Abstract
The prevalence of hepatitis B surface antigen (HBsAg) in a population aged 15 y or older was high in China, but an immunization strategy for this population was unavailable. We investigated the seroprevalence of hepatitis B and immune response to HBV vaccine in Chinese college students (n = 2040 participants), 11.1%, 80.8%, and 8.1% had confirmed, unknown and no HBV vaccination history, respectively. The seropositive rates for HBsAg, anti-HBs sole and anti-HBs plus anti-HBc were 12.6%, 25.7%, and 30.1%, respectively. The HBsAg seropositive rate was significantly lower in participants with confirmed HBV vaccination history than in those with unknown or no vaccination history (5.3%, 13.6%, and 12.6%, respectively, P = 0.0019). The anti-HBs alone seropositive rate was significantly higher in participants with confirmed HBV vaccination history than in those with unknown or no vaccination history (37.6%, 25.3%, and 13.8%, respectively, P < 0.0001). Participants negative for HBsAg, anti-HBs, and anti-HBc at baseline (n = 600) were given three doses of recombinant HBV vaccine (GlaxoSmithKline) at month 0, 1, and 6. Robust immune response was elicited after two and three doses (seroprotective rate: 91.9% and 99.0%, respectively, and geometric mean concentration [GMC]: 95.8 and 742.6 IU/L, respectively). Fourteen months after the third dose, the anti-HBs seroprotective rate of the group remained more than 97%. The seroprotective rates and GMCs did not differ significantly by vaccination history. This study suggested that three doses of 20 μg HBV vaccine were needed for college students negative for HBsAg, anti-HBs, and anti-HBc to ensure high seroprotective rates and concentrations.
Introduction
Hepatitis B is a serious infectious disease caused by hepatitis B virus (HBV). HBV is mainly transmitted by blood or blood products, mother-to-child, sexual exposure, and close contact involving open wounds on skins or mucous membranes. Vaccination is by far the most cost-effective way to prevent HBV infection, and in turn, to reduce the incidence of acute and chronic hepatitis B, hepatic cirrhosis, and heptocellular carcinoma.Citation1
Hepatitis B (HB) prevention has long been focused on newborns, because China is a highly endemic area where hepatitis B mother-to-child transmission is very common. In 1992 the Chinese Ministry of Health recommended HBV vaccine for routine immunization of infants but parents had to pay for vaccinations. China integrated HBV vaccines into the Expanded Program of Immunization (EPI) in 2002, when the cost of HBV vaccines was paid by the government while administration fees of HBV vaccinations (up to $1.10 per dose) had to be charged to the parents. Since 2005, China’s government has required that all infants should be immunized with the HBV vaccine free of charge. According to the national serological surveys, in 1992 and 2006; it was estimated that from 1992 to 2006, the children infected with HBV had been reduced by nearly 80 million, and the children carriers of hepatitis B surface antigen (HBsAg) had been reduced by 19 million. However, the prevalence of HBsAg in adolescents and adults more than 15 y of age was still as high as 8.57%, where the decline was not significant. HBsAg carrier rate showed a rising trend with increasing age. The 2 surveys showed that the carrier rate for the whole population had slightly declined from 9.75% to 7.18%, which was still at a high level.Citation2
In order to reduce the whole population’s prevalence of HBV infection, worldwide experience has confirmed that vaccinations should also be given to children and adolescents to block the horizontal transmission, in addition to routine vaccinations in newborns for interruption of mother-to-child transmission. Taiwan started vaccinations in neonates born to mothers of HBV carriage in 1984 and then gradually expanded the vaccination target population, which achieved good results.Citation3 Currently, Canada, the United States, Australia, Greece, Malta, Germany, France, Belgium, Italy, Spain, and other countries with low prevalence of HBV infection have included adolescents into routine immunization.Citation4,Citation5
At present, children less than 15 y of age have been regarded as a key group for hepatitis B immunization in China. However, to date, there is no special immunization strategy for the population 15 y of age and older. In this study, we investigated the seroprevalence of hepatitis B and immune response to HBV vaccine among Chinese college students to uncover the need for universal mass vaccination or booster immunization only for students with HBV vaccination.
Results
Demography
A total of 2040 participants were recruited including 366 males (17.9%) and 1674 females (82.1%) with a male-to-female ratio of 1: 4.57. All of the participants were born between 1982 and 1992 and aged 17–27 y with a median age of 19.7 y. With respect to ethnicity, 60.8%, 30.8%, and 5.9% of the participants were Han, Zhuang, and others respectively. All participants were born in Guangxi Zhuang Autonomous Region and 70% were from a rural area ().
