Abstract
Rotaviruses (RV) are a major cause of severe gastroenteritis (GE) in children aged <5 y. For the first time in China, we assessed the efficacy of two oral doses of the human rotavirus vaccine (RIX4414) in infants during the first two years of life (113808/NCT01171963). Healthy infants aged 6–16 weeks were randomized (1:1) to receive two oral doses of either the RIX4414 vaccine/placebo according to a 0, 1 month schedule. Vaccine efficacy (VE) against severe RVGE was assessed from two weeks post-Dose 2 up until the end of the second RV season and calculated with its 95% confidence intervals (CI). The primary efficacy objective was met if the lower limit of the 95% CI on VE was ≥10%. Unsolicited symptoms reported during the 31-d post-vaccination follow-up period and serious adverse events (SAEs) reported throughout the study were assessed. Of 3333 enrolled infants, 3148 were included in the according-to-protocol efficacy cohort. Over two consecutive RV seasons, fewer severe RVGE episodes were reported in the RIX4414 group (n = 21) vs. the placebo group (n = 75). VE against severe RVGE was 72% (95% CI: 54.1–83.6); the lower limit of the 95% CI on VE was >10%. The number of unsolicited symptoms and SAEs reported was similar between both groups. Thirteen deaths (RIX4414 = 6; placebo = 7) occurred during the study. All SAEs and deaths in the RIX4414 group were considered unrelated to vaccination. Two oral doses of RIX4414 vaccine provided a substantial level of protection against severe RVGE in Chinese children during the first two years of life.
Introduction
Rotaviruses (RV) are globally recognized as the leading cause of severe, dehydrating gastroenteritis (GE) in children younger than five years,Citation1 with a peak age of clinical illness due to RV between 4 and 24 mo.Citation2 The worldwide annual RV disease burden is estimated at approximately 114 million GE episodes, 2.5 million medical visits, 2.4 million hospitalizations and 527 000 deaths.Citation3-Citation5 With the second largest birth cohort in the world, China ranks second in terms of global RV-related deaths.Citation6 Annually, RV causes approximately 4900 deaths, nearly 330 000 hospitalizations and almost 2 640 000 outpatient visits in children younger than 5 y in the country.Citation7 Passive surveillance conducted by the Asian Rotavirus Surveillance Network between 2001 and 2003 identified RV in 44% of children hospitalized for diarrhea in China and G3 as the predominant strain.Citation8 Thereafter, a hospital-based surveillance study conducted between 2003 and 2007 showed that RV was responsible for approximately 47.8% of diarrheal hospitalizations in children younger than five years, with the majority occurring in infants younger than two years of age.Citation9
There is no specific drug treatment for RVGE and as improved hygiene and sanitation has not reduced the incidence of RV infection,Citation10 vaccination has been identified as the most effective intervention to control the associated disease burden. It is estimated that if a vaccine was introduced as a two-dose schedule in the national immunization program in China, it could substantially reduce the disease and economic burden of RV diarrhea.Citation7 Two, live-attenuated, orally administered RV vaccines are currently licensed and available for use in several countries: a monovalent human rotavirus vaccine (RIX4414; Rotarix™, GlaxoSmithKline Vaccines) and a pentavalent human-bovine rotavirus vaccine (Rotateq®, Merck and Co.).Citation11,Citation12 Phase III studies conducted in Europe, Latin America, Africa, and Asia have demonstrated that the RIX4414 vaccine is efficacious in the first two years of life of infants and well-tolerated.Citation13-Citation17 Other available rotavirus vaccines include Rotavin-M1™ in Vietnam (Center for Research and Production of Vaccines and Biologicals [POLYVAC]),Citation18,Citation19 and the Lanzhou lamb RV vaccine in China (LLR; Lanzhou Institute of Biomedical Products).Citation20 RV vaccination is currently not mandatory in China and is not included in the expanded program of immunization (EPI).Citation21,Citation22 The LLR vaccine was licensed in China in 2001 and while more than 26 million doses of the vaccine have been administered in the private market, the schedule of one dose between 6 and 12 mo of age followed by one dose every year until three years of age, is considered too complex for a national immunization program.Citation7
The aim of this phase-3, multi-center study was to evaluate the efficacy, immunogenicity, reactogenicity, and safety of RIX4414 in Chinese infants during the first two years of life. This paper describes the efficacy and safety of RIX4414, and a subsequent paper will present the reactogenicity and immunogenicity results.
