Abstract
The type of T cell polarization and simultaneous production of multiple cytokines have been correlated with vaccine efficacy. ELISpot is a T cell detection technique optimized for the measurement of a secreted cytokine at the single cell level. The FluoroSpot assay differs from ELISpot by the use of multiple fluorescent-labeled anticytokine detection antibodies, allowing optimal measurement of multiple cytokines. In the present study, we show that an IFNγ/IL-10 FluoroSpot assay is more sensitive than flow cytometry to detect Tr1 regulatory T cells, an immunosuppressive T cell population characterized by the production of IL-10 and IFNγ. As many tolerogenic vaccines are designed to induce these Tr1 cells, this FluoroSpot test could represent a standard method for the detection of these cells in the future.
The use of an IFNγ/IL-2 FluoroSpot assay during influenza vaccine monitoring showed that the influenza-specific IL-2-producing T-cell response was the dominant response both before and after vaccine administration. This study therefore questions the rationale of using the single-color IFNγ ELISpot as the standard technique to monitor vaccine-specific T-cell response. Using this same test, a trend was also observed between baseline levels of IFNγ T cell response and T cell vaccine response. In addition, a lower IFNγ+IL-2+ T-cell response after vaccine was observed in the group of patients treated with TNFα inhibitors (P = 0.08).
This study therefore supports the use of the FluoroSpot assay due to its robustness, versatility and the complementary information that it provides compared with ELISpot or flow cytometry to monitor vaccine-specific T-cell responses.
Introduction
Various T-cell subsets have been described based on their cytokine profiles. Type 1 cytokines (IL-2, IFNγ) promote cell-mediated immunity and enhance the cytotoxicity of macrophages against intracellular pathogens.Citation1 A type 1 cytokine profile has been associated with a better clinical outcome in chronic infections and cancer.Citation2-Citation5 In contrast, type 2 T cells, especially CD4+ T cells, produce another set of cytokines (IL-4, IL-5, IL-10, IL-13) and appear to be important for promoting differentiation of B cells and for control of extracellular pathogens and neutralization of viruses before their entry into cells.Citation6 CD4+ T helper (Th) cells producing IL-17A and possibly other cytokines (IL-22, G-CSF, IL-17F) are involved in the regulation of inflammation by inducing pro-inflammatory cytokinesCitation7 and in the defense against certain fungi (Candida albicans).Citation8
IL-10-producing T cells, often called Tr1 T cells, were defined in mice by H Groux and coworkers as cells that prevent colitis transferred by pathogenic effector T cells.Citation9 These cells may also produce TGFβ, but differ from Th2 cells by their simultaneous production of IFNγ but not IL-2 or Th2 cytokines (IL-4, IL-5). In contrast with Foxp3 regulatory T cells, whose suppressor activity is inhibited by inflammation, Tr1 T cells have been shown to control tissue inflammation.Citation10 Tr1 can be generated in vitro in the presence of IL-10 together with vitamin D3, IFNα, or other immunosuppressive drugs such as dexamethasone or rapamycin or with anti-CD46 antibodies in the presence of IL-2.Citation11,Citation12 In vitro generated Tr1 cells were able to inhibit the development of Th2-driven allergen-induced airway inflammation, serum IgE and airway hyperresponsiveness.Citation13,Citation14
Targeting specific receptors or signaling pathways on dendritic cells or mucosal routes of immunization may promote the induction of IL-10 regulatory T cells which may confer protection in allergy.Citation15-Citation17 More generally, depending on the adjuvant selected or the route of immunization, vaccine-specific T cells engage toward a specific program of polarization possibly correlated with vaccine efficacy.Citation18,Citation19 Determination of the profile of cytokines release by vaccine-induced T cells may therefore help to define the type of protection and clinical activity associated with the vaccine.
Various techniques have been developed to quantitate cytokine release by T cells. Some of these techniques (ELISpot [Enzyme-linked immunospot], intracellular flow cytometry) allow determination of the number of cytokine-producing T cells, while other techniques measure the cytokines produced in supernatants (Elisa, Luminex, Cytometric beads array) of a bulk population of T cells without assessment of individual cellular cytokine production.Citation20-Citation22 The classical ELISpot is designed to measure secretion of a single cytokine by a single cell. However, it has been shown that detection of the cytokine secretion profile and not just the secretion of a single cytokine is required to define the subset of T helper cells and that simultaneous production of various cytokines by T cells is associated with improved vaccine efficacy.Citation23 All these arguments support the simultaneous detection of cytokines at a single cell level. Flow cytometry is able to meet this requirement, but it only detects intracellular blocked cytokines and does not take into account post-translational regulation resulting from the switch from an inactive intracellular form to an active released form of cytokines such as IL-1, IL-18, and TGFβ. In addition, in contrast to the ELISpot, intracellular flow cytometry does not detect accumulation of the cytokines produced. It should be noted that cytometric bead array technology (CBA) can simultaneously quantitate multiple proteins, but in supernatants and not at a single cell level, which precludes determination of the frequency of cytokine-producing cells. Immunoenzymatic Dual-Color ELISpots have been described by various groups. Using this test, it has been confirmed that the breadth and magnitude of HIV-specific T cells secreting both IFNγ and IL-2 were positively correlated with CD4 cell counts and negatively correlated with viral load.Citation24 However, with colorimetric development, difficulties in the interpretation of mixed color spots have been reported, especially when one color is dominant.Citation25,Citation26 We therefore developed a FluoroSpot assay based on the use of multiple fluorescent-labeled anti-cytokine detection antibodies.Citation25 This assay clearly provides better discrimination and characterization of dual cytokine-producing cells than an enzymatic reaction.Citation25 The aim of this study was to: (1) develop new tools (cytokine transfected cells) to validate an IFNγ-IL-10 FluoroSpot assay in order to more clearly characterize immunoregulatory Tr1 cells; (2) compare the sensitivity of various FluoroSpot assays with that of conventional techniques able to determine the precursor frequency of specific T cells (intracellular flow cytometry, Elispot); (3) demonstrate the robustness and versatility of the FluoroSpot assay in routine practice to monitor an influenza vaccine clinical trial in patients with inflammatory bowel disease, as the CD4 +T cell response against influenza vaccine may also participate in protection against influenza.Citation27
We feel that this study provides new tools and arguments to support the development of this new technology in order to identify subpopulation of immune cells based on their cytokine profile and to quantitate specific polyfunctional T cells monitored after a clinical trial. It provides an integrated body of results concerning the FluoroSpot assay.
