Abstract
Pertussis remains an important cause of infant death worldwide and is an ongoing public health concern even in countries with high vaccination coverage. A cross-sectional seroepidemiological study was undertaken to estimate true incidence rates and gain further insight into the epidemiology and burden of pertussis in China. During 2011, a total of 1080 blood samples were obtained from healthy individuals between 0 and 86 y of age in Zhengzhou, Central China. Serum IgG antibodies against pertussis toxin (PT) and filamentous hemagglutinin (FHA) were measured quantitatively using ELISA. The results showed that the geometric mean titers of PT and FHA IgG were 6.48 IU/mL (95% CI: 5.70–7.41 IU/mL) and 11.39 IU/mL (95% CI: 10.22–12.87 IU/mL) among subjects less than 4 y of age, indicating that pertussis antibody levels were low despite high vaccination coverage. Of the 850 subjects ≥4 y of age, 56 (6.6%) had anti-PT IgG titers above 30 IU/mL, and 11 (1.3%) had antibodies titers above 80 IU/mL. The estimated age-specific incidence of infection with B. pertussis revealed a peak incidence in the 31 to 40 y age group, followed by the 41 to 60 y age group. Taken together, these results indicate that pertussis is common in Chinese subjects in Zhengzhou, especially in adults, suggesting that the disease burden is underestimated in China. Therefore, our study stresses the importance of strengthening the diagnostic capacity and improving surveillance system for delineating current epidemiological profiles of pertussis. Most importantly, it may be advisable to re-evaluate the current Chinese pertussis immunization schedule and implement to booster doses for older children, adolescents and adults.
Introduction
Pertussis (Whooping cough) is an acute respiratory infectious disease caused by the bacterium Bordetella pertussis. The morbidity and mortality associated with pertussis has been dramatically reduced in many countries following the introduction of whole cell pertussis vaccines (WPVs) in the 1950s.Citation1,Citation2 However, according to the World Health Organization (WHO) estimates, there were 195 000 deaths from whooping cough worldwide in 2008 and most of the deaths occurred in developing countries.Citation3 A resurgence of pertussis has also been observed in developed countries despite the high vaccination coverage since the 1990s. Between 2010 and 2012, there were large outbreaks in UK and North America.Citation4-Citation6 Pertussis therefore remains one of the leading causes of vaccine-preventable deaths in the world today.
Pertussis is a class B reportable infectious disease in China. Vaccination against pertussis has been used since the early 1960s. Three doses of pertussis vaccines combined with diphtheria and tetanus toxoids (DTP) are routinely administrated to infants at 3, 4, and 5 mo of age, and since 1982 a booster dose has been given at 18 mo of age.Citation7-Citation9 Vaccination coverage using the initial three doses was low before 1980s but has increased with time. Coverage between 1983 and 2012 ranged from 58% to 99% (). According to data from the Chinese infectious disease reporting system, the incidence of pertussis has been less than 1 per 100 000 since the 1990s with only 2183 and 2517 pertussis cases being reported in 2011 and 2012, respectively.Citation10 Pertussis is clinically diagnosed by physicians, and the currently available molecular laboratory methods are rarely used. The reported low incidence may, therefore, be related to the diagnostic criteria used, and is suggestive of significant underestimation disease prevalence. Thus, pertussis remains endemic in China with local outbreaks being reported in some regions.Citation7,Citation11
Figure 1. The number of reported pertussis cases and pertussis vaccination coverage in Zhengzhou, 2001–2012 (A) and the whole of China, 1983–2012 (B).
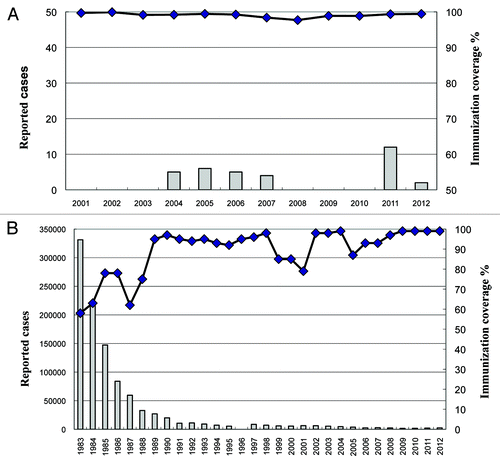
It is recommended that rational surveillance is used to monitor and estimate the epidemiologic trends in pertussis.Citation12,Citation13 Antibody responses indicate the presence of both symptomatic and asymptomatic infection, and probably give a true indication of prevalence. Indeed, seroepidemiological surveys are widely used to study the incidence of bacterial and viral infections.Citation14-Citation18 Several sero-prevalence studies for pertussis have been undertaken in cities in southern China.Citation19-Citation21 However, China is a large country with over 1.3 billion population, so it is necessary to obtain seroepidemiological data from other regions in order to fully understanding the burden of disease. Zhengzhou, is located in central part of China and the total population is reached 9.1 million in 2012. In the past ten years, vaccination coverage has been more than 99% while the reported case is less than 15 in this city every year (). To our knowledge, the pertussis seroepidemiological study among the general population of central China has not been reported. Furthermore, filamentous hemagglutinin (FHA), another virulence factor produced by B. pertussis, is used as the main component of many acellular pertussis vaccines (APVs) and is believed to be important for protection.Citation22,Citation23 But until now, little is known about the seroprevalence of anti-FHA IgG in China. The present cross sectional study was aimed to investigate the age-specific seroprevalence of pertussis in Zhengzhou, Central China, by evaluating antibody levels of pertussis toxin (PT) and FHA in healthy children and adults, in order to gain further insight into the epidemiology and burden of this disease.