Results
Demography
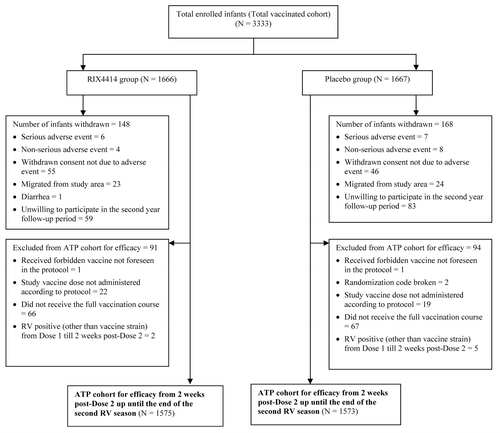
A total of 3333 infants (RIX4414 = 1666; placebo = 1667) were enrolled between Aug 2010 and Dec 2010; and were followed until the end of the second RV season (May 2012). The according-to-protocol (ATP) cohort for efficacy over two consecutive RV seasons included 3148 infants (RIX4414 = 1575; placebo = 1573). The ATP efficacy cohort for the second RV season included 2979 infants (RIX4414 = 1500; placebo = 1479). The reasons for exclusion from the analyses are shown in . The demographic characteristics were similar between the two groups with respect to age, gender, and ethnic origin. The mean age of infants was 9.6 weeks (standard deviation [SD] = 2.62) at Dose 1 and 14.1 weeks (SD = 2.72) at Dose 2; 51.1% of infants were male and all were of Chinese origin.
Vaccine efficacy (VE)—ATP efficacy cohort
From two weeks post-Dose 2 up until the end of the second RV season, 1.3% (n = 21) of infants in the RIX4414 group and 4.8% (n = 75) of infants in the placebo group recorded severe RVGE caused by circulating wild-type RV (P < 0.001). The resulting VE against severe RVGE was 72% (95% confidence interval [CI]: 54.1–83.6) (); the primary objective was achieved since the lower limit of the 95% CI on VE was >10%. VE against severe RVGE caused by G1 and non-G1 RV types was 64% (95% CI: 20.4–85.2) and 77.8% (95% CI: 58.0–89.2), respectively. G1P[8] (RIX4414 = 8; placebo = 20) and G2P[4] (RIX4414 = 11; placebo = 40) were the most frequent G1 and non-G1 RV types isolated from infants with severe RVGE episodes, respectively (). Circulation of G3P[8] and G9P[8] was low in the study population; each RV type was detected in three RVGE episodes, all in infants from the placebo group.
Table 1. Efficacy of RIX4414 over two consecutive RV seasons—from two weeks post-Dose 2 up until the end of the second RV season (ATP cohort for efficacy)
From two weeks post-Dose 2 up until the end of the second RV season, significantly fewer RVGE episodes of any severity were recorded in the RIX4414 group (4.4%, n = 70) as compared with the placebo group (10.6%, n = 167), resulting in a VE of 58.1% (95% CI: 44.3–68.8) (P < 0.001) against RVGE of any severity. The most frequent G1 and non-G1 RV types isolated from RVGE episodes of any severity were G1P[8] (RIX4414 = 20; placebo = 38) and G2P[4] (RIX4414 = 42; placebo = 102), respectively (). G3P[8] was detected in 11 episodes from infants in the placebo group; G9P[8] was detected in one episode from an infant in the RIX4414 group and five episodes from infants who received placebo ().