HBV vaccination history
Among the 2040 participants, 226 (11.1%) had a confirmed HBV vaccination history, of whom 215 (10.5%) had completed a serial of three doses and 11 (0.5%) had not. Among the participants, 1648 (80.8%) had an unknown HBV vaccination history and 166 participants (8.1%) had never received any HBV vaccine. Of the 215 participants receiving full doses of vaccination, 65 (30.2%) received their first dose within 24 h after birth, while the rest received their first dose beyond 24 h after birth (). An amount of 129 (60%) of the 215 participants received a plasma-derived HBV vaccine in their full doses of vaccination before 2000 and the remaining 86 (40%) received recombinant vaccines in their full doses of vaccination after 2000.
Table 1. Baseline seropositive rates of HBsAg and anti-HBc and seroprotective rate of anti-HBs by HB vaccination history
Baseline seroprevalence
The baseline seroprevalence for HBsAg, anti-HBs, and anti-hepatitis B core antigen (anti-HBc) is shown in .
The HBsAg seropositive rate in participants with confirmed HBV vaccination was significantly lower than in those without HB vaccination history (P = 0.0097) or with unknown HB vaccination history (P = 0.0004). The anti-HBs alone seroprotective rate was significantly higher in participants with confirmed HBV vaccination than in those without HBV vaccination (P < 0.0001) or with unknown HBV vaccination history (P < 0.0001). Anti-HBc prevalence was significantly lower in participants with confirmed or unknown HBV vaccination than in those without HBV vaccination history (both P < 0.01). There was no statistical difference between those with confirmed HBV vaccination and with unknown HB vaccination history (P > 0.05).
Of the 215 participants with confirmed HBV vaccination history, 65 participants received their first dose within 24 h after birth and had a lower HBsAg seropositive rate than those receiving their first dose beyond 24 h after birth, although there was no statistical difference (1.5% vs.7.3%, P = 0.1123).
Immune response to HBV vaccine in participants seronegative for HBsAg, anti-HBs, and anti-HBc at baseline
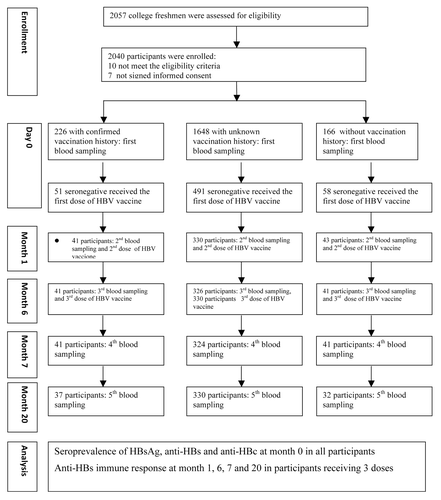
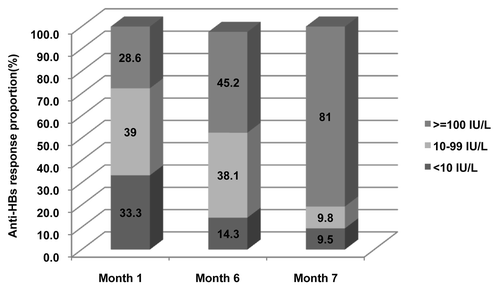
The 600 participants seronegative for HBsAg, anti-HBs, and anti-HBc at baseline were given a HBV vaccine, of whom 414, 407, 406, and 399 participants had post-vaccination serological results available at month 1, 6, 7, and 20, respectively, and were included in immunologic analysis (; ). Regardless of the participants HBV vaccination histories, one dose induced mild immune response while robust immune response was elicited with anti-HBs seroprotective rate of more than 87% after 2 doses (month 6) and more than 98% after three doses (month 7). Fourteen months after the third dose, the anti-HBs seroprotective rates remained more than 97%. At each time point after HBV vaccination, the seroprotective rates and geometric mean concentrations (GMCs) did not show statistical difference among participants having confirmed, none or unknown HBV vaccination history. The anti-HBs response distribution as expressed with proportion of participants having anti-HBs concentration <10 IU/L, 10–100 IU/L, ≥100 IU/L among participants with different HBV vaccination history, was statistically different at month 7 (P = 0.0079) but not at month 1, 6, and 20 (P = 0.6333–0.9266). After 1, 2, and three doses, 21.7%, 51.1%, and 96.1% of participants had anti-HBs concentration more than 100 IU/L. Even 14 mo after the third dose, 81.0% of participants had anti-HBs concentrations more than 100 IU/L.
Figure 2. Anti-HBs seroprotective rate after HB vaccination given at month 0, 1, and 6 among participants seronegative for HBsAg, anti-HBs, and anti-HBc at baseline.