Results
Validation and comparative analysis of an IFNγ/IL-10 FluoroSpot vs. intracellular detection of IFNγ/IL-10 by flow cytometry
In order to validate an IFNγ/IL-10 FluoroSpot assay, we first confirmed that the CHO cell line cotransfected with cDNA encoding human IFNγ and IL-10 produced both IL-10 and IFNγ by monoparametric IFNγ and IL-10 ELISpot (Fig. S1).
The FluoroSpot assay was then used to assess the capacity of these cells to simultaneously produce the two cytokines. This test showed that the majority (more than 90%) of the cells produced the two cytokines at the single cell level (). To compare the sensitivity of this test with that of cytokine detection by intracellular flow cytometry, we mixed various proportions of parental and transfected CHO cells in a constant total number of cells (105 cells/well). The FluoroSpot assay clearly detected IFNγ and IL-10, when as few as 10 CH0γ10 cells were incubated in the assay (). In contrast, at least 5,000 CH0γ10 cells were required to detect these cytokines by flow cytometry after cell permeabilization (). As expected, flow cytometry and FluoroSpot analysis both showed that most CH0γ10 cells produced both IFNγ and IL-10 ( to be compared with ).
Figure 1. Detection of dual IFNγ− and IL-10-producing cells using the FluoroSpot assay and flow cytometry. Increasing numbers of transfected CHOγ10 cells constitutively secreting IFNγ and IL-10 cytokines were mixed with non-transfected CHO cells. Each cellular mix was incubated overnight in medium in FluoroSpot plates (105 cells/well). An amount of 5 µg/ml Brefeldin A was added 6 h after the start of incubation for intracellular cytokine staining (ICS) by flow cytometry. Each FluoroSpot condition was performed in triplicate. (A) Image of an IFNγ/IL-10 FluoroSpot with dual-color spots: green filter (IFNγ green spot), red filter (IL-10 red spots), and composite picture of both filters. (B) Same composite picture as in (A), counted with Immunospot® 5.16 (dual color spots are circled in white). Comparative analysis of signal obtained for IFNγ (C), IL-10 (D), and dual IFNγ+/IL10+ detection using the FluoroSpot assay or flow cytometry (ICS).
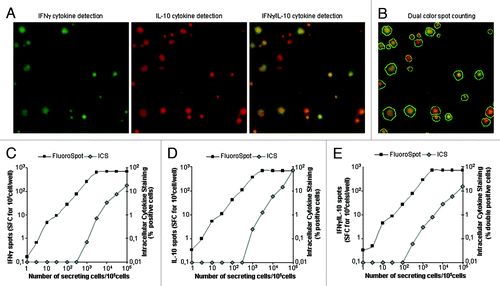
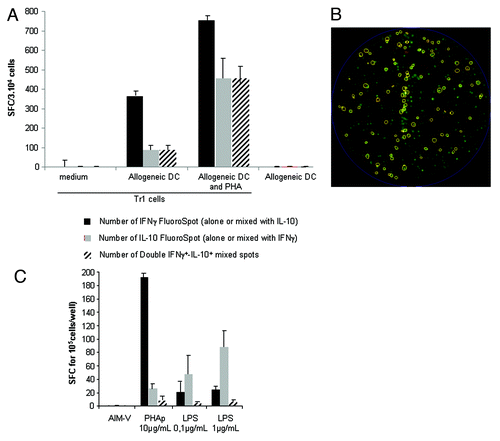
Tr1-generated T cells characterized by dual production of IFNγ and IL-10 were easily detected by this FluoroSpot assay (). Resting Tr1 cells did not produce IFNγ and IL-10. Bulk culture of Tr1 T cells activated by allogeneic DC either alone or combined with PHA comprised both bona fide Tr1 cells, which simultaneously produced IFNγ and IL-10 (). Few cells produced IL-10 alone, while some T cells, likely conventional T cells, produced IFNγ alone, indicating that the protocol used for Tr1 generation did not result in pure populations of Tr1 T cells. PBMC from healthy donors stimulated by various mitogens (PHA, LPS) produced various proportions of IFNγ and IL-10 with no significant dual IFNγ− and IL-10-producing cells ()
Figure 2. Detection of Tr1 cells by an IFNγ-IL-10 FluoroSpot assay. Tr1 cells were generated by coculturing CD4+CD25- cells with allogeneic DC (see Methods). One week after the second stimulation, cells were recovered and cultured with medium or activated for 24 h with allogeneic DC alone or in combination with PHAp. The IFNγ-IL-10 FluoroSpot assay was revealed 24 h later. (A) Quantitative and qualitative analysis of IFNγ-IL-10 FluoroSpot assay from Tr1-generated T cells. (B) Example of IFNγ-IL-10 FluoroSpot in activated Tr1 T cells. IFNγ and IL-10 FluoroSpots are green and red, respectively. Mixed dual IFNγ-IL-10 FluoroSpot are circled. (C) PBMC were stimulated with PHAp and LPS and the cytokines produced were revealed by an IFNγ-IL-10 FluoroSpot assay. Error bars represent the SD of triplicate wells in the FluoroSpot. These experiments were performed in duplicate.
Comparative analysis of IFNγ/IL-2 FluoroSpot and intracellular detection of IFNγ and IL-2
PBMC stimulated by PMA ionomycin produced substantial amounts of IFNγ and IL-2 detected by both FluoroSpot assay and intracellular detection of cytokine by flow cytometry. The dual IFNγ-IL-2 FluoroSpot assay was more sensitive to detect IFNγ, as no IFNγ signal was detected by flow cytometry when 10 000 PBMC were stimulated, while the FluoroSpot assay detected 24 spots (Fig S1). The sensitivity of the two tests to detect IL-2 or dual IFNγ and IL-2 production appeared to be equivalent (Fig. S1).