The percentage of infants hospitalized for RVGE was lower in the RIX4414 group (0.3%; n = 4) than in the placebo group (1.3%; n = 21) (P < 0.001); VE against RVGE hospitalizations was 81% (95% CI: 43.6–95.3) from two weeks post-Dose 2 until the end of the second RV season (). VE results for severe RVGE, RVGE of any severity and RVGE hospitalizations observed in the first and second year of life are shown in . Similar incidences were observed for GE and severe GE due to any cause in the RIX4414 and placebo groups (). The percentage of infants hospitalized for GE due to any cause was 2.7% (n = 43) in the RIX4414 group and 4.6% (n = 73) in the placebo group.
VE—Total vaccinated cohort (TVC)
From Dose 1 up until the end of the second RV season, the percentage of infants recording severe RVGE caused by circulating wild-type RV was lower in the RIX4414 group (1.5% [95% CI: 1.0–2.2]) than in the placebo group (4.6% [95% CI: 3.6–5.7]) (P < 0.001), resulting in a VE of 67.1% (95% CI: 47.7–79.9).
Seasonality
The highest number of RVGE episodes was observed in January 2012 followed by December 2011 in the RIX4414 and placebo groups.
Safety
During the 31-d post-vaccination follow-up period, at least one unsolicited adverse event was recorded in 310 infants in the RIX4414 group (18.6%) and 368 infants in the placebo group (22.1%). Upper respiratory tract infection was the most common unsolicited adverse event recorded in both groups (RIX4414 = 119; placebo = 124) followed by nasopharyngitis (RIX4414 = 103; placebo = 123). From Dose 1 until the end of the second RV season, at least one serious adverse event (SAE) was recorded in 183 infants (11.0%) in the RIX4414 group and 246 infants (14.8%) in the placebo group. The most frequently reported SAE was bronchitis in the RIX4414 (n = 80) and placebo groups (n = 103). Two cases of intussusception were reported (RIX4414 = 1; placebo = 1), 142 and 65 d after the second dose, respectively. Both cases resolved and were considered as not being related to vaccination by the investigators. All SAEs in the RIX4414 group were considered unrelated to vaccination. Diarrhea, reported as an SAE, in one infant in the placebo group was considered related to vaccination by the investigator.
There were 13 deaths (RIX4414 = 6; placebo = 7) during the study. The causes of death in each group are listed in . All deaths were considered unrelated to vaccination by the investigators. Eighteen infants withdrew from the study due to unsolicited symptoms and SAEs (RIX4414 = 8; placebo = 10).
Table 2. Cause of death from Dose 1 up until the end of the second RV season
Discussion
The disease burden of RV in China is highest in children younger than two years with approximately 42% of RV infections occurring between 7 and 12 mo of age.Citation23 This was the first clinical trial in China to evaluate the efficacy of the RIX4414 vaccine during the first two years of life. Children in this study were enrolled from rural areas in the Guangxi Province located in southern China where several levels of medical facilities are available including village physicians, township hospitals, and county and city hospitals. Part of the study population resided in remote and/or mountainous areas. While basic medical treatment is offered at the village level; treatment for severe disease may be accessed at the township level and higher.