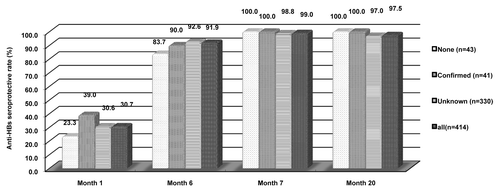
Table 2. Anti-HBs response after HB vaccination given at month 0, 1, and 6 among participants seronegative for HBsAg, anti-HBs, and anti-HBc at baseline
Response to additional three doses of HBV vaccine in non-responders
Among the 330 participants with unknown HBV vaccination history and negative for all the three serological markers, four non-responders after the initial three doses of the HBV vaccine were administrated an additional three doses from month 20 onward at the regimen of month 0, 1, and 6, and achieved seroprotective anti-HBs. One participant had high anti-HBs response (3385.5 IU/L), one had middle response (306.1 IU/L), and another 2 participants had low response (17.5 IU/L and 64.4 IU/L).
Response to HBV vaccine in participants seropositive for anti-HBc alone
In addition to the participants negative for all the three serological markers, the 42 students with anti-HBc positive alone were also given three doses of HBV vaccine. The anti-HBs response is shown in . Sixty-six percent (28/42) of participants had seroprotective anti-HBs and 28.6% (12/42) had higher than 100 IU/L anti-HBs one month after the first dose of the HBV vaccine. After 2 doses, 85.7% (36/42) of participants had seropretective response and 45.2% (19/42) participants had anti-HBs with higher than 100 IU/L. Thirty-eight subjects (90.5%) had seroprotective response after three doses, of which 34 (89.5%) participants had anti-HBs concentrations higher than 100 IU/L. The GMCs at month 1, 6, and 7 were 19.3 (9.0–41.5), 67.9 (38.2–120.4), and 328.4 (188.8–571.1), respectively. There were four non-responders and an additional three doses of HBV vaccine were given to them. One non-responder negative for HBV DNA achieved a low level of anti-HBs concentration (84.5 IU/L). The remaining three non-responders who were positive for HBV DNA did not develop seroprotective anti-HBs.
Discussion
According to the seroepidemiologic survey conducted in China in 2006, the prevalence rates of HBsAg and anti-HBs in the population aged 20 to 25 y were 12% and 46%, respectively, highlighting the importance of having an immunization strategy of HBV vaccines for this population.Citation2 Most of college students enjoy participating in sports activities which are associated with an increased risk of accidental injuries. They are in a period of sexual activity and may engage in unprotected sexual activities. In addition, they are in close contact with each other due to crowded living, by which they may share daily necessities such as toothbrushes, razors, etc. According to the Technical Guide for Adult Hepatitis B Immunization in China, college students are at high risk for HBV.Citation6 Previous studies showed that the prevalence rates of HBsAg in Chinese college students were 4% to 13%.Citation7-Citation10 Therefore, immunizing college students with HBV vaccines should be considered as an important part of the immunization strategy to lower the prevalence of HBsAg.
The HBV vaccination histories in children were not routinely recorded in China before 1992. Most studies obtained HBV vaccination histories by questionnaires, which could introduce recall bias. In order to get the HBV vaccination history as exact as possible in this study, the record of HBV vaccination was retrieved through the Centre for Disease Control and Prevention (CDC) system. There was no significant difference in the HBsAg prevalence between participants who had definitely not received hepatitis B vaccination and those with an unknown history of vaccination, possibly because most of the students with an unknown vaccination history might not have been vaccinated or failed to complete the full-course immunization. HBV vaccination was not recommended before 1992 in China. So the HBV vaccine coverage was very low, especially in rural and remote areas. Guangxi is located in a poor and remote area of China. According to a national survey conducted in 1999 in 31 provinces of China, the HBV vaccine coverage rates in children younger than 3 y of age in different provinces or autonomous regions of China were uneven. Four provinces had the coverage ≥90%, 9 provinces 75–89%, 8 provinces 50–74%, and 10 provinces <50% (including Guangxi).Citation11
Our study found that the HBsAg prevalence rate (12.6%) in the college students studied was higher, and anti-HBs prevalence (25.7%) was lower as compared with the results of the national serosurvey conducted in 2006 (7.2% and 50.1%, respectively).Citation2 Although the HBsAg prevalence in participants with confirmed HBV vaccination history was up to 5.3%, it was significantly lower than that in participants without HBV vaccination history (12.6%) and those with unknown vaccination history (13.6%). Seventy (32.5%) participants who had a confirmed history of vaccination, with three doses, were positive for anti-HBc, in comparison with 13/81 (16.0%) in Qian’s study.Citation12 This difference was likely because of the failure in timely HBV vaccination with the first dose. As pointed out in the