 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pneumococcal disease epidemiology has changed after introduction of pneumococcal conjugate vaccines. Seven-valent vaccine (PCV7) has been effective in reducing invasive pneumococcal disease (IPD). In Europe, PCV13 effectiveness was estimated at 78% (95% CI: −18–96%) for 2-priming doses. In Italy, PCV7 was introduced in 2006 in the childhood immunization schedule and replaced with PCV13 in 2010. In Apulia, vaccination coverage has reached 95.1% (birth-cohort 2010). We estimated PCV program effectiveness and its impact on S. pneumoniae diseases.
PCV Effectiveness: We used the screening method. We calculated the Proportion of Population Vaccinated from immunization registries and detected cases through a laboratory-confirmed surveillance among hospitalized children ≤60 months. A confirmed IPD case was a child with PCR positive for S. pneumoniae. Differences among children were assessed with the Chi-square or the Fisher exact test (P value < 0.05).
PCV Impact: We constructed time series using outcome-specific Poisson regression models: hospitalization rate in pre-PCV era and hospitalization risk ratios (RRs) with 95% CIs for both PCV7 and PCV7/PCV13 shifting era. We calculated hospitalization RR with 95% CIs comparing pre-PCV years with vaccination period. The PCV effectiveness was 84.3% (95% CI: 84.0–84.6%). In May 2010-January 2013, we enrolled 159 suspected IPD of whom 4 were confirmed. Two (fully vaccinated) were caused by serotype 9V, 1 (not vaccinated) by serotype 3, 1 (vaccinated with 2 PCV13 doses) by 15B/C. The most important reduction was for pneumococcal pneumonia (RR: 0.43, 95% CI: 0.21–0.90). The PCV program show promising results in terms of both PCV13 effectiveness and its impact in reducing IPD in children <5 years.
Introduction
Pneumococcal disease remains a major public health problem worldwide,Citation1 although its epidemiology has dramatically changed, particularly among young children, in the US and in a number of EU countries after the introduction of pneumococcal conjugate vaccines into national childhood immunization programmes.Citation2,Citation3 Since its introduction in 2000, the 7-valent pneumococcal conjugate vaccine (PCV7) has been effective in reducing invasive pneumococcal disease (IPD) and non-invasive infections (pneumonia and otitis media, especially complicated cases) in all age groups.Citation4-Citation7 Recently, many countries have introduced higher valence conjugate vaccines (PCV10 or PCV13) following an increase of non-vaccine-serotype IPD cases due to serotype replacement.Citation8-Citation12 Both new vaccines have been licensed based on a putative correlate of protection.Citation13 The recently published results of a clinical trial evaluating safety, immunogenicity, and impact of PCV13 provide evidence that PCV13 is effective in preventing vaccine-type IPD in Alaska Native children.Citation14 In Europe, PCV13 effectiveness has been estimated to be 78% (95% CI: −18% to 96%) and 73% (95% CI: 29–90%) for two priming doses and for one dose over a year respectively.Citation15 Moreover, PCV13 has shown an impact on overall pneumococcal nasopharyngeal carriage in young children <2 y with acute otitis media (AOM).Citation16
In Italy, between 2006 and 2010, all 21 regions had recommended or introduced PCV7 in their childhood immunization schedules.Citation17 In May 2010, a national recommendation by the Ministry of Health replaced PCV7 with PCV13.Citation18 Since February 2012, PCV13 has been included in the list of Essential Health Interventions. The vaccine is actively offered, free of charge, to all newborns with 3 doses at 3, 5–6, and 11–13 mo of age (the 2p + 1 schedule: 3, 5, and booster at 12 mo).Citation19,Citation20 In addition, (1) children who had received 1 or more doses of PCV7 should complete immunization with PCV13 (sequential schedule: 1 PCV7 dose + 2 PCV13 doses; 2 PCV7 doses + 1 PCV13 dose); (2) children aged 16–24 mo who had received 3 doses of PCV7 before age 12 mo are recommended to receive 1 PCV13 catch up dose. Children who had never been vaccinated with PCV7 should receive (1) 2 doses of PCV13 with an interval of at least two months if aged between 12 and 23 mo; (2) 1 dose if aged between 2 and 5 y; (3) 3 doses (at least two months apart from each other) plus a booster between 12 and 15 mo, if preterm infants. For children <60 mo of age with underlying medical conditions (1) those who had received 3 doses of PCV7 within 12 mo of age should receive two additional doses of PCV13 at 12–15 and at 16–24 mo; (2) those who had received any incomplete schedule of less than 3 doses of PCV7 should complete with the PCV13 sequential schedule (1 PCV7 dose + 3 PCV13 doses; 2 PCV7 doses + 2 PCV13 doses) by their 15 mo; (3) children between 24 and 60 mo of age, who had completed vaccination with PCV7 by the age of 12 mo should receive two doses with an interval of two months ().Citation18
Table 1. Italian national recommendations for PCV13 immunization schedule in newborns and in children <60 mo of age
In Apulia region, PCV7 was introduced in January 2006 and it has been replaced by PCV13 in May 2010. According to the “National Immunization Plan 2012–2014,” the objective for pneumococcal conjugate vaccination is to achieve and maintain a vaccination coverage of at least 95% among children under 24 mo of age.Citation21,Citation22 Vaccination coverage is routinely computed from the regional Computerized Immunization Registry. PCV vaccination coverage in children under 24 mo of age has progressively increased from 75.3% in birth cohort 2006 (PCV7 only) to 95.1% in birth cohort 2010 (PCV7/PCV13). Preliminary data for the 2011 birth cohort show a PCV13 vaccination coverage of 90%.