Comparative analysis of the sensitivity of the IFNγ/IL-2 FluoroSpot vs. the monoparametric IFNγ and IL-2 ELISpot
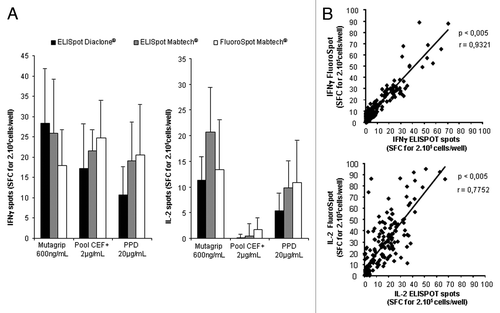
presents a representative experiment, in which the FluoroSpot assay did not appear to be less sensitive than the two commercial ELISpot assays used to quantitate IFNγ or IL-2 in the supernatants of these cells (). Monoparametric IFNγ and IL-2 ELISpot and FluoroSpot assays were then compared in a series of samples corresponding to 30 patients vaccinated with Mutagrip®, with testing of the anti-influenza response at d0 and d21 after vaccine administration. A strong correlation was observed between IFNγ ELISpot and FluoroSpot assays (r = 0.9; P < 0.005) and between IL-2 ELISpot and FluoroSpot assays (r = 0.77; P < 0.005) ().
Figure 3. Comparison of FluoroSpot and ELISpot to detect T-cell response using various commercial kits. IFNγ and IL-2 responses of PBMC (2.105) from healthy donors or patients after various stimulatory conditions were detected with ELISpot kits from Diaclone® and Mabtech® and FluoroSpot kits from Mabtech®. Spot counts were compared. (A) Comparative analysis of spots counts using these three techniques in representative healthy donors. Three healthy donors were selected for these experiments. (B) Correlation between IFNγ and IL-2 spots detected with Diaclone® ELISpot and Mabtech® FluoroSpot after stimulation of PBMC from patients vaccinated with Mutagrip® influenza seasonal vaccine (baseline levels and vaccine-induced T cells were pooled for this analysis). An amount of 160 samples were included for this comparison and some samples were tested at various dilutions. Error bars represent the SD of triplicate wells in the ELISpot or FluoroSpot
Quantitative and qualitative T-cell response to influenza vaccine using the FluoroSpot assay
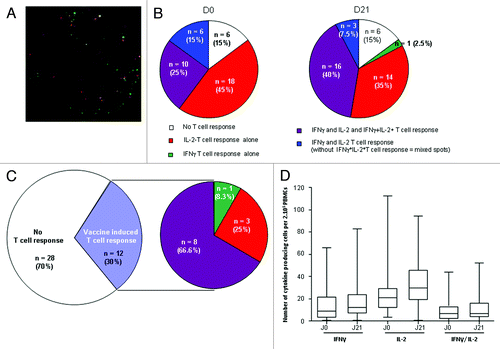
The dual IFNγ and IL-2 FluoroSpot assay showed that most patients (34/40 [85%]) presented a baseline T-cell response against the influenza vaccine (). This response was dominated by an IL-2 T-cell response (Fig. 4 A-C), as no patients presented an isolated IFNγ T-cell response before vaccination, whereas an isolated IL-2 T-cell response was detected in 45% of patients (). Anti-Mutagrip T cells simultaneously producing IFNγ and IL-2 were observed in 25% of patients. In some patients (15%), T cells produced IL-2 and IFNγ with no mixed spots, indicating that the two types of cytokines were not produced by the same cells (, left).
Figure 4. Qualitative influenza vaccine-specific T-cell response using dual IFNγ/IL-2 FluoroSpot assay. Forty PBMC (2.105) from patients were pulsed with Mutagrip composed of a mixture of influenza antigens before (D0) or 21 d after seasonal influenza vaccination with Mutagrip and the reaction was revealed with double IFNγ/L-2 FluoroSpot assay (A) Representative image of a T-cell response to Mutagrip (green, IFNγ FluoroSpot; red, IL-2 FluoroSpot; yellow, IFNγ/IL-2 mixed FluoroSpot). (B) Qualitative analysis at D0 and D21 of anti-Mutagrip T cell response using dual IFNγ/IL-2 FluoroSpot assay. IFNγ and IL-2 T cell responses correspond to monoparametric response to IFNγ and IL-2, whereas IFNγ+IL-2+ T-cell response corresponds to T cells producing both cytokines with mixed spots. (C) Left: Analysis of the number and percentage of patients responding to Mutagrip at day 21 using the dual IFNγ/IL-2 FluoroSpot assay. Right: Qualitative analysis of T-cell response in patients with vaccine-induced T-cell response. (D) Box and whisker plots of the IFNγ, IL-2 and IFNγ-IL-2 response before and 21 d after the vaccine is shown for the whole population. FluoroSpot assay was performed in triplicate.
Overall, IL-2 FluoroSpot allowed the detection of all baseline positive influenza-specific T cell responses (34/34 = 85%), while IFNγ FluoroSpot detected only 16 of the 34 T-cell responses (47%).
On day 21 after vaccine, the anti-Mutagrip T cell response resembled the baseline T-cell response except for a higher percentage (40%) of T cells simultaneously producing IFNγ and IL-2 (, right).
On day 21 after vaccine, when both IL-2 and IFNγ T cell responses were combined, 12 out of 40 patients (30%) exhibited induction or enhanced vaccine-specific T-cell response (, right). This vaccine-induced T-cell response was again dominated by the IL-2-T-cell response, as 25% of responses were only detected by IL-2 FluoroSpot, whereas 66.6% of T cells from patients responding to Mutagrip simultaneously produced both IFNγ and IL-2 (). A box and whisker plot analysis confirmed that the best response after vaccine was observed for IL-2 ().
A trend was observed between baseline levels of IFNγ T-cell response and T-cell vaccine response, as 37.5% of patients with a baseline IFNγ T-cell response presented an IFNγ T cell response against the vaccine, whereas only 12.5% of patients with no IFNγ T cell response responded to the vaccine by induction or enhancement of the IFNγ T cell response (P = 0.1). Some of the chronic inflammatory bowel disease patients in this series were treated with immunosuppressive therapy (33/40) comprising corticosteroids either alone (n = 15) or in combination (n = 18) with TNFα inhibitors. A lower IFNγ+IL-2+T cell vaccine response was observed in the group of patients treated with TNFα inhibitors (p = 0.08)(). A similar trend was observed for the IFNγ, but not for the IL-2 T cell response. (data not shown).