Results
A total of 1080 subjects were enrolled in the study during 2011. The population included 532 (49.3%) male and 548 (50.7%) female subjects (Table S1). Their ages ranged from 0 to 86 y (median: 16.0 y). GMTs for anti-PT IgG and anti-FHA antibodies among all subjects were 8.46 IU/mL (95% CI: 8.01, 8.94 IU/mL) and 9.63 IU/ml (95% CI: 9.02, 10.27 IU/mL), respectively (Table S1). No significant differences were observed between male and female subjects in the different-age groups. However, antibody titers to PT and FHA were both related to age (P < 0.01).
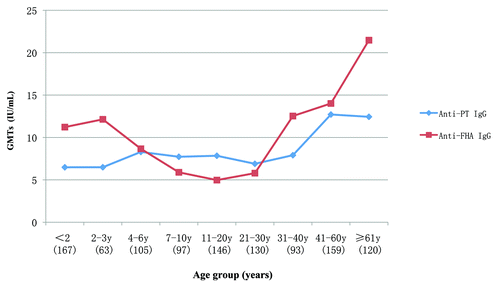
The age-specific GMTs distributions showed that the GMTs level of anti-PT IgG in the group aged 41 to 60 y was higher (12.71 IU/mL; 95% CI: 10.95, 14.76 IU/mL) when compared with all other age groups except in the ≥61 y group (12.44 IU/mL; 95% CI: 11.46, 13.50 IU/mL, P = 0.841). Similarly, the GMTs of anti-FHA were significantly higher in the >61 age group (21.47 IU/mL; 95% CI: 18.67, 24.70 IU/mL) than that in any other age group (P < 0.01) (Table S1).
GMTs curves for PT and FHA IgG titers were significantly correlated with one another (r = 0.755, P < 0.05) (). Changes in FHA IgG largely paralleled those for PT, but some differences were apparent in individual subjects. To further investigate the relationship of anti-PT and FHA IgG concentrations, subjects with one or both antibody titers ≥30 IU/mL were compared by age group. Only two subjects exceeded the 95th percentile for both anti-PT and FHA IgG titers (Fig. S2, upper right). One subject was 2 y old and the other was 45. No subjects in the both <2 and 11–20 y age groups had PT or FHA titers above the 95th percentile. Of the 73 subjects who had anti-PT IgG titers (≥30 IU/mL), 27 (37.0%) also had anti-FHA IgG titers ≥30 IU/mL. Conversely, only 18.8% (27/144) of subjects with anti-FHA IgG titers ≥30 IU/ml had anti-PT titers ≥30 IU/mL. Half of the subjects with anti-PT antibodies <30 IU/mL were ≥41 y of age, representing 25.3% of the sampled population.
Figure 2. Geometric mean titers of anti-PT and anti-FHA IgG by age group. The number in brackets represents the amount of subject at each age group.
The frequency of anti-PT IgG titers stratified by age is shown in . Of the 850 subjects older than 4 y of age, 56 (6.6%) had anti-PT IgG titers ≥30 IU/ml, and 11 (1.3%) had titers ≥80 IU/mL. The highest proportion of anti-PT IgG titers ≥30 IU/mL occurred in the 31 to 40 y age group, followed by the 20 to 31 and 41 to 60 age groups. A significantly higher prevalence of anti-PT IgG concentrations above 30 IU/mL were observed among individuals aged ≥21 y (42/502; 8.4%) than among those of 4 to 20 y (14/348, 4.0%, P < 0.05). The highest frequency of titers ≥ 80 IU/mL was seen in the 41 to 60 y age group (3.8%). No differences in these parameters were found between female and male subjects.
Table 1. Distribution of anti-PT IgG titers by age group
Although there has been higher pertussis vaccine coverage in children in Zhengzhou since 2001 (), the anti-PT IgG (92.6%, 213/230) and/or anti-FHA titers (90.0%, 207/230) in most of subjects under 4 y of age were below 30 IU/mL (). The GMTs of PT and FHA IgG were 6.48 IU/mL (95% CI: 5.70–7.41 IU/mL) and 11.39 IU/mL (95% CI: 10.22–12.87 IU/mL), respectively. Only one 2 y old child was found to have anti-PT IgG concentrations ≥80 IU/mL. This subject also had a high anti-FHA IgG titer (385 IU/mL).