WHO’s position paper, perinatal or early childhood transmission was the main cause of chronic HBV infection throughout the world. Thus, the first dose of hepatitis B vaccine should be administered after birth as soon as possible (within 24 h).Citation13 Delay in the birth dose resulted in an increased risk of HBV infection.Citation14 In this study, among the 215 participants who had confirmed HBV vaccination of three doses, 65 (30%) and 150 (70%) participants received the first dose within and beyond 24 h after birth, respectively. One out of the 65 participants (1.5%) and 11 out of the 150 participants (7.3%) were positive for HBsAg. Although there was no statistically significant difference (P = 0.1123), the HBsAg prevalence was lower in the participants receiving the first dose within 24 h after birth than beyond 24 h after birth. Therefore, the rate of timely vaccination with the first dose was very important to prevent HBV infection. In addition to the timeliness of the first dose, the study in Thailand found the risk of infants born to mothers with chronic HBV infection becoming chronically infected was associated with a delayed second dose of HBV vaccine, even if the first dose had been immunized promptly.Citation15 In this study, this issue could not be analyzed due to the small sample size of participants with delayed second dose of HBV vaccine. Incomplete HBV vaccination also resulted in increased risk of chronic HBV infection.Citation16 Our study found 11 out of the 226 participants (4.9%) with confirmed HBV vaccination histories had not completed their three doses of HBV vaccine. Although the 11 participants did not become chronic HBV infection, 8 of them (72.7%) had anti-HBc positive, indicating a natural infection with HBV. For the participants with three doses of HBV vaccination, only 32.5% (70/215) had anti-HBc marker.
In China, recombinant HBV vaccine totally replaced plasma-derived HBV vaccine after 2000. In this study, all students were administrated with plasma-derived HBV vaccine in their childhood. A lot of studies have demonstrated that both types of HBV vaccines performed very well with respect to safety, immunogenicity and protective efficacy.Citation17-Citation19 Therefore, the reason of relatively low seroprotective rates of anti-HBs alone in this study population may account for the lower HBV vaccine coverage rates but not for the usage of plasma-derived HBV vaccines. Currently, international public health authorities did not recommend a booster for immune competent people after primary immunization because of the robust anamnestic response to HBV in most people for many years after priming, even when antibodies were undetectable upon exposure.Citation20-Citation23 Nevertheless, some studies in Taiwan have revealed the absence of an anamnestic anti-HBs response in approximately 25% adolescents after one booster dose.Citation24-Citation26 Chronic HBV infections were reported after vaccine-induced protecting antibodies had disappeared.Citation27 This absence of immune memory may raise concerns about the need of booster dose for high-risk groups.
High HBsAg prevalence, low anti-HBs seroprotective rates, low concentration at baseline, and low HBV vaccination coverage rates in the study population indicated the necessity of immunization with HBV vaccines for college students. How to immunize this population should consider the following factors: primary HBV vaccination history, the condition of primary HBV vaccinations (such as HBV vaccination timing, the doses and the type of HBV vaccine), and the anti-HBs titer.Citation28 Taking into account nearly 81% of the participants having unknown vaccination history, it was less practical to figure out an immunization strategy on the basis of previous vaccination history. Therefore, we investigated the anti-HBs response in the participants negative for HBsAg, anti-HBs, and anti-HBc and with different HBV vaccination histories. About 30% of the participants were negative for HBsAg, anti-HBs, and anti-HBc, and were susceptible for HBV infection; they should be deemed the target population of HBV immunization. We found that among students with none, confirmed or unknown HBV vaccination history, anti-HBs GMCs, and seroprotective rates were not different after one, two, or three doses of HBV vaccine. One dose of HBV vaccine only induced 30% anti-HBs seroprotective rate. After two doses, 90% of participants produced protective anti-HBs but the anti-HBs GMC was less than 100 IU/L. Three doses induced more than 95% seroprotective rate and the GMC was 742.6 IU/L. The results were similar to the study conducted in Taiwan college students where at least two doses were needed for at-risk youths who received complete plasma-derived HBV vaccine in neonatal and infant periods but were seronegative for HBsAg, anti-HBc, and anti-HBs in adolescence to ensure higher than 90% of anti-HBs seroprotection rates.Citation29 However, anti-HBs of 10–99 IU/L was considered as low response. Some studies suggested that all individuals with anti-HBs