Our study confirmed that two doses of the RIX4414 vaccine provided a substantial level of protection against severe RVGE (VE = 72% [95% CI: 54.1–83.6]) over two consecutive RV seasons. This result was comparable to the VE observed in similarly-designed two year efficacy studies conducted in Latin America (80.5% [95% CI: 71.3–87.1]);Citation14 lower than that observed in Europe (90.4% [95% CI: 85.1–94.1]) and high-income Asian countries: Hong Kong, Singapore, and Taiwan (96.1% [95% CI: 85.1–99.5);Citation13,Citation17 and higher than that observed over a two-year period in impoverished settings in South Africa (59% [95% CI: 1–83]) and Malawi (38.1% [95% CI: 9.8–57.3]).Citation15,Citation16 While the VE in Japan (91.6% [95% CI: 62.4–99.1])Citation24 appears to be higher than the VE in China; it must be highlighted that the 95% CIs overlapped. Furthermore, the VE observed in the first year of life in this study was well conserved into the second year of life (P < 0.001) indicative of the persistence of protective efficacy through the second year of life. Additionally, as previously reported, we noted a significant level of protection against RVGE hospitalizations with the RIX4414 vaccine (81%; P < 0.001) over two consecutive RV seasons in the present study.Citation13,Citation14 Another finding was the comparable incidence of GE and severe GE due to any cause between the RIX4414 and placebo groups; suggesting that other bacterial and viral enteropathogens could be contributing to GE related illness.
G2P[4] was documented as the predominant strain in episodes of severe RVGE and RVGE of any severity in the current study. This observation is inconsistent with previously observed predominant strain, G3P[8] in China,Citation6,Citation9 suggesting that the distribution of RV strains differ from year-to-year. Given this interesting aspect of rotaviruses, it is important to assess the ability of the RV vaccine to protect against circulating RV types that may help in controlling the disease burden.Citation24 In accordance with previous studies, RIX4414 afforded significant protection against severe RVGE and RVGE of any severity caused by G1 and non-G1 RV types.Citation13,Citation14,Citation17 Significant VE was observed against G2P[4] in episodes of severe RVGE (72.5%) and RVGE of any severity RVGE (58.9%) in this study. This is of particular interest as this strain is heterotypic to the RIX4414 vaccine strain for both G and P types, suggesting that in China the RIX4414 vaccine can provide broad protection against non-G1 and non P[8] types. Notably, in some earlier studies, VE against G2 could not be satisfactorily assessed due to the low circulation of G2 type.Citation14,Citation17 However, significant protection against severe RVGE due to G2P[4] (P = 0.0086) has been observed in Europe over two consecutive RV seasons.Citation13 In addition, an integrated analysis on VE against severe RVGE by RV types indicated good clinical protection against G2P[4].Citation25
With respect to seasonality, the highest number of RVGE episodes in this study was recorded in January 2012 and December 2011; these findings are consistent with a previous study in China that indicated RV peaks in winter.Citation23
In terms of safety, there was no evidence of a clinically significant difference between the RIX4414 and placebo groups with respect to unsolicited symptoms reported 31 d post-vaccination and SAEs reported from Dose 1 up until the end of the second RV season. The safety data in this study are consistent with an integrated safety summary report.Citation26 This integrated safety analysis noted that all SAEs were assessed as unrelated to vaccination and that there was no imbalance between the RIX4414 and placebo groups with respect to the number of deaths.Citation26 Two cases of intussusception were recorded during the current study (one in each group), both of which resolved. While recent studies have shown some evidence of a temporal increase in the risk of intussusception following the first vaccine dose, it is still uncertain whether RV vaccination has any impact on the overall incidence of intussusception.Citation27,Citation28 Our study results provide comparable safety profiles between the RIX4414 vaccine and placebo in Chinese infants.
The study results need to be interpreted in the context of certain limitations. First, the study was not powered to explore differences between the groups with respect to RVGE of any severity, and GE and severe GE due to any cause. Second, we should consider the possibility that the medical advice-seeking behavior of parents may have been influenced by factors including the challenge of traveling from remote areas to township or higher levels of hospitals.
We conclude from our study that two oral doses of RIX4414 coadministered with routine childhood vaccines is efficacious against severe RVGE caused by circulating RV strains in China during the first two years of life. These data may help public health officials in making an informed decision regarding the adoption of RV vaccination in their country.