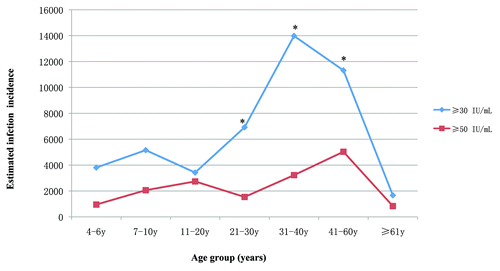
It is generally accepted that anti-PT IgG is a specific antibody for estimating B. pertussis infection in individuals.Citation24-Citation26 There are no available cutoff values for anti-PT IgG in the diagnosis of B. pertussis infection in China. Anti-PT IgG cutoff values of 30 IU/mL used in the previous studies was employed to estimate recent B. pertussis infection.Citation20,Citation21,Citation24 To avoid the interference from antibodies induced by inoculating pertussis vaccines, we limited this part of our analysis of B. pertussis infection incidence to subject ≥4 y of age. The estimated age-specific incidence of infection with B. pertussis in our population revealed a peak incidence (13 978 per 100 000 population) in the 31 to 40 y age group, followed by the 41 to 60 y age group (11 320 per 100 000 population) (). Furthermore, we also calculated the incidence of infection using the anti-PT IgG cut-off value (≥50 IU/mL) recommended by EU Pertstrain group.Citation27 It was revealed that 21 (2.5%) subjects had titers ≥50 IU/mL at age groups ≥4 y. A similar trend of incidence rates were observed although the estimated incidence based on the cut-off level (≥50 IU/mL) from other counties were lower than that of the cut-off value (≥30 IU/mL) in some age groups () (P < 0.05). The pertussis incidence rate estimated using different the cut-off values in this study was therefore, much higher than that reported from Chinese infectious disease reporting system, suggesting that pertussis in Chinese adults may be more common than is predicted from these official statistics.
Discussion
According to the Chinese Immunization Program Schedule, children are to receive four doses of pertussis vaccines in the first 2 y of life. High pertussis vaccination coverage has been maintained since 2000s. Thus, theoretically, subject <2 y age should have higher anti-PT and FHA antibodies than any of the other age groups.
Consistent with the previous studies,Citation19,Citation21 the GMTs of IgG-PT in the <4 y age group was low: 6.48 IU/mL (95% CI: 5.70–7.41 IU/mL). In this study, we also assessed the seroprevalence of anti-FHA IgG in the general population, and low GMTs of anti-FHA IgG (11.39 IU/mL; 95% CI: 10.22–12.87 IU/mL) was found in subjects less than 4 y of age. The data further indicates that pertussis antibody levels remain low in infants despite high vaccination coverage. It has been reported that immune response is related to the type of pertussis vaccine used.Citation28 Currently, two different commercial types of vaccines against pertussis are available: inactivated WPVs and APVs. APVs can be divided into purified and co-purified types depending on the methods used in the production process. Co-purified vaccines are mainly produced in Asian countries such as Japan and China.Citation8,Citation29 It has previously been demonstrated that anti-PT and FHA IgG titers are lower in WPVs recipients than purified APVs recipients.Citation28,Citation30 The antibody response induced by co-purified APVs has also been found to be lower than that achieved with purified APVs even though this type of APVs confers protection against intranasal and intracerebral challenges with B. pertussis (our unpublished data). Although APVs were included in the Chinese National Expanded Program on Immunization from 2007 onwards, WPVs and co-purified APVs are still used in Zhengzhou due to economic constraints. The lower humoral immune response of WPVs and co-purified APVs may be an explanation for the relatively lower pertussis antibody levels in our study. Another explanation might be the small sample size.