Table 1. Relationships between immunosuppressive therapy (with immunosuppressors [IS] and/or anti-TNFα antibodies) and vaccine-induced IFNγ+IL-2+T cell responses −
Discussion
This study clinically validated two FluoroSpot assays for simultaneous detection of dual IFNγ- and IL-10- or IFNγ- and IL-2-producing T cells. Using a new stable IFNγ- and IL-10-producing cell line, we showed that the FluoroSpot assay is more sensitive to detect IFNγ and IL-10 than flow cytometry. These findings extend previous results concerning the higher sensitivity of ELISpot compared with flow cytometry to detect intracellular cytokines.Citation28 This FluoroSpot assay allows easy detection of regulatory Tr1 cells generated in vitro. It will be important to extend these results in future studies by detecting these cells directly in vivo. The availability of a validated IFNγ/IL-10 FluoroSpot test with positive control could represent a future reference method to detect regulatory Tr1 cells based on their IL-10 and IFNγ production. Although some molecules (Lag3, ICOS, LAP, CD46, CD49b) expressed on CD4+ T cell subpopulations may identify IL-10-producing Tr1 cells, these results remain controversial and no consensus has been reached concerning the phenotype of Tr1 cells.Citation29-Citation31 Nevertheless, measurement of Tr1 cells may provide valuable information in various clinical settings.Citation32
Long-term tolerance to stem cell allografts, islet transplant and kidney or liver allografts has been associated with induction of Tr1 cells.Citation33-Citation35 We have shown that, depending on the modality of vaccination and especially the use of DC-based vaccine, the induced antitumor CD8+ T cells may produce IL-10.Citation36 With the development of tolerogenic vaccines, tracking of these populations may represent a possible surrogate marker of their clinical efficacy. Clinical improvement observed after allergen immunotherapy for allergic diseases such as rhinitis and asthma is associated with the induction of IL-10- and TGF-β-producing Tr-1 cells.Citation37
In a second part of this work, we compared an IFNγ/IL-2 FluoroSpot to conventional monoparametric IL-2 and IFNγ ELISpot assays. A very good correlation was demonstrated between FluoroSpot and ELISpot assays with an equivalent sensitivity, as already reported.Citation38 Previous studies have also shown that the FluoroSpot assay—like the ELISpot assay—was more sensitive than ELISA, possibly due to direct binding of the secreted cytokine to coated antibodies with no risk of interference from soluble cytokine receptors present in various human fluids.Citation25,Citation39-Citation42
Another advantage of FluoroSpot compared with ELISpot is that it allows counting of double or triple cytokine-producing cellsCitation26,Citation43,Citation44 and requires half as many cells to simultaneously measure both cytokines compared with the number of cells required to detect each cytokine separately. Comparison of the dual IFNγ and IL-2 FluoroSpot with flow cytometry showed that this test is more sensitive to detect IFNγ, as previously shown with the IFNγ and IL-10 dual color FluoroSpot.
To address the robustness and versatility of this FluoroSpot assay, this assay was used to measure anti-influenza T cell response during an influenza vaccine protocol. The influenza-specific IL-2-producing T-cell response was found to be the dominant response both before and after vaccination. Previous studies have reported that influenza-specific T cells preferentially produce IL-2 over IFNγ.Citation45,Citation46 After influenza vaccination, the CD4+ T cell response was also dominated by IL-2+IFNγ- T cells.Citation47,Citation48 It is noteworthy that the single-color IFNγ ELISpot has become the standard technique to assess antiviral T-cell response. For example, in HCV infection, viral clearance was associated with high magnitude of IFNγ T cell response.Citation49,Citation50 However, this study as well as other studies showed that single IFNγ detection for immune monitoring is insufficient to provide a global assessment of the T cell response and is not always correlated with virus clearance.Citation51,Citation52
The significance of this differential cytokine production by influenza-specific T cells is unclear. Central memory T cells are known to preferentially produce IL-2.Citation53 The majority of influenza virus-specific T cells present a central memory phenotype,Citation45,Citation54-Citation56 which may explain their dominant IL-2 production. In addition, human CD4+T cells primed by protein vaccines mainly secreted IL-2 but not IFNγ, whereas most CD4+ T cells in the same individuals initially primed by infection with live pathogens secreted IFNγ.Citation46,Citation57 Lastly, primed precursor uncommitted T Helper cells secreted IL-2 without IFNγ and differentiated into Th1 or Th2 phenotypes following in vitro stimulation with appropriate polarizing conditions.Citation46 However, influenza-specific IL-2+IFNγ- did not appear to be derived from uncommitted cells, as these cells maintained a Th1-like phenotype even under Th2 polarizing conditions.Citation46
In general, the ability of T cells to secrete IL-2 is correlated with their proliferative capacityCitation58-Citation61 and memory potential, but with limited effector functionality.Citation51
The proportions of dual IFNγ+IL-2+-producing influenza-specific T cells increased from 29% of total positive T-cell response before vaccination to 47% after vaccination.
This proportion of dual-positive IFNγ+IL-2+ T cells after vaccination is situated in the same range as that found after H5N1 vaccine administration in healthy subjects.Citation47,Citation62 Multifunctional T cells producing IL-2 have been associated with protection against infection by pathogenic Leishmania major and are induced by the most potent live vaccines, such as vaccinia and yellow fever vaccines.Citation23,Citation63,Citation64 Using a dual-color ELISpot, Bernard et al. showed that dual IFNγ and IL-2-T cell responses were more frequent in acute than in chronic HIV infection, while the proportion of T cells secreting only IFNγ increased with time from infection.Citation65 These results may be explained by early loss of IL-2 secretion with persistence of IFNγ secretion in exhausted T cells during chronic infection.Citation51,Citation52,Citation66
This FluoroSpot assay demonstrated a lower IFNγ+-IL-2+ T-cell response after vaccination in the group of patients treated with TNFα inhibitors. To our knowledge, no previous study has assessed the influence of TNFα inhibitors on T-cell response after vaccination. However, the humoral response did not appear to be significantly affected by TNFα inhibitors.Citation67 It would be interesting to conduct an in vitro study to investigate the consequences of TNFα inhibition on IFNγ and IL-2 production.