Methodology
Study design and participants
We conducted a phase III, randomized, double-blind (i.e., concealed from parents/guardians, study personnel, and investigators), placebo-controlled study at four centers in China between August 2010 and May 2012 (113808/NCT01171963). Healthy Chinese infants 6–16 weeks of age were randomized (1:1) into two treatment groups to receive either two oral doses of RIX4414 vaccine or placebo according to a 0, 1 mo schedule. According to EPI, infants in both groups received concomitant pediatric vaccination.
Infants were excluded from the study if they had received: any investigational drug or vaccine from 30 d before Dose 1 or during the study, immunoglobulins and/or blood products since birth or during the study, frequent immunosuppressants or other immune-modifying drugs since birth. Administration of any vaccine unforeseen by the study protocol within 14 d before any dose of the study vaccines or concurrent participation in other clinical studies were reasons for exclusion. Infants with any confirmed or suspected immunosuppressive or immunodeficient condition, a history of allergy to any of the vaccine components, clinically significant chronic gastrointestinal disease, congenital or hereditary immunodeficiency, confirmed RVGE, major congenital defects, or serious chronic illness were also excluded. Vaccination was postponed for infants who had GE within seven days of receiving the study vaccine/placebo and for infants with minor illness, as determined by the investigator.
The study was conducted in accordance with Good Clinical Practice, including the Declaration of Helsinki; and adhered to all applicable local regulations in China. Parents/guardians provided written informed consent before any study procedures were performed.
Study vaccine
The liquid RIX4414 vaccine contained at least 106.0 median cell culture infectious dose (CCID50) of live, attenuated human rotavirus strain. The placebo had the same constituents and appearance as the active vaccine but without the vaccine viral strain. The RIX4414 vaccine and placebo were administered orally.
Assessment of efficacy
We performed active surveillance of GE episodes beginning from Dose 1 of RIX4414 vaccine/placebo until the end of the second RV season. A GE episode was defined as the occurrence of diarrhea (≥3 looser than normal stools within 24 h) with or without vomiting. Two occurrences of GE were classified as separate episodes if there was an interval of ≥5 symptom-free days between the two GE episodes. For each GE episode, a diary card was completed by parents/guardians until the GE symptoms had ceased. Active follow-up of GE episodes was performed by study staff via telephone contact or face-to-face home visits, every two weeks. For each GE episode, stool samples were collected as early as possible (or preferably within two days of the onset of GE). Parents/guardians were requested to record during each GE episode date and time of stool sample collection, number of looser than normal stools, axillary temperature, number of vomiting episodes, and details of any rehydration treatment or medical attention (specifically: the date and details of doctor and/or emergency room visits, hospitalization) in the diary card. Information from the diary cards was used to assess the severity of each GE episode using the 20-point Vesikari scale,Citation29 where a score of <7 was defined as mild, 7–10 was moderate, and ≥11 was severe.
The collected stool samples were analyzed to identify RV using an enzyme-linked immunosorbent assay (ELISA; RotaClone™ assay, Meridian Biosciences). All RV positive stool samples were further tested using reverse transcriptase polymerase chain reaction followed by reverse hybridization and sequencing to determine the G and P types and differentiate the presence of wild-type G1 RV from the vaccine strain virus. These tests were performed at LabCorp Clinical Trials (formerly, Clearstone Laboratory).
Assessment of safety
The safety of the vaccine was assessed in terms of the unsolicited symptoms reported during the 31-d follow-up after either dose of RIX4414 or placebo; and SAEs which were recorded from the administration of Dose 1 and up until the end of the second RV season. An SAE is defined as any life-threatening medical event that may require medical or surgical intervention to prevent hospitalization, disability, or a congenital anomaly/birth defect in the study subject or death.
Statistical analyses
A target sample size of 3250 infants (1625 in each group) was required to reach 2600 evaluable subjects (1300 in each group) for the efficacy evaluation. Assuming a 2% rate of RVGE during follow-up in the placebo group and a true VE of 80%, this study had 95.8% power to observe a lower limit of 95% CI for VE >10%.