PT is the most specific antigen for B. pertussis, and for this reason ELISA estimates of IgG antibodies against PT using are used to diagnose pertussis infection. Depending on the vaccination program and epidemiology of pertussis, various cutoff points have been proposed for adolescents and adults in different countries.Citation21,Citation24-Citation26,Citation31 Melker et al.Citation24 analyzed IgG-PT in sera from 11 336 subjects including healthy individuals, those with clinical suspicion of pertussis and from Dutch patients with pertussis. Their results indicated titers of 80 IU/mL were achieved after 4.5 mo and that titers <32 IU/mL were achieved within 1 y of the onset of disease. More recently the EU Pertstrain group has recommended a cut-off between 50 and 120 IU/mL in different countries as providing proof of recent B. pertussis infection in patients not vaccinated during the previous 12 mo.Citation27 However, cutoff values for diagnosis of B. pertussis infection are not available in China, despite the availability of data from several seroepidemiological studies undertaken in recent years.Citation19-Citation21
In agreement with previous studies in China,Citation19,Citation21 we used a cutoff 30 IU/mL to indicate probable recent contact with B. pertussis in this study. In order to avoid interference with vaccination induced antibodies, subjects <4 y of age were excluded for calculation of incidence. Since the decay rate for pertussis-specific antibodies may be some years longer than the article suggested, the exclusion of subjects below age 4 y would not completely exclude a confounding effect from the vaccine-induced antibody titers although there was low mean value of anti-PT IgG of studies populations. At this cut-off value, the serological prevalence rate was 6.6% in subjects ≥4 y of age in our study which was comparable to the 6.6% prevalence previously reported for subjects aged 2 to 20 yCitation19 and the 9.4% prevalence among subjects >7 y of age.Citation21 By comparison, in the past two decades, the annual reported pertussis cases were less than 5000 in China. The estimated incidence rate based on these seroepidemiological data was more than a thousand times higher than the reported <1/100 000. The difference might be attributed to some cases had not been recorded in the passive national reporting system due to atypical symptoms and difficulties in laboratory diagnosis.Citation32 However, it should be kept in mind that PT IgG levels reflect exposure to B. pertussis rather than clinical disease. Taken together, these findings indicate that pertussis is common and that the true burden of the disease is under-estimated in China. Therefore, it is suggested that a functionally active surveillance system should be introduced in the future.
Furthermore, we also tried to estimate the incidence using other cut-off level (≥50 IU/mL). Significant low incidence was found when compared with that of the cut-off value (≥30 IU/mL). Considering this cutoff point (≥50 IU/mL) based on seroepidemiological data from western countries where preschool children and adolescents receive boosters may not be suitable for use in China where booster doses are not routinely given. In order to define the appropriate cut-offs for serological diagnosis of pertussis in China, nation-wide multi-center studies should be conducted to further understand the seroepidemiology of pertussis throughout the country.
Pertussis is traditionally considered a childhood disease. However, a recent resurgence of pertussis has been noted in developed countries even with high vaccination coverage, and there has been a shift in incidence toward adolescents and adults.Citation6,Citation33,Citation34 In our study, the prevalence of elevated anti-PT IgG concentrations above 30 IU/mL was significantly higher in subjects ≥21 y of age than in the 4 to 20 y age group. The peak prevalence in the 31 to 40 y age group, differed to findings from a study undertaken in Guangdong, in Southern of China where the highest prevalence was seen in 17 to 19 and 41 to 50 y old subjects.Citation21 The exact cause of difference is unclear, and it may be associated with the low number of subjects included in two different geographic seroepidemiological studies. Taken as a whole these seroepidemiological data, are indicative of a significant number of unnoticed pertussis cases in the adolescent and adult population of China.
The disease is generally milder in older children and adults than in children, but infected adolescents and adults have been shown to serve as a reservoir for disease transmission to young children and infants, who are vulnerable to severe pertussis and its life-threatening complications.Citation35,Citation36 In light of this risk and the parallel decline in immunity over time,Citation37,Citation38 pertussis booster vaccines have been introduced in preschool, adolescents or adult subjects in many countries.Citation12,Citation13,Citation39-Citation41 Vaccinating older children, and adults with a booster dose, not only protects the vaccinated individuals but may also reduce the risk of pertussis transmission from older individuals to infants. In Singapore, a booster dose of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) has been administered to school children at 10 to 11 y of age since 2008. This intervention markedly reduced the incidence of pertussis in young infants in Singapore.Citation41 Studies in California demonstrated that children with pertussis had a lower odds of having received the five-dose tetanus toxoid, diphtheria toxoid and acellular pertussis vaccine (DTaP) than control children. The risk increased in parallel with the interval since the last DTaP dose.Citation42 Recently, Witt et al. demonstrated less than expected effectiveness of DTaP in 8- to 12-y-olds by estimating the vaccine effectiveness by age group, suggesting the earlier or more numerous booster doses of APVs as part of routine immunization.Citation43 As the exact parameters of herd immunity may not be established for pertussis, it is suggested that maximal effective use of available vaccines could provide population-level immunity to prevent or reduce transmission.Citation6,Citation43,Citation44 Therefore, to reduce the burden of pertussis, it is recommended that booster doses of vaccine are introduced in old children, adolescent and adults in China.