concentration below 100 IU/L should receive a booster dose every 2 y.Citation30 Therefore, we recommended three doses for the college students negative for HBsAg, anti-HBs, and anti-HBc to obtain high anti-HBs seroprotective rate and concentration. Another study in Taiwan also indicated that including three doses of the booster vaccination including three doses may be essential for adolescents negative for anti-HBs, HBsAg, and anti-HBc to maintain anti-HBs concentration and reduce HBsAg prevalence.Citation31 One study showed that high concentrations of anti-HBs could provide long-lasting protection against HBV infection.Citation32 In this study, all participants were recruited from one medical college in the university, which resulted in enrolling a higher proportion of female students (82.4%). A predominantly female sample may increase anti-HBs response since it has been observed that being male was associated with an increased risk for nonresponse or a low response to HBV vaccination.Citation33
Anti-HBc acts as a serum marker for HBV infection. There should be an anamnestic response of the anti-HBs titers equal to or higher than 10 IU/L after one dose of the HBV vaccine in a population with isolated positive anti-HBc due to prior infection and immunity after one dose of the HBV vaccine.Citation34 In this study, 28 (66.7%) of 42 participants with isolated positive anti-HBc had ≥10 IU/L anti-HBs after one dose of HBV vaccine, which indicated that 66.7% participants with isolated positive anti-HBc had true anti-HBc positive and had already eliminated HBV in their body. Ten (23.8%) of 42 participants with isolated positive anti-HBc had no seroprotective anti-HBs after one dose of HBV vaccine but achieved ≥10 IU/L anti-HBs after full course of the HBV vaccine. Thus it was deduced that 23.8% (10/42) participants may have had a false anti-HBc positive and had never been infected with HBV. The observation was consistent with the previous reports of unspecificity problem concerning the Abbott Architect anti-HBc.Citation35,Citation36 Ollier et al. reported an unspecificity problem concerning the Abbott ARCHITECT® old anti-HBc test (Abbott ARCHITECT® anti-HBc, Abbott Laboratories).Citation35 Seiskari et al. studied the specificity of ARCHITECT® new anti-HBc II assay, and concluded that the samples with very low reactivity may be adequate to confirm, especially if the other HBV markers or clinical findings do not support the diagnosis.Citation36 Four of (9.5%) of 42 participants with isolated positive anti-HBc could not induce seroprotective anti-HBs after three doses of the HBV vaccine. Three of them were HBV DNA positive and regarded as occult HBV infection. The fourth participant with negative HBV DNA achieved a low level of anti-HBs concentration (84.5 IU/L) after additional three doses of HBV vaccine and his anti-HBc positivity also may be regarded as a false positive and he was susceptible to HBV infection. Through the immune response of these participants with isolated positive anti-HBc to HBV vaccine, we found that 26.2% (11/42) participants should be considered susceptible to a HBV infection. Currently, there is a lack of consensus on whether those having positive anti-HBc alone and waned anti-HBs concentrations should receive a HBV vaccination. Su et al. recommended a single booster dose of HBV vaccine for participants with isolated positive anti-HBc who had been fully vaccinated with a HBV vaccine as infants.Citation37 The present study found that three doses of HBV vaccine could induce a higher protection rate and anti-HBs concentration in students with isolated positive anti-HBc compared with one dose of the HBV vaccine.
One of the strengths of our study was that it was the first study to analyze seroprevalence of hepatitis B and immune response to HBV vaccination in Chinese college students based on the confirmed HB vaccination history. Another strength was conducting the study on the immune response for isolated positive anti-HBc participants after HBV vaccination. It was demonstrated that the isolated anti-HBc positive subjects may be divided into three groups according to their immune responses to the HBV vaccine: (1) occult HBV infection (no immune response to three doses of HBV vaccine), (2) true anti-HBc positive with resolved HBV infection (anamnestic response to HBV vaccination), and (3) false anti-HBc positive without HBV infection (primary response to HBV vaccination). A predominantly female sample and the lack of HBV infection status of participants’ mothers were thought as limitation in our study.
In summary, immunization with three doses of 20 µg hepatitis B vaccine for college students negative for HBsAg, anti-HBs, and anti-HBc was recommended to ensure high anti-HBs seroprotective rate and concentration. However, to immunize all college students or only those negative for HBsAg, anti-HBs, and anti-HBc warranted more studies. The cost-effectiveness analysis may facilitate the development of a reasonable hepatitis B immunization strategy for college students in China.
