx.doi.org/10.1007/s12250-012-3247-8; PMID: 22684473