It was noted that GMTs of the antibody level against FHA shared a similar trend to PT-IgG, although anti-FHA IgG titers appeared to be higher than those of PT. Unlike PT antibodies, to be specific for B. pertussis, FHA antibodies not only appear after contact with B. pertussis but could also be induced after contact with other bacteria such as B. parapertussis and Haemophilus influenze.Citation45 It was, therefore, speculated that subjects ≥4 y of age with single high FHA titers might have been infected recently with or exposure to B. parapertussis or other organisms produced cross-reacting antigens. Furthermore, the kinetics of FHA antibody are also thought to decline more slowly than PT antibodies which may also explain these findings.Citation46,Citation47
The limitation of this study include single sample collected during the study period as a single-sample analysis can have limitations regarding analytical reliability. Therefore, dual sample per subject should be got for giving a more convincing basis for drawing conclusions in the further seroepidemiological studies.
In conclusion, our results indicate that serum antibody levels to two antigenic components PT and FHA among subjects <4 y of age remains low despite higher immunization coverage in China. High serological prevalence of anti-PT IgG titers above 30 IU/mL were found in subjects ≥4 y of age and the estimated age-specific incidence of infection with B. pertussis revealed a peak incidence in the 31 to 40 y age group, followed by the 41 to 60 y age group, suggesting that pertussis was common in Zhengzhou, especially in adults. Taken together, these findings suggest that the pertussis burden may be underestimated in China. Therefore, our study stresses the importance of strengthening the diagnostic capacity and improving surveillance system for delineating current epidemiological profiles of pertussis. Most importantly, it is necessary to re-evaluate the currently recommended Chinese pertussis immunization schedule, and to consider booster doses for older children, adolescent or adults.
Materials and Methods
Study population
A cross-sectional serological survey was conducted between January and December, 2011 at the Center for Disease Control and Prevention of Henan Province, The First Hospital of Zhengzhou University, and The Second Hospital of Zhengzhou University in Zhengzhou, China. No pertussis outbreaks were reported in Zhengzhou during the period studied. Subjects with cough, fever, and diagnosed infectious diseases were excluded from the analysis. All subjects, who were asymptomatic, were selected randomly from three units at the beginning of the survey. Data collected on each subject included: age, gender, date of sampling and geographical information. Two to four milliliters of venous blood was drawn and serum was extracted from the blood sample on the same day. All sera were transported using the cold chain system and stored at −20 °C until analysis. The study protocol was approved by the Local Ethics Committee and written individual informed consent was obtained from each participant or guardian before enrollment.
Enzyme immunoassays for quantification of antibodies
IgG antibodies against PT and FHA were measured quantitatively by ELISA as described previously.Citation48 The antibody titers were calculated using a parallel line assay method in which dose-response curves for reference and test sera were constructed on a log-log scale. WHO Human International Standard for Pertussis Antiserum, (NIBSC 06/140) used was used for the assay. This contained 335 IU anti-PT IgG and 130 IU anti-FHA IgG per ampoule.Citation49 Antibody activities were expressed in international units (IU/mL). The lower limit of detection for both PT and FHA was 1 IU/mL.
Statistical analysis
Statistical analysis was performed using SPSS version 13.0 software (SPSS Inc.). Serum anti-PT and anti-FHA antibody levels were expressed as geometric mean antibody titers (GMTs) with 95% confidence intervals (CIs). In light of the potential effect of vaccination on the pertussis seroepidemiological analysis, subjects were categorized according to the age as follows: <2 y, 2 to 3 y, 4 to 6 y, 7 to 10 y, 11 to 20 y, 21 to 30 y, 31 to 40 y, 41 to 60 y, and ≥61 y. Considering unavailable anti-PT IgG cutoff values for diagnosis of B. pertussis infection and comparable to recent seroepidemiological surveys in China, a cut-off value of 30 IU/mL used in the previous studies was employed to estimate the incidence of infection at age groups ≥4 y.Citation20,Citation21,Citation24 Mean concentrations of anti-PT and anti-FHA IgG in different age groups and genders were examined using one-way analysis of variance (ANOVA). The Chi-square test was used to evaluate differences in seroprevalence across age groups. Values of P < 0.05 were considered statistically significant.
| Abbreviations: | ||
| PT | = | pertussis toxin |
| FHA | = | filamentous hemagglutinin |
| WPVs | = | whole cell pertussis vaccines |
| WHO | = | World Health Organization |
| DTP | = | pertussis vaccines combined with diphtheria and tetanus toxoids |
| APVs | = | acellular pertussis vaccines |
| Tdap | = | tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine |
| DTaP | = | tetanus toxoid, diphtheria toxoid and acellular pertussis vaccine |
| GMTs | = | geometric mean antibody titers |
| CIs | = | confidence intervals |
| ANOVA | = | one-way analysis of variance |
Additional material
Download Zip (342.6 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the Center for Disease Control and Prevention of Henan Province, The First Hospital of Zhengzhou University, and The Second Hospital of Zhengzhou University for their assistance in collecting blood samples. This work was partly supported by National Major Scientific and Technological Special Project for “Significant Infectious Disease Control” (No. 2012ZX10004701) from the Ministry of Science and Technology, People’s Republic of China.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/vaccines/article/26335
References
- Kerr JR, Matthews RC. Bordetella pertussis infection: pathogenesis, diagnosis, management, and the role of protective immunity. Eur J Clin Microbiol Infect Dis 2000; 19:77 - 88; http://dx.doi.org/10.1007/s100960050435; PMID: 10746492
- de Greeff SC, Mooi FR, Westerhof A, Verbakel JM, Peeters MF, Heuvelman CJ, Notermans DW, Elvers LH, Schellekens JF, de Melker HE. Pertussis disease burden in the household: how to protect young infants. Clin Infect Dis 2010; 50:1339 - 45; http://dx.doi.org/10.1086/652281; PMID: 20370464
- World Health Organization. Geneva: Programmes and projects/Immunization surveillance, assessment and monitoring/Vaccine-preventable diseases/Pertussis., Available at: http://www.who.int/immunization_monitoring/diseases/pertussis/en/index.html. [Last accessed March 23, 2013].