Methods
Participants
All freshmen in one college in Liuzhou city of Guangxi Zhuang Autonomous Region except for those with acute febrile diseases or born outside of this province were enrolled in September 2009, and tested for baseline HBsAg, anti-HBs, and anti-HBc for the seroprevalence study. Those participants negative for all the three markers or positive for anti-HBc only were enrolled for the HBV vaccination study and given intramuscularly three doses of HBV vaccine (Engerix-B, recombinant hepatitis B surface antigen, 20 µg/mL/vial, GlaxoSmithKline) at month 0, 1, and 6, then tested for anti-HBs at month 1, 6, 7, and 20. Students having any of the following conditions were excluded: acute illness, immune compromised conditions, renal insufficiency, pregnancy, and allergic history to HB vaccine or yeast. Non-responders were defined as having an anti-HBs below 10 IU/L one month after three doses of HBV vaccine,Citation38 and were administrated,Citation38 an additional three doses of HBV vaccine according to the schedule of month 0, 1, and 6. Records on HBV vaccination history were retrieved from the Guangxi CDC.
This study was conducted in accordance with Good Clinical Practice and the Helsinki Declaration. Written informed consent was obtained from all the participants and their parents. All relevant documents were approved by the ethical review committee of Guangxi CDC.
Serological assay
HBsAg, anti-HBs, and anti-HBc were determined by chemiluminescent microparticle immunoassay (CMIA) using the Architect i2000SR analyzer (Abbott Diagnostic). Serum HBsAg and anti-HBs were determined quantitatively, while anti-HBc was determined qualitatively. HBsAg was considered positive if the concentration ≥0.05 IU/L. Anti-HBs ≥10 IU/L were considered seroprotective, ≥10 IU/L and <100 IU/L were considered low response, and anti-HBs concentrations more than 100 IU/L were medium or high response.Citation38 The upper detection limits for HBsAg and anti-HBs were 250 and 1000 IU/L, respectively. When calculating the GMC, a concentration of 0.1 IU/L was used for those lower than 0.1 IU/L and of 1000 IU/L for those higher than 1000 IU/L. Anti-HBc was interpreted with the ratio of the sample signal to cutoff ratio (S/CO). S/CO ≥1.0 was deemed anti-HBc positive per manufacturer’s specification. For anti-HBc alone, double testing was implemented. HBsAg was detected using a HBsAg ad/ay serotype reference panel. Sensitivity results calculated by linear regression ranged from 0.15 to 0.29 ng/mL and were estimated to be 99.52% (418/420) with a 95% confidence interval of 98.29% to 99.94%. Anti-HBs had a sensitivity of 97.53% (594/609) with a 95% confidence range of 95.57% to 98.62%. Anti-HBc had a sensitivity of 100% (406/406) with a 95% confidence interval range of 99.10–100%.
Statistical analysis
The results on seroprevalence and immune response to HBV vaccination were presented with point estimates and two-sided 95% confidence intervals (CI). The outcomes for anti-HBs were expressed as seroprotective rates and GMC. GMC1 were calculated on all the participants with specific vaccination history and GMC2 were calculated on the participants other than HBsAg positive. Chi-square tests or Fisher exact tests were used for analyzing categorical data when relevant. Student t-tests or analysis of variance (ANOVAs) were used for analyzing continuous data when relevant. The statistical analysis was conducted by an independent statistician using SAS version 9.2. Hypothesis testing was two-sided with α value of 0.05. Bonferroni adjustments were used for multiple comparisons, by which the α’s value was adjusted by comparison time.
| Abbreviation: | ||
| ANOVA | = | analysis of variance |
| anti-HBc | = | antibody against hepatitis B core antigen |
| Anti-HBs | = | antibody against hepatitis B surface antigen |
| CDC | = | Centre for Disease Control and Prevention |
| CI | = | confidence interval |
| CMIA | = | chemiluminescent microparticle immunoassay |
| GMC | = | geometric mean concentration |
| HB | = | hepatitis B |
| HBcAg | = | hepatitis B core antigen |
| HBsAg | = | hepatitis B surface antigen |
| HBV | = | hepatitis B virus |
| S/CO | = | sample signal-to-cutoff ratio |
Disclosure of Potential Conflicts of Interest
All authors declare no conflict of interest.
Role of the Funding Source
This study was supported by Major Science and Technology Special Project of China twelfth Five-year Plan (2012ZX10002001).
Acknowledgments
We thank the students for participating in this study; the study nurses, and other staff members of Guangxi University of Science and Technology. The authors thank Dr Lisa Nicholson, Dr Yin Peng, and Dr Yuan-Zheng Qiu for critically proofreading this manuscript. And also the authors thank Dr Qian Zhang and Dr Ming-Huan Zheng for the support of the statistical analyses in the study.