Overall, this study provides new quality control tools for the use of the FluoroSpot assay and demonstrates its higher sensitivity compared with that of flow cytometry to measure the frequency of cytokine-producing cells. This study also validated the feasibility of the FluoroSpot assay for use in the clinic for the detection of T cell subpopulations based on their cytokine production and for quantitation of polyfunctional specific T cells during vaccine monitoring. The cellular origin of cytokine production could not be determined due to the limited blood volume of patient samples. However, it is likely that influenza-specific dual IL-2-IFNγ-producing cells correspond to CD4+ T cells, as the seasonal influenza vaccine composed of recombinant proteins without adjuvant is known not to induce CD8+ T cells.Citation68 In vitro, in the antigen presentation assay used, at the vaccine dosage selected (10 μg/ml), cross-presentation did not occur, which precluded activation of CD8+ T cells.Citation69
Unfortunately, FluoroSpot assay results could not be correlated with humoral response, as vaccines were used as antigens for the FluoroSpot assay, whereas purified HA proteins were used to measure humoral response. The clinical value of the FluoroSpot assay to detect double cytokine-producing cells could not be assessed in this study.
A recent study reported that the frequency of total pre-existing influenza-specific T cells was strongly inversely correlated with virus shedding, illness duration, and total symptom scores after influenza virus challenge.Citation70 These findings may indicate the need for more systematic assessment of influenza-specific T-cell response to predict patient protection. This study therefore supports the use of the FluoroSpot assay due to its robustness, versatility, and the complementary information it provides compared with ELISpot to monitor vaccine-specific T-cell response. Its higher sensitivity compared with cytometry and its ability to detect multifunctional specific T cells shown in the present study represent other arguments to introduce this new assay in clinical laboratory.
Material and Methods
Patients
Patients with inflammatory bowel disease (Crohn disease or ulcerative colitis) were vaccinated with one dose (0.5 ml) of the Mutagrip seasonal (2009–2010 winter) influenza vaccine (Sanofi-Pasteur) composed of the following three influenza strains (A/Brisbane/59/2007 [H1N1]-like, A/Brisbane/10/2007 [H3N2], and B/Brisbane/60/2008-like). Patients were either treated or not treated with TNFα inhibitors (infliximab or adalimumab) either alone or in combination with other immunosuppressive therapies (thiopurine, corticosteroids, and methotrexate).
Cells
PBMC and ascites lymphocytes
Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coat (BC) of healthy donors (HD) provided by the French blood institute (EFS) or from 40 inflammatory bowel disease patients vaccinated with Mutagrip® (Sanofi-Pasteur). Ascitic fluid was collected from a patient with peritoneal carcinomatosis. Ascites-infiltrating cells were then isolated. Written informed consent was obtained from each patient. This protocol was approved by the CPP Ile de France III ethics committee. The phase 3 clinical trial was registered at the clinical trials.gov website under number: NCT 01022749
CHO (Chinese hamster ovary)-IFNγ-IL10 (CH0γ10)
The IFNγ- and IL-10-producing CHO cell line was derived from the CHO-IFNγ cell line.Citation71 This cell line was co-transfected with a pcDNA3.1 plasmid encoding for human IL-10 and a neomycin resistance gene obtained from P. Van der Bruggen (Institut Ludwig, Brussels). The plasmid was first linearized with Sca1 A and 100 μg of this plasmid were then electroporated at 300 V and 150 μ Farads (GenePulser II, Biorad). After electroporation, the cells were cultured in RPMI (Roswell Park Memorial Institute)-10% fetal calf serum (FCS), 5% Glutamine and Pyruvate and selected by adding 4.5 μM methotrexate (CHO-IFNγ) and 0.5 mg/ml G418 (neomycin). All cell lines were cultured at 37 °C under 95% relative humidity and 5% CO2 conditions.
Intracellular cytokine staining assay
Flow cytometry analysis was performed as previously described.Citation20 For dual detection of IFNγ and IL10, IFNγ and IL-10 were stained by Fluorescein-IsoThioCyanate (FITC)-conjugated anti-IFNγ mAb (B-B1, IgG1) and PhycoErythrin (PE) conjugated-anti-IL-10 mAb (B-N10, IgG1) (GenProbe-Diaclone), respectively. For dual detection of IFNγ and IL-2, IFNγ and IL-2 were stained by PE conjugated-anti-IFNγ mAb (4S.B3, IgG1) and FITC conjugated anti-IL-2 mAb (5344.111, IgG1)(Becton-Dickinson), respectively. The fixative and permeabilization procedure was performed using the BD cytofix/cytoperm kit as recommended by the supplier (Becton Dickinson). Isotype controls were included in each experiment.
IFNγ, IL-2, and IL-10 ELISpot assays
Assays were performed according to the manufacturers’ recommendations (GenProbe-Diaclone or Mabtech Nacka) as previously described.Citation20 See Supplementary Methods for more details.
IFNγ/IL-2 and IFNγ/IL-10 FluoroSpot assays
All FluoroSpot assays were performed using FluoroSpot kits from Mabtech, according to the manufacturer’s instructions. See supplementary methods for more details.
ELISpot and FluoroSpot analysis
ELISpot and FluoroSpot well images were captured with an Immunospot® Series 5 UV Analyzer (Cellular Technology Limited [C.T.L.]) and analyzed with ImmunoSpot®5.0 professional analysis software (C.T.L.) or ImmunoSpot®5.16 professional analysis software (C.T.L.) for ELISpot or FluoroSpot, respectively.
Generation of Tr1 cells
Tr1 cells were obtained by co-culture of CD4+CD25− cells with allogeneic monocyte-derived dendritic cells (Mo-DCs) as previously described by Levings et al.Citation72 See supplementary methods for more details.
Serology
Antibody titers against the 3 influenza vaccine antigens were assayed using a hemagglutination inhibition test modified from the Center for Disease Control (CDC) guidelines. Briefly, sera were treated with receptor destroying enzyme (RDE) (Denka Seiken Tokyo, Japan) to remove nonspecific inhibitors. 2-fold dilutions of treated sera, starting at 1:10, were tested against four hemagglutinin units of each antigen (Influenza A/Uruguay/716/2007 [H3N2], Influenza A/Brisbane 59/07 [H1N1], Influenza B/Brisbane/60/08, NISBC, UK) on human O Rh− red blood cells. The HAI antibody titer was defined as the highest serum dilution that completely inhibited hemagglutination. All sera from an individual patient were analyzed on the same microtiter plate. Sera with titers <10 were assigned a titer of five for calculation purposes.