The primary efficacy endpoint was VE against severe RVGE from two weeks post-Dose 2 up until the end of the second RV season. The primary efficacy objective was met if the lower limit of the 95% CI on VE was >10%. Due to the low number of severe RVGE cases observed during the first RV season, this study was extended up until the end of the second RV season for severe RVGE cases. Infants who completed the first RV season and for whom consent to continue till the end of the second RV season was not provided were considered drop-outs. The ATP efficacy cohort included infants who received two doses of RIX4414/placebo; who had entered the efficacy surveillance period; who had no rotavirus other than the vaccine strain in their GE stool samples collected between Dose 1 and two weeks post-Dose 2 of RIX4414/placebo, and who complied with the protocol throughout. The total vaccinated cohort was used in the safety analysis and included infants who received at least one dose of RIX4414/placebo.
The percentage of subjects reporting any and severe RVGE episodes (overall and by G type), RVGE requiring hospitalization, GE, and severe GE due to any cause were calculated for over two consecutive RV seasons, and for the first and second RV seasons with 95% CI. The seasonality pattern of RVGE cases was also recorded.
All statistical analyses were performed using SAS Drug and Development web portal version 3.5 and SAS version Proc StatXact 8.1.
| Abbreviations: | ||
| ATP | = | according-to-protocol |
| CCID50 | = | median cell culture infectious dose |
| CI | = | confidence interval |
| ELISA | = | enzyme-linked immunosorbent assay |
| EPI | = | expanded program on immunization |
| GE | = | gastroenteritis |
| LLR | = | Lanzhou lamb rotavirus |
| RV | = | rotavirus |
| SAE | = | serious adverse event |
| SAS | = | statistical analysis system |
| SD | = | standard deviation |
| VE | = | vaccine efficacy |
Disclosure of Potential Conflicts of Interest
Authors Huang T, Li Y, Luo D, Tao J, Fu B, Si G, Nong Y, and Mo Z declare to have received institutional funding/grants from GlaxoSmithKline Biologicals SA for the conduct of Rota trials. GS also declare to have received support for travel to meetings for the study or other purposes from GlaxoSmithKline Biologicals SA. Li RC declares no conflict of interest. Liao X, Luan I, Tang H, Rathi N, Karkada N, Htay Han H are employed by GlaxoSmithKline group of companies; Rathi N and Htay Han H also hold stock ownership/restricted shares from the sponsoring company.
Acknowledgments and Funding
The authors thank all the investigators, study nurses, and staff members who were involved in this clinical trial and the participating parents and children. The authors acknowledge the following members of GlaxoSmithKline (current and ex-employees): Ben Wang, Cindy Zhang, Luca Zhang, Miki Sun, Weiwei Zhu, Ying Lu, Yu He, Katherine Li, Vivian Chen, Helen Zhang, Janly Huang, Vern Liu, Kelly Wang, Tmily Wang, Emily Zhong, Jessie Lan, Lilith Zou, Mingwei Zhang, Tina Zhao for their monitoring support; Clay Churchill and Jenny Jiang (for providing inputs on the protocol design), Ramya Basavaraju (for global study management), Mudit Mehrotra (for study data management), Ilse Vastiau (for providing inputs on the study report), Syed Yasin (for writing the study report), Ashmita Ravishankar (for medical writing), and Manjula K (for publication coordination and editorial support).
This study was sponsored and funded by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis; and also took charge of all costs associated with the development and the publishing of the manuscript.
Trial Registration Number
NCT01171963
Trademarks
Rotarix is a registered trademark of the GlaxoSmithKline group of companies. Rotateq is a registered trademark of Merck and Co., USA. Rotavin-M1 is a registered trademark of Center for research and production of vaccines and biologicals [POLYVAC], Vietnam. Lanzhou lamb rotavirus vaccine is a registered trademark of Lanzhou Institute of Biomedical Products, China. RotaClone is a registered trademark of Meridian Biosciences, USA.