- van Hoek AJCH, Campbell H, Amirthalingam G, Andrews N, Miller E. The number of deaths among infants under one year of age in England with pertussis: results of a capture/recapture analysis for the period 2001 to 2011. Euro Surveill 2013; 18:20414; PMID: 23470020
- Winter K, Harriman K, Zipprich J, Schechter R, Talarico J, Watt J, Chavez G. California pertussis epidemic, 2010. J Pediatr 2012; 161:1091 - 6; http://dx.doi.org/10.1016/j.jpeds.2012.05.041; PMID: 22819634
- Cherry JD. Epidemic pertussis in 2012--the resurgence of a vaccine-preventable disease. N Engl J Med 2012; 367:785 - 7; http://dx.doi.org/10.1056/NEJMp1209051; PMID: 22894554
- Wang J, Yang Y, Li J, Mertsola J, Arvilommi H, Shen X, He Q. Infantile pertussis rediscovered in China. Emerg Infect Dis 2002; 8:859 - 61; http://dx.doi.org/10.3201/eid0808.010442; PMID: 12141975
- Wang L, Lei D, Zhang S. Acellular pertussis vaccines in China. Vaccine 2012; 30:7174 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.10.009; PMID: 23084855
- Zhu F. Pertussis booster vaccine in China. Hum Vaccin 2011; 7:272 - 5; http://dx.doi.org/10.4161/hv.7.2.13861; PMID: 21307651
- Center for Disease Control and Prevention. Infectious Disease Report, Beijing, China. Available at: http://www.chinacdc.cn/tjsj/fdcrbbg/. [Last accessed March 24, 2013].
- Guo Lichun LX. Huang Haitao, Xu Qingyang, Sun Zhengyi, Jiang Guangqi. Epidemiological investigation for an outbreak of pertussis in families. Chinese Journal of Vaccines and Immunization 2012; 18:180
- Tan T, Halperin S, Cherry JD, Edwards K, Englund JA, Glezen P, Greenberg D, Rothstein E, Skowronski D. Pertussis immunization in the global pertussis initiative North American region: recommended strategies and implementation considerations. Pediatr Infect Dis J 2005; 24:Suppl S83 - 6; http://dx.doi.org/10.1097/01.inf.0000160919.94330.1a; PMID: 15876933
- Wirsing von König CH, Campins-Marti M, Finn A, Guiso N, Mertsola J, Liese J. Pertussis immunization in the global pertussis initiative European region: recommended strategies and implementation considerations. Pediatr Infect Dis J 2005; 24:Suppl S87 - 92; http://dx.doi.org/10.1097/01.inf.0000160920.75623.a3; PMID: 15876934
- Anastassopoulou CG, Kafatos G, Nardone A, Andrews N, Pebody RG, Mossong J, Davidkin I, Gelb D, DE Ory F, Thierfelder W, et al. The European Sero-Epidemiology Network 2 (ESEN2): standardization of assay results for hepatitis A virus (HAV) to enable comparisons of seroprevalence data across 15 countries. Epidemiol Infect 2009; 137:485 - 94; http://dx.doi.org/10.1017/S0950268808001155; PMID: 18694528
- Nardone A, de Ory F, Carton M, Cohen D, van Damme P, Davidkin I, Rota MC, de Melker H, Mossong J, Slacikova M, et al. The comparative sero-epidemiology of varicella zoster virus in 11 countries in the European region. Vaccine 2007; 25:7866 - 72; http://dx.doi.org/10.1016/j.vaccine.2007.07.036; PMID: 17919788
- Stroffolini T, Chiaramonte M, Craxì A, Franco E, Rapicetta M, Trivello R, De Mattia D, Mura I, Giammanco A, Rigo G, et al. Baseline sero-epidemiology of hepatitis B virus infection in children and teenagers in Italy. A survey before mass hepatitis B vaccination. J Infect 1991; 22:191 - 9; http://dx.doi.org/10.1016/0163-4453(91)91723-B; PMID: 2026895
- Yeboah-Manu D, Röltgen K, Opare W, Asan-Ampah K, Quenin-Fosu K, Asante-Poku A, Ampadu E, Fyfe J, Koram K, Ahorlu C, et al. Sero-epidemiology as a tool to screen populations for exposure to Mycobacterium ulcerans. PLoS Negl Trop Dis 2012; 6:e1460; http://dx.doi.org/10.1371/journal.pntd.0001460; PMID: 22253937