Statistical analysis
The number of cells specifically responsive to the antigens or mitogens and expressed as spot-forming cells (SFC) was calculated after subtracting negative controls (PBMC incubated with medium). A response was considered positive when the number of spots in the wells stimulated with antigens was 2-fold higher than the number of spots in the wells without antigen with a cut-off of 10 SFC/2.10Citation5 cells above mean background. Vaccinal response was considered positive when, for the same patient, at d21, the number of spots in the wells stimulated with antigens was 2-fold higher than the number of spots in the wells without antigen with a cut-off of 10 SFC/2.10Citation5 cells above mean background and if the d21/d0 ratio for an antigen was greater than or equal to 2. For ICS, % of positive cells was obtained after subtracting negative controls (PBMC incubated with corresponding isotype control Ab). Determination factor (r) and the regression curve were obtained using linear regression calculation on paired values. For all data, P values were calculated using Student’s t test and are shown when significant.
Additional material
Download Zip (810 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the French Ministry of Health (Programme Hospitalier de Recherche Clinique National), Ligue contre le Cancer, Agence Nationale de la Recherche (ANR), Labex Immuno-Oncology, Institut National du Cancer (Carpem project), Canceropole-Region Ile de France.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/vaccines/article/26593
References
- Tartour E, Mathiot C, Fridman WH. Current status of interleukin-2 therapy in cancer. Biomed Pharmacother 1992; 46:473 - 84; http://dx.doi.org/10.1016/0753-3322(92)90005-R; PMID: 1306361
- Andersen P, Doherty TM, Pai M, Weldingh K. The prognosis of latent tuberculosis: can disease be predicted?. Trends Mol Med 2007; 13:175 - 82; http://dx.doi.org/10.1016/j.molmed.2007.03.004; PMID: 17418641
- Zeremski M, Petrovic LM, Talal AH. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J Viral Hepat 2007; 14:675 - 87; PMID: 17875002
- Adotevi O, Vingert B, Freyburger L, Shrikant P, Lone YC, Quintin-Colonna F, Haicheur N, Amessou M, Herbelin A, Langlade-Demoyen P, et al. B subunit of Shiga toxin-based vaccines synergize with alpha-galactosylceramide to break tolerance against self antigen and elicit antiviral immunity. J Immunol 2007; 179:3371 - 9; PMID: 17709554
- Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, Badoual C, Gey A, Ravel P, Marcheteau E, et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood 2011; 118:4853 - 62; http://dx.doi.org/10.1182/blood-2011-01-329656; PMID: 21908423
- De Carli M, D’Elios MM, Zancuoghi G, Romagnani S, Del Prete G. Human Th1 and Th2 cells: functional properties, regulation of development and role in autoimmunity. Autoimmunity 1994; 18:301 - 8; http://dx.doi.org/10.3109/08916939409009532; PMID: 7858116
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med 2009; 361:888 - 98; http://dx.doi.org/10.1056/NEJMra0707449; PMID: 19710487
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011; 332:65 - 8; http://dx.doi.org/10.1126/science.1200439; PMID: 21350122
- Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MGA. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997; 389:737 - 42; http://dx.doi.org/10.1038/39614; PMID: 9338786
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med 2002; 195:603 - 16; http://dx.doi.org/10.1084/jem.20011629; PMID: 11877483
- Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, Roncarolo MG. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes 2006; 55:40 - 9; http://dx.doi.org/10.2337/diabetes.55.01.06.db05-0613; PMID: 16380475
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 2003; 421:388 - 92; http://dx.doi.org/10.1038/nature01315; PMID: 12540904
- Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med 2002; 8:1024 - 32; http://dx.doi.org/10.1038/nm745; PMID: 12145647
- Cottrez F, Hurst SD, Coffman RL, Groux H. T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol 2000; 165:4848 - 53; PMID: 11046008
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012; 18:1254 - 61; http://dx.doi.org/10.1038/nm.2883; PMID: 22842478
- Mascarell L, Lombardi V, Louise A, Saint-Lu N, Chabre H, Moussu H, Betbeder D, Balazuc AM, Van Overtvelt L, Moingeon P. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol 2008; 122:603 - 9, e5; http://dx.doi.org/10.1016/j.jaci.2008.06.034; PMID: 18774396
- Jarnicki AG, Conroy H, Brereton C, Donnelly G, Toomey D, Walsh K, Sweeney C, Leavy O, Fletcher J, Lavelle EC, et al. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol 2008; 180:3797 - 806; PMID: 18322186
- Sun JB, Czerkinsky C, Holmgren J. Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand J Immunol 2010; 71:1 - 11; http://dx.doi.org/10.1111/j.1365-3083.2009.02321.x; PMID: 20017804
- Zygmunt BM, Rharbaoui F, Groebe L, Guzman CA. Intranasal immunization promotes th17 immune responses. J Immunol 2009; 183:6933 - 8; http://dx.doi.org/10.4049/jimmunol.0901144; PMID: 19890060
- Chauvat A, Benhamouda N, Loison E, Gougeon ML, Gey A, Levionnois E, Ravel P, Abitbol V, Roncelin S, Marcheteau E, et al. Pitfalls in anti-influenza T cell detection by Elispot using thimerosal containing pandemic H1N1 vaccine as antigen. J Immunol Methods 2012; 378:81 - 7; http://dx.doi.org/10.1016/j.jim.2012.02.008; PMID: 22366633
- Bercovici N, Haicheur N, Massicard S, Vernel-Pauillac F, Adotevi O, Landais D, Gorin I, Robert C, Prince HM, Grob JJ, et al. Analysis and characterization of antitumor T-cell response after administration of dendritic cells loaded with allogeneic tumor lysate to metastatic melanoma patients. J Immunother 2008; 31:101 - 12; http://dx.doi.org/10.1097/CJI.0b013e318159f5ba; PMID: 18157017