References
- Bernstein DI. Rotavirus overview. Pediatr Infect Dis J 2009; 28:Suppl S50 - 3; http://dx.doi.org/10.1097/INF.0b013e3181967bee; PMID: 19252423
- Chandran A, Fitzwater S, Zhen A, Santosham M. Prevention of rotavirus gastroenteritis in infants and children: rotavirus vaccine safety, efficacy, and potential impact of vaccines. Biologics 2010; 4:213 - 29; PMID: 20714358
- O’Ryan M, Linhares AC. Update on Rotarix: an oral human rotavirus vaccine. Expert Rev Vaccines 2009; 8:1627 - 41; http://dx.doi.org/10.1586/erv.09.136; PMID: 19943758
- World Health Organization. Rotavirus vaccines. Wkly Epidemiol 2007; 82:285 - 95
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 2009; 200:Suppl 1 S9 - 15; http://dx.doi.org/10.1086/605025; PMID: 19817620
- Fang Z-Y, Wang B, Kilgore PE, Bresee JS, Zhang L-J, Sun L-W, Du ZQ, Tang JY, Hou AC, Shen H, et al. Sentinel hospital surveillance for rotavirus diarrhea in the People’s Republic of China, August 2001-July 2003. J Infect Dis 2005; 192:Suppl 1 S94 - 9; http://dx.doi.org/10.1086/431505; PMID: 16088812
- Liu N, Yen C, Fang Z-Y, Tate JE, Jiang B, Parashar UD, Zeng G, Duan ZJ. Projected health impact and cost-effectiveness of rotavirus vaccination among children <5 years of age in China. Vaccine 2012; 30:6940 - 5; http://dx.doi.org/10.1016/j.vaccine.2012.05.084; PMID: 22705174
- Nelson EAS, Bresee JS, Parashar UD, Widdowson MA, Glass RI, Asian Rotavirus Surveillance Network. Rotavirus epidemiology: the Asian Rotavirus Surveillance Network. Vaccine 2008; 26:3192 - 6; http://dx.doi.org/10.1016/j.vaccine.2008.03.073; PMID: 18485546
- Duan Z-J, Liu N, Yang S-H, Zhang J, Sun L-W, Tang J-Y, Jin Y, Du ZQ, Xu J, Wu QB, et al. Hospital-based surveillance of rotavirus diarrhea in the People’s Republic of China, August 2003-July 2007. J Infect Dis 2009; 200:Suppl 1 S167 - 73; http://dx.doi.org/10.1086/605039; PMID: 19817597
- Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, Jiang B, Gentsch JR. Rotavirus vaccines: current prospects and future challenges. Lancet 2006; 368:323 - 32; http://dx.doi.org/10.1016/S0140-6736(06)68815-6; PMID: 16860702
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al, Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11 - 22; http://dx.doi.org/10.1056/NEJMoa052434; PMID: 16394298
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al, Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23 - 33; http://dx.doi.org/10.1056/NEJMoa052664; PMID: 16394299
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757 - 63; http://dx.doi.org/10.1016/S0140-6736(07)61744-9; PMID: 18037080
- Linhares AC, Velázquez FR, Pérez-Schael I, Sáez-Llorens X, Abate H, Espinoza F, López P, Macías-Parra M, Ortega-Barría E, Rivera-Medina DM, et al, Human Rotavirus Vaccine Study Group. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371:1181 - 9; http://dx.doi.org/10.1016/S0140-6736(08)60524-3; PMID: 18395579
- Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, Neuzil KM, Steele AD. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine 2012; 30:Suppl 1 A44 - 51; http://dx.doi.org/10.1016/j.vaccine.2011.08.080; PMID: 22520136
- Cunliffe NA, Witte D, Ngwira BM, Todd S, Bostock NJ, Turner AM, Chimpeni P, Victor JC, Steele AD, Bouckenooghe A, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine 2012; 30:Suppl 1 A36 - 43; http://dx.doi.org/10.1016/j.vaccine.2011.09.120; PMID: 22520135