- Simonsen J, Strid MA, Mølbak K, Krogfelt KA, Linneberg A, Teunis P. Sero-epidemiology as a tool to study the incidence of Salmonella infections in humans. Epidemiol Infect 2008; 136:895 - 902; http://dx.doi.org/10.1017/S0950268807009314; PMID: 17678562
- Wang CQ, Zhu QR. Seroprevalence of Bordetella pertussis antibody in children and adolescents in China. Pediatr Infect Dis J 2011; 30:593 - 6; http://dx.doi.org/10.1097/INF.0b013e31820eaf88; PMID: 21422963
- Zhang Q, Han F, Nie Q, Ren H, Zhang B, Liu Q, He Q, Shao Z. Seroprevalence of antibodies to pertussis and diphtheria among healthy adults in China. J Infect 2011; 63:441 - 6; http://dx.doi.org/10.1016/j.jinf.2011.07.018; PMID: 21864569
- Zhang Q, Zheng H, Liu M, Han K, Shu J, Wu C, Xu N, He Q, Luo H. The seroepidemiology of immunoglobulin G antibodies against pertussis toxin in China: a cross sectional study. BMC Infect Dis 2012; 12:138; http://dx.doi.org/10.1186/1471-2334-12-138; PMID: 22892100
- Roberts M, Cropley I, Chatfield S, Dougan G. Protection of mice against respiratory Bordetella pertussis infection by intranasal immunization with P.69 and FHA. Vaccine 1993; 11:866 - 72; http://dx.doi.org/10.1016/0264-410X(93)90363-3; PMID: 8356847
- Kimura A, Mountzouros KT, Relman DA, Falkow S, Cowell JL. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun 1990; 58:7 - 16; PMID: 2294058
- de Melker HE, Versteegh FG, Conyn-Van Spaendonck MA, Elvers LH, Berbers GA, van Der Zee A, Schellekens JF. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J Clin Microbiol 2000; 38:800 - 6; PMID: 10655388
- Krause DS. Cross-section seroepidemiology of Bordetella pertussis. J Infect Dis 1996; 174:896; http://dx.doi.org/10.1093/infdis/174.4.896; PMID: 8843241
- García-Corbeira P, Dal-Ré R, Aguilar L, García-de-Lomas J. Seroepidemiology of Bordetella pertussis infections in the Spanish population: a cross-sectional study. Vaccine 2000; 18:2173 - 6; http://dx.doi.org/10.1016/S0264-410X(00)00029-3; PMID: 10717335
- Guiso N, Berbers G, Fry NK, He Q, Riffelmann M, Wirsing von König CH, EU Pertstrain group. What to do and what not to do in serological diagnosis of pertussis: recommendations from EU reference laboratories. Eur J Clin Microbiol Infect Dis 2011; 30:307 - 12; http://dx.doi.org/10.1007/s10096-010-1104-y; PMID: 21069406
- Canthaboo C, Williams L, Xing DK, Corbel MJ. Investigation of cellular and humoral immune responses to whole cell and acellular pertussis vaccines. Vaccine 2000; 19:637 - 43; http://dx.doi.org/10.1016/S0264-410X(00)00253-X; PMID: 11090715
- Sato H, Sato Y. Experience with diphtheria toxoid-tetanus toxoid-acellular pertussis vaccine in Japan. Clin Infect Dis 1999; 28:Suppl 2 S124 - 30; http://dx.doi.org/10.1086/515063; PMID: 10447030
- Mills KH, Ryan M, Ryan E, Mahon BP. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect Immun 1998; 66:594 - 602; PMID: 9453614
- Pebody RG, Gay NJ, Giammanco A, Baron S, Schellekens J, Tischer A, Olander RM, Andrews NJ, Edmunds WJ, Lecoeur H, et al. The seroepidemiology of Bordetella pertussis infection in Western Europe. Epidemiol Infect 2005; 133:159 - 71; http://dx.doi.org/10.1017/S0950268804003012; PMID: 15724723
- Halperin SA. The control of pertussis--2007 and beyond. N Engl J Med 2007; 356:110 - 3; http://dx.doi.org/10.1056/NEJMp068288; PMID: 17215528
- Güriş D, Strebel PM, Bardenheier B, Brennan M, Tachdjian R, Finch E, Wharton M, Livengood JR. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990-1996. Clin Infect Dis 1999; 28:1230 - 7; http://dx.doi.org/10.1086/514776; PMID: 10451158
- Lee GM, Lett S, Schauer S, LeBaron C, Murphy TV, Rusinak D, Lieu TA, Massachusetts Pertussis Study Group. Societal costs and morbidity of pertussis in adolescents and adults. Clin Infect Dis 2004; 39:1572 - 80; http://dx.doi.org/10.1086/425006; PMID: 15578353