- Haicheur N, Escudier B, Dorval T, Negrier S, De Mulder PH, Dupuy JM, Novick D, Guillot T, Wolf S, Pouillart P, et al. Cytokines and soluble cytokine receptor induction after IL-12 administration in cancer patients. Clin Exp Immunol 2000; 119:28 - 37; http://dx.doi.org/10.1046/j.1365-2249.2000.01112.x; PMID: 10606961
- Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13:843 - 50; http://dx.doi.org/10.1038/nm1592; PMID: 17558415
- Peretz Y, Ndongala ML, Boulet S, Boulassel MR, Rouleau D, Côté P, Longpré D, Routy JP, Falutz J, Tremblay C, et al. Functional T cell subsets contribute differentially to HIV peptide-specific responses within infected individuals: correlation of these functional T cell subsets with markers of disease progression. Clin Immunol 2007; 124:57 - 68; http://dx.doi.org/10.1016/j.clim.2007.04.004; PMID: 17521962
- Gazagne A, Claret E, Wijdenes J, Yssel H, Bousquet F, Levy E, Vielh P, Scotte F, Goupil TL, Fridman WH, et al. A Fluorospot assay to detect single T lymphocytes simultaneously producing multiple cytokines. J Immunol Methods 2003; 283:91 - 8; http://dx.doi.org/10.1016/j.jim.2003.08.013; PMID: 14659902
- Rebhahn JA, Bishop C, Divekar AA, Jiminez-Garcia K, Kobie JJ, Lee FE, Maupin GM, Snyder-Cappione JE, Zaiss DM, Mosmann TR. Automated analysis of two- and three-color fluorescent Elispot (Fluorospot) assays for cytokine secretion. Comput Methods Programs Biomed 2008; 92:54 - 65; http://dx.doi.org/10.1016/j.cmpb.2008.06.002; PMID: 18644656
- McKinstry KK, Strutt TM, Swain SL. Hallmarks of CD4 T cell immunity against influenza. J Intern Med 2011; 269:507 - 18; http://dx.doi.org/10.1111/j.1365-2796.2011.02367.x; PMID: 21362069
- Karulin AY, Hesse MD, Tary-Lehmann M, Lehmann PV. Single-cytokine-producing CD4 memory cells predominate in type 1 and type 2 immunity. J Immunol 2000; 164:1862 - 72; PMID: 10657635
- Fujio K, Okamura T, Yamamoto K. The Family of IL-10-secreting CD4+ T cells. Adv Immunol 2010; 105:99 - 130; http://dx.doi.org/10.1016/S0065-2776(10)05004-2; PMID: 20510731
- Pot C, Apetoh L, Kuchroo VK. Type 1 regulatory T cells (Tr1) in autoimmunity. Semin Immunol 2011; 23:202 - 8; http://dx.doi.org/10.1016/j.smim.2011.07.005; PMID: 21840222
- Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 2013; 19:739 - 46; http://dx.doi.org/10.1038/nm.3179; PMID: 23624599
- Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest 2006; 116:3252 - 7; http://dx.doi.org/10.1172/JCI29251; PMID: 17099776
- Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, de Waal Malefyt R, de Vries JE, Roncarolo MG. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med 1994; 179:493 - 502; http://dx.doi.org/10.1084/jem.179.2.493; PMID: 7905018
- Huurman VA, Velthuis JH, Hilbrands R, Tree TI, Gillard P, van der Meer-Prins PM, Duinkerken G, Pinkse GG, Keymeulen B, Roelen DL, et al. Allograft-specific cytokine profiles associate with clinical outcome after islet cell transplantation. Am J Transplant 2009; 9:382 - 8; http://dx.doi.org/10.1111/j.1600-6143.2008.02479.x; PMID: 19067657
- VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier RP, Orosz CG. Human allograft acceptance is associated with immune regulation. J Clin Invest 2000; 106:145 - 55; http://dx.doi.org/10.1172/JCI9171; PMID: 10880058
- Connerotte T, Van Pel A, Godelaine D, Tartour E, Schuler-Thurner B, Lucas S, Thielemans K, Schuler G, Coulie PG. Functions of Anti-MAGE T-cells induced in melanoma patients under different vaccination modalities. Cancer Res 2008; 68:3931 - 40; http://dx.doi.org/10.1158/0008-5472.CAN-07-5898; PMID: 18483279
- Nouri-Aria KT, Durham SR. Regulatory T cells and allergic disease. Inflamm Allergy Drug Targets 2008; 7:237 - 52; http://dx.doi.org/10.2174/187152808786848405; PMID: 19075789
- Hallengärd D, Haller BK, Maltais AK, Gelius E, Nihlmark K, Wahren B, Bråve A. Comparison of plasmid vaccine immunization schedules using intradermal in vivo electroporation. Clin Vaccine Immunol 2011; 18:1577 - 81; http://dx.doi.org/10.1128/CVI.05045-11; PMID: 21752954
- Montero-Julian FA, Brailly H, Sautès C, Joyeux I, Dorval T, Mosseri V, Yasukawa K, Wijdenes J, Adler A, Gorin I, et al. Characterization of soluble gp130 released by melanoma cell lines: A polyvalent antagonist of cytokines from the interleukin 6 family. Clin Cancer Res 1997; 3:1443 - 51; PMID: 9815830
- Badoual C, Bouchaud G, Agueznay NelH, Mortier E, Hans S, Gey A, Fernani F, Peyrard S, -Puig PL, Bruneval P, et al. The soluble alpha chain of interleukin-15 receptor: a proinflammatory molecule associated with tumor progression in head and neck cancer. Cancer Res 2008; 68:3907 - 14; http://dx.doi.org/10.1158/0008-5472.CAN-07-6842; PMID: 18483276
- Guerkov RE, Targoni OS, Kreher CR, Boehm BO, Herrera MT, Tary-Lehmann M, Lehmann PV, Schwander SK. Detection of low-frequency antigen-specific IL-10-producing CD4(+) T cells via ELISPOT in PBMC: cognate vs. nonspecific production of the cytokine. J Immunol Methods 2003; 279:111 - 21; http://dx.doi.org/10.1016/S0022-1759(03)00240-0; PMID: 12969552
- Tartour E, Mosseri V, Jouffroy T, Deneux L, Jaulerry C, Brunin F, Fridman WH, Rodriguez J. Serum soluble interleukin-2 receptor concentrations as an independent prognostic marker in head and neck cancer. Lancet 2001; 357:1263 - 4; http://dx.doi.org/10.1016/S0140-6736(00)04420-2; PMID: 11418153
- Ahlborg N, Axelsson B. Dual- and triple-color fluorospot. Methods Mol Biol 2012; 792:77 - 85; http://dx.doi.org/10.1007/978-1-61779-325-7_6; PMID: 21956502
- Smedman C, Ernemar T, Gudmundsdotter L, Gille-Johnson P, Somell A, Nihlmark K, Gårdlund B, Andersson J, Paulie S. FluoroSpot analysis of TLR-activated monocytes reveals several distinct cytokine secreting subpopulations. Scand J Immunol 2011; 75:249 - 58; http://dx.doi.org/10.1111/j.1365-3083.2011.02641.x; PMID: 21955279