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, Teoh YL, Tang H, Boudville I, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 2009; 27:5936 - 41; http://dx.doi.org/10.1016/j.vaccine.2009.07.098; PMID: 19679216
- Luan T, Trang NV, Phuong NM, Nguyen HT, Ngo HT, Nguyen HT, Tran HB, Dang HN, Dang AD, Gentsch JR, et al. Development and characterization of candidate rotavirus vaccine strains derived from children with diarrhoea in Vietnam. Vaccine 2009; 27:Suppl 5 F130 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.08.086; PMID: 19931712
- Anh DD, Trang NV, Thiem VD, Anh NTH, Mao ND, Wang Y, et al. A dose-escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin-M1) in Vietnamese children. Vaccine 2012; 30S:A114 - 21; http://dx.doi.org/10.1016/j.vaccine.2011.07.118
- Fu C, He Q, Xu J, Xie H, Ding P, Hu W, Dong Z, Liu X, Wang M. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine 2012; 31:154 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.10.078; PMID: 23127516
- Wang X-Y, Riewpaiboon A, von Seidlein L, Chen X-B, Kilgore PE, Ma J-C, Qi SX, Zhang ZY, Hao ZY, Chen JC, et al. Potential cost-effectiveness of a rotavirus immunization program in rural China. Clin Infect Dis 2009; 49:1202 - 10; http://dx.doi.org/10.1086/605632; PMID: 19739973
- Ouyang Y, Ma H, Jin M, Wang X, Wang J, Xu L, Lin S, Shen Z, Chen Z, Qiu Z, et al. Etiology and epidemiology of viral diarrhea in children under the age of five hospitalized in Tianjin, China. Arch Virol 2012; 157:881 - 7; http://dx.doi.org/10.1007/s00705-012-1235-9; PMID: 22318672
- Lou J-T, Xu X-J, Wu Y-D, Tao R, Tong M-Q. Epidemiology and burden of rotavirus infection among children in Hangzhou, China. J Clin Virol 2011; 50:84 - 7; http://dx.doi.org/10.1016/j.jcv.2010.10.003; PMID: 21041114
- Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Van Doorn LJ, Muto H, Smolenov I, Suryakiran PV, Han HH. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011; 29:6335 - 41; http://dx.doi.org/10.1016/j.vaccine.2011.05.017; PMID: 21640780
- De Vos B, Han HH, Bouckenooghe A, Debrus S, Gillard P, Ward R, Cheuvart B. Live attenuated human rotavirus vaccine, RIX4414, provides clinical protection in infants against rotavirus strains with and without shared G and P genotypes: integrated analysis of randomized controlled trials. Pediatr Infect Dis J 2009; 28:261 - 6; http://dx.doi.org/10.1097/INF.0b013e3181907177; PMID: 19289978
- Cheuvart B, Friedland LR, Abu-Elyazeed R, Han HH, Guerra Y, Verstraeten T. The human rotavirus vaccine RIX4414 in infants: a review of safety and tolerability. Pediatr Infect Dis J 2009; 28:225 - 32; http://dx.doi.org/10.1097/INF.0b013e31819715fa; PMID: 19209095
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, Booy R, Bines JE, PAEDS/APSU Study Group. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine 2011; 29:3061 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.01.088; PMID: 21316503
- Velázquez FR, Colindres RE, Grajales C, Hernández MT, Mercadillo MG, Torres FJ, Cervantes-Apolinar M, DeAntonio-Suarez R, Ortega-Barria E, Blum M, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J 2012; 31:736 - 44; http://dx.doi.org/10.1097/INF.0b013e318253add3; PMID: 22695189
- Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990; 22:259 - 67; http://dx.doi.org/10.3109/00365549009027046; PMID: 2371542