In light of the National Immunization Plan objective and eight years after the introduction of PCV Universal Mass Vaccination, we estimated the overall effectiveness of the pneumococcal immunization program (VE) in preventing IPD during and after the PCV7/PCV13 shifting in the immunization schedule. Moreover, we assessed the impact of the vaccination program on the morbidity of S. pneumoniae related disease and infections.
Results
Vaccination program effectiveness
The overall PCV (PCV7/PCV13) vaccination program effectiveness was 84.3% (95% CI: 84.0–84.6%) for a proportion of cases vaccinated of 50% (two laboratory confirmed cases caused by vaccine serotypes and adequately vaccinated over a total of four laboratory confirmed cases) and a proportion of the population vaccinated of 86.4% (average coverage for PCV7 or PCV13 in birth cohorts 2006–2011). Between May 2010 and January 2013, we enrolled 159 patients (median age: 25 mo; range: 0–60 mo; 52.2% male) as suspected IPD cases. The most frequent clinical presentation was pneumonia (47.2%) and the main reported risk factor was “attending a community” (41.5%). No deaths were reported. Of the enrolled patients, 124 (78%) were fully vaccinated with either PCV7 (73; 45.9%) or PCV13 (51; 32.1%); 12 (7.5%) were partially vaccinated with either PCV7 or PCV13 and 23 (14.5%) were not vaccinated. Patients’ demographic characteristics, risks factors, clinical pictures, and disease outcome were similar among the enrolled children regardless their vaccination status, with the only exception of “age ≤24 months” and “attending a community” ().
Table 2. Description of enrolled patients (n = 159) by demographics, clinical picture, outcome, and risk factors, according to vaccination history, Apulia region, Italy, May 2010–January 2013
We identified 4 (4/159; 2.5%) confirmed IPD cases; 153 suspected cases were negative for S. pneumoniae and 2 (1.2%) samples were not suitable for testing. Of the 4 confirmed IPD cases, 2 were caused by serotype 9V and were fully vaccinated, 1 with 3 PCV7 doses and 1 with 3 PCV7 doses + 1 dose of PCV13 respectively. The remaining 2 were caused 1 by serotype 3 (not vaccinated) and 1 by 15 B/C (vaccinated with 2 PCV13 doses, ).
Table 3. Confirmed IPD cases by enrollment date, sex, age, clinical picture, vaccination history, sample type, and isolated serotypes, Apulia region, Italy, May 2010–January 2013
Impact of the vaccination program
We observed a pattern in reduction for all examined outcomes (). The most important reduction was recorded for pneumococcal pneumonia (RR: 0.43, 95% CI: 0.21–0.90), followed by IPD (RR: 0.72, 95% CI: 0.21–2.43), AOM (RR: 0.75, 95% CI: 0.65–0.88), and all-cause pneumonia (RR: 0.92, 95% CI: 0.86–0.99, ).
Figure 1. Hospitalization rate ratio for IPD, Streptococcus pneumoniae pneumonia, all-cause pneumonia, and AOM, by time period (baseline, PCV7 vaccination era, PCV7/PCV13 transition era), Apulia region, Italy, 2001–2011.
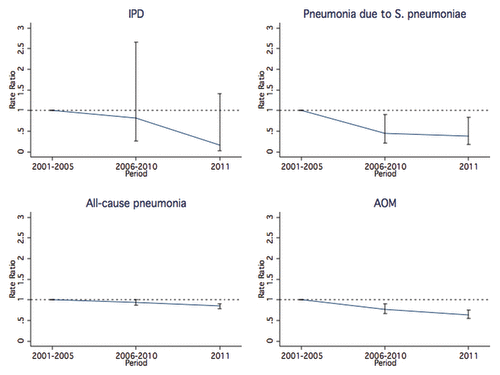
Table 4. Hospitalization rates per 100 000, rate ratios (RRs), and 95% CIs for IPD