- Elrefaei M, McElroy MD, Preas CP, Hoh R, Deeks S, Martin J, Cao H. Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol 2004; 173:2184 - 9; PMID: 15265956
- Divekar AA, Zaiss DM, Lee FE, Liu D, Topham DJ, Sijts AJ, Mosmann TR. Protein vaccines induce uncommitted IL-2-secreting human and mouse CD4 T cells, whereas infections induce more IFN-gamma-secreting cells. J Immunol 2006; 176:1465 - 73; PMID: 16424174
- Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A 2009; 106:3877 - 82; http://dx.doi.org/10.1073/pnas.0813390106; PMID: 19237568
- Powers DC, McElhaney JE, Florendo OA Jr., Manning MC, Upshaw CM, Bentley DW, Wilkinson BE. Humoral and cellular immune responses following vaccination with purified recombinant hemagglutinin from influenza A (H3N2) virus. J Infect Dis 1997; 175:342 - 51; http://dx.doi.org/10.1093/infdis/175.2.342; PMID: 9203655
- Flynn JK, Dore GJ, Hellard M, Yeung B, Rawlinson WD, White PA, Kaldor JM, Lloyd AR, Ffrench RA, ATAHC Study Group. Early IL-10 predominant responses are associated with progression to chronic hepatitis C virus infection in injecting drug users. J Viral Hepat 2011; 18:549 - 61; http://dx.doi.org/10.1111/j.1365-2893.2010.01335.x; PMID: 20626625
- Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med 2001; 194:1395 - 406; http://dx.doi.org/10.1084/jem.194.10.1395; PMID: 11714747
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 2008; 8:247 - 58; http://dx.doi.org/10.1038/nri2274; PMID: 18323851
- Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev 2006; 211:236 - 54; http://dx.doi.org/10.1111/j.0105-2896.2006.00395.x; PMID: 16824132
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708 - 12; http://dx.doi.org/10.1038/44385; PMID: 10537110
- Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol 2007; 81:8468 - 76; http://dx.doi.org/10.1128/JVI.00228-07; PMID: 17553885
- Lucas M, Day CL, Wyer JR, Cunliffe SL, Loughry A, McMichael AJ, Klenerman P. Ex vivo phenotype and frequency of influenza virus-specific CD4 memory T cells. J Virol 2004; 78:7284 - 7; http://dx.doi.org/10.1128/JVI.78.13.7284-7287.2004; PMID: 15194806
- Danke NA, Kwok WW. HLA class II-restricted CD4+ T cell responses directed against influenza viral antigens postinfluenza vaccination. J Immunol 2003; 171:3163 - 9; PMID: 12960344
- Chapman TJ, Castrucci MR, Padrick RC, Bradley LM, Topham DJ. Antigen-specific and non-specific CD4+ T cell recruitment and proliferation during influenza infection. Virology 2005; 340:296 - 306; http://dx.doi.org/10.1016/j.virol.2005.06.023; PMID: 16054188
- Lichterfeld M, Yu XG, Mui SK, Williams KL, Trocha A, Brockman MA, Allgaier RL, Waring MT, Koibuchi T, Johnston MN, et al. Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. J Virol 2007; 81:4199 - 214; http://dx.doi.org/10.1128/JVI.01388-06; PMID: 17287271
- Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med 2003; 198:1909 - 22; http://dx.doi.org/10.1084/jem.20031598; PMID: 14676302
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 2003; 9:540 - 7; http://dx.doi.org/10.1038/nm866; PMID: 12692546
- Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc Natl Acad Sci U S A 2005; 102:7239 - 44; http://dx.doi.org/10.1073/pnas.0502393102; PMID: 15872023
- Pedersen GK, Madhun AS, Breakwell L, Hoschler K, Sjursen H, Pathirana RD, Goudsmit J, Cox RJ. T-helper 1 cells elicited by H5N1 vaccination predict seroprotection. J Infect Dis 2012; 206:158 - 66; http://dx.doi.org/10.1093/infdis/jis330; PMID: 22551811
- Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med 2006; 203:413 - 24; http://dx.doi.org/10.1084/jem.20051720; PMID: 16461338
- Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell 2006; 124:849 - 63; http://dx.doi.org/10.1016/j.cell.2006.02.019; PMID: 16497593
- Ndongala ML, Kamya P, Boulet S, Peretz Y, Rouleau D, Tremblay C, Leblanc R, Côté P, Baril JG, Thomas R, et al. Changes in function of HIV-specific T-cell responses with increasing time from infection. Viral Immunol 2010; 23:159 - 68; http://dx.doi.org/10.1089/vim.2009.0084; PMID: 20373996
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 2003; 77:4911 - 27; http://dx.doi.org/10.1128/JVI.77.8.4911-4927.2003; PMID: 12663797
- Agarwal N, Ollington K, Kaneshiro M, Frenck R, Melmed GY. Are immunosuppressive medications associated with decreased responses to routine immunizations? A systematic review. Vaccine 2012; 30:1413 - 24; http://dx.doi.org/10.1016/j.vaccine.2011.11.109; PMID: 22197580
- Hillaire ML, Osterhaus AD, Rimmelzwaan GF. Induction of virus-specific cytotoxic T lymphocytes as a basis for the development of broadly protective influenza vaccines. J Biomed Biotechnol 2011; 2011:939860; http://dx.doi.org/10.1155/2011/939860; PMID: 22007149
- Lee RS, Tartour E, van der Bruggen P, Vantomme V, Joyeux I, Goud B, Fridman WH, Johannes L. Major histocompatibility complex class I presentation of exogenous soluble tumor antigen fused to the B-fragment of Shiga toxin. Eur J Immunol 1998; 28:2726 - 37; http://dx.doi.org/10.1002/(SICI)1521-4141(199809)28:09<2726::AID-IMMU2726>3.0.CO;2-W; PMID: 9754560
- Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012; 18:274 - 80; http://dx.doi.org/10.1038/nm.2612; PMID: 22286307
- Gey A, Hamdi S, Vielh P, Mehtali M, Fridman WH, Tartour E. Development of a direct in situ RT-PCR method using labeled primers to detect cytokine mRNA inside cells. J Immunol Methods 1999; 227:149 - 60; http://dx.doi.org/10.1016/S0022-1759(99)00078-2; PMID: 10485262
- Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood 2005; 105:1162 - 9; http://dx.doi.org/10.1182/blood-2004-03-1211; PMID: 15479730