- Baughman AL, Cortese MM, Slade BA. Incidence of Bordetella pertussis infection in adolescents and adults. Clin Infect Dis 2007; 44:149 - 50, author reply 150-1; http://dx.doi.org/10.1086/510081; PMID: 17143835
- Mitka M. Age range widens for pertussis vaccine: boosters advised for adolescents and adults. JAMA 2006; 295:871 - 2; http://dx.doi.org/10.1001/jama.295.8.871; PMID: 16493092
- Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J 2005; 24:Suppl S58 - 61; http://dx.doi.org/10.1097/01.inf.0000160914.59160.41; PMID: 15876927
- Lavine JS, Bjørnstad ON, de Blasio BF, Storsaeter J. Short-lived immunity against pertussis, age-specific routes of transmission, and the utility of a teenage booster vaccine. Vaccine 2012; 30:544 - 51; http://dx.doi.org/10.1016/j.vaccine.2011.11.065; PMID: 22119924
- Wiese-Posselt M, Tertilt C, Zepp F. Vaccination recommendations for Germany. Dtsch Arztebl Int 2011; 108:771 - 9, quiz 780; PMID: 22163258
- de Greeff SC, Lugnér AK, van den Heuvel DM, Mooi FR, de Melker HE. Economic analysis of pertussis illness in the Dutch population: implications for current and future vaccination strategies. Vaccine 2009; 27:1932 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.01.106; PMID: 19368774
- Lai FY, Thoon KC, Ang LW, Tey SH, Heng D, Cutter JL, Phoon MC, Chow VT. Comparative seroepidemiology of pertussis, diphtheria and poliovirus antibodies in Singapore: waning pertussis immunity in a highly immunized population and the need for adolescent booster doses. Vaccine 2012; 30:3566 - 71; http://dx.doi.org/10.1016/j.vaccine.2012.03.059; PMID: 22475863
- Misegades LK, Winter K, Harriman K, Talarico J, Messonnier NE, Clark TA, Martin SW. Association of childhood pertussis with receipt of 5 doses of pertussis vaccine by time since last vaccine dose, California, 2010. JAMA 2012; 308:2126 - 32; http://dx.doi.org/10.1001/jama.2012.14939; PMID: 23188029
- Witt MA, Katz PH, Witt DJ. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin Infect Dis 2012; 54:1730 - 5; http://dx.doi.org/10.1093/cid/cis287; PMID: 22423127
- Skowronski DM, Janjua NZ, Tsafack EP, Ouakki M, Hoang L, De Serres G. The number needed to vaccinate to prevent infant pertussis hospitalization and death through parent cocoon immunization. Clin Infect Dis 2012; 54:318 - 27; http://dx.doi.org/10.1093/cid/cir836; PMID: 22156850
- Isacson J, Trollfors B, Taranger J, Lagergård T. Acquisition of IgG serum antibodies against two Bordetella antigens (filamentous hemagglutinin and pertactin) in children with no symptoms of pertussis. Pediatr Infect Dis J 1995; 14:517 - 21; http://dx.doi.org/10.1097/00006454-199506000-00009; PMID: 7667057
- Long SS, Welkon CJ, Clark JL. Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J Infect Dis 1990; 161:480 - 6; http://dx.doi.org/10.1093/infdis/161.3.480; PMID: 2313126
- He Q, Viljanen MK, Olander RM, Bogaerts H, De Grave D, Ruuskanen O, Mertsola J. Antibodies to filamentous hemagglutinin of Bordetella pertussis and protection against whooping cough in schoolchildren. J Infect Dis 1994; 170:705 - 8; http://dx.doi.org/10.1093/infdis/170.3.705; PMID: 8077734
- Xu Y, Luo P, Wang L, Wei C, Hou Q, Zhang S. Development and validation of ELISA assays for determination of serum antibody against pertussis toxin, filamentous hemagglutinin, pertactin of B. pertussis. Chinese Journal of Vaccine and Immunization 2011; 17:205 - 8
- Xing D, Wirsing von König CH, Newland P, Riffelmann M, Meade BD, Corbel M, Gaines-Das R. Characterization of reference materials for human antiserum to pertussis antigens by an international collaborative study. Clin Vaccine Immunol 2009; 16:303 - 11; http://dx.doi.org/10.1128/CVI.00372-08; PMID: 19109448