References
- World Health Organization. Department of Communicable Disease Surveillance and Response. Hepatitis B 2002; 2:1-76; http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009; 27:6550 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.08.048; PMID: 19729084
- Chien YC, Jan CF, Kuo HS, Chen CJ. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev 2006; 28:126 - 35; http://dx.doi.org/10.1093/epirev/mxj010; PMID: 16782778
- Menzies R, McIntyre P. Vaccine preventable diseases and vaccination policy for indigenous populations. Epidemiol Rev 2006; 28:71 - 80; http://dx.doi.org/10.1093/epirev/mxj005; PMID: 16763071
- Van Damme P. Hepatitis B: vaccination programmes in Europe--an update. Vaccine 2001; 19:2375 - 9; http://dx.doi.org/10.1016/S0264-410X(00)00457-6; PMID: 11257363
- Cui FQ. Chinese Prevention Medicine Association; National Immunization Program, Chinese Center for Disease Control and Prevention. [Technical guide for adult hepatitis B immunization in China] Zhonghua Liu Xing Bing Xue Za Zhi 2011; 32:1199 - 203; PMID: 22336599
- Du G, Niu J, Liu Z. Survey of HBV infection among the new college students at Shanxi Agriculture University. Journal of Changzhi Medical College 2003; 17:18 - 20
- Zeng L. A study on the infect condition of HBV for 3246 freshman and countermeasures research. Journal of Guangzhou Physical Education Institute 2006; 26:88 - 94
- Lv F. Analysis outcomes of HBV serum marker in 4932 college students. Health Medicine Research and Practice in Higher Institutions 2006; 3:8 - 9
- Huang H. Hepatitis B viral infection status of grade 2008 freshman of Hunan University of technology. Pract Prev Med 2009; 16:406 - 7
- Zhu X, Zhang XL, Wang LX. National EPI vaccination and hepatitis B coverage rate and the related factors: results from the 1999 national coverage survey. Chin J Vac Imminization 2000; 6:1 - 5
- Wu Q, Zhuang GH, Wang XL, Wang LR, Li N, Zhang M. Antibody levels and immune memory 23 years after primary plasma-derived hepatitis B vaccination: results of a randomized placebo-controlled trial cohort from China where endemicity is high. Vaccine 2011; 29:2302 - 7; http://dx.doi.org/10.1016/j.vaccine.2011.01.025; PMID: 21277403
- World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec 2009; 84:405 - 19; http://www.who.int/wer PMID: 19817017
- Marion SA, Tomm Pastore M, Pi DW, Mathias RG. Long-term follow-up of hepatitis B vaccine in infants of carrier mothers. Am J Epidemiol 1994; 140:734 - 46; PMID: 7942775
- Tharmaphornpilas P, Rasdjarmrearnsook AO, Plianpanich S, Sa-nguanmoo P, Poovorawan Y. Increased risk of developing chronic HBV infection in infants born to chronically HBV infected mothers as a result of delayed second dose of hepatitis B vaccination. Vaccine 2009; 27:6110 - 5; http://dx.doi.org/10.1016/j.vaccine.2009.08.034; PMID: 19716459
- van der Ploeg CP, Kateman H, Vermeer-de Bondt PE, Verkerk PH. [Increased risk of hepatitis B due to incomplete or untimely immunisation in one-quarter of infants of hepatitis-B-virus carriers]. Ned Tijdschr Geneeskd 2004; 148:1820 - 4; PMID: 15495511
- Lee PI, Lee CY. Practical considerations in converting from plasma-derived to recombinant hepatitis B vaccines. BioDrugs 1998; 10:11 - 25; http://dx.doi.org/10.2165/00063030-199810010-00002; PMID: 18020583
- Guirong S, Jingpu S, Shuqiu L, Qundi Z, Wandi Z, Yuezhong Z, et al. A comparative study of yeast-derived plasma-derived hepatitis B vaccine immunity in adults. Journal of China Medical University 1987; 16:75 - 8
- Qirong Z, Jianshe W. Comparison of the efficacy between plasma-derived and recombinant yeast-derived hepatitis B vaccine for interruption of maternal-infantile transmission of hepatitis B infection. Zhonghua Er Ke Za Zhi 2000; 38:682 - 4
- Banatvala J, Van Damme P, Van Hattum J, European Consensus Group on Hepatitis B Immunity. Boosters for hepatitis B. Lancet 2000; 356:337 - 8; http://dx.doi.org/10.1016/S0140-6736(05)73618-7; PMID: 11071210
- Immunization Practices Advisory Committee. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep 1991; 40:RR-13 1 - 25; PMID: 1835756
- John TJ, Cooksley G, Steering Committee for the Prevention and Control of Infectious Diseases in Asia. Hepatitis B vaccine boosters: is there a clinical need in high endemicity populations?. J Gastroenterol Hepatol 2005; 20:5 - 10; http://dx.doi.org/10.1111/j.1440-1746.2004.03398.x; PMID: 15610440
- Van Damme P, Van Herck K. A review of the long-term protection after hepatitis A and B vaccination. Travel Med Infect Dis 2007; 5:79 - 84; http://dx.doi.org/10.1016/j.tmaid.2006.04.004; PMID: 17298912
- Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, Su IJ, Kuo HS, Huang LM, Chen DS, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15-18 years after neonatal immunization. J Infect Dis 2008; 197:1419 - 26; http://dx.doi.org/10.1086/587695; PMID: 18444799
- Wang LY, Lin HH. Short-term response to a booster dose of hepatitis B vaccine in anti-HBs negative adolescents who had received primary vaccination 16 years ago. Vaccine 2007; 25:7160 - 7; http://dx.doi.org/10.1016/j.vaccine.2007.07.022; PMID: 17707557
- Kao JT, Wang JH, Hung CH, Yen YH, Hung SF, Hu TH, Lee CM, Lu SN. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine 2009; 27:1858 - 62; http://dx.doi.org/10.1016/j.vaccine.2009.01.027; PMID: 19186203
- Chang HC, Yen CJ, Lee YC, Chiu TY, Jan CF. Seroprevalence of hepatitis B viral markers among freshmen--20 years after mass hepatitis B vaccination program in Taiwan. J Formos Med Assoc 2007; 106:513 - 9; http://dx.doi.org/10.1016/S0929-6646(07)60001-1; PMID: 17660140
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011; 53:68 - 75; http://dx.doi.org/10.1093/cid/cir270; PMID: 21653306
- Jan CF, Huang KC, Chien YC, Greydanus DE, Davies HD, Chiu TY, Huang LM, Chen CJ, Chen DS. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology 2010; 51:1547 - 54; http://dx.doi.org/10.1002/hep.23543; PMID: 20209603
- Yoshida T, Saito I. Hepatitis B booster vaccination for healthcare workers. Lancet 2000; 355:1464 - 6; http://dx.doi.org/10.1016/S0140-6736(05)74663-8; PMID: 10791552
- Lu JJ, Cheng CC, Chou SM, Hor CB, Yang YC, Wang HL. Hepatitis B immunity in adolescents and necessity for boost vaccination: 23 years after nationwide hepatitis B virus vaccination program in Taiwan. Vaccine 2009; 27:6613 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.08.007; PMID: 19698812
- Banatvala J, Van Damme P, Oehen S. Lifelong protection against hepatitis B: the role of vaccine immunogenicity in immune memory. Vaccine 2000; 19:877 - 85; http://dx.doi.org/10.1016/S0264-410X(00)00224-3; PMID: 11115711
- Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B Vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med 1998; 15:1 - 8; http://dx.doi.org/10.1016/S0749-3797(98)00003-8; PMID: 9651632
- Koh HJ, Kim SD, Choi JH, Kim SR, Lee JS. [A study of immune response to hepatitis B vaccine & HBV DNA in isolated anti-HBc positive subjects] J Prev Med Pub Health 2005; 38:170 - 4
- Ollier L, Laffont C, Kechkekian A, Doglio A, Giordanengo V. Detection of antibodies to hepatitis B core antigen using the Abbott ARCHITECT anti-HBc assay: analysis of borderline reactive sera. J Virol Methods 2008; 154:206 - 9; http://dx.doi.org/10.1016/j.jviromet.2008.09.006; PMID: 18848582
- Seiskari T, Lehtisaari H, Haapala AM, Aittoniemi J. From Abbott ARCHITECT anti-HBc to Anti-HBc II--improved performance in detecting antibodies to hepatitis B core antigen. J Clin Virol 2010; 47:100 - 1; http://dx.doi.org/10.1016/j.jcv.2009.11.010; PMID: 19962345
- Su FH, Bai CH, Chu FY, Lin YS, Su CT, Yeh CC. Significance and anamnestic response in isolated hepatitis B core antibody-positive individuals 18 years after neonatal hepatitis B virus vaccination in Taiwan. Vaccine 2012; 30:4034 - 9; http://dx.doi.org/10.1016/j.vaccine.2012.04.031; PMID: 22531558
- Floreani A, Baldo V, Cristofoletti M, Renzulli G, Valeri A, Zanetti C, Trivello R. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine 2004; 22:607 - 10; http://dx.doi.org/10.1016/j.vaccine.2003.09.001; PMID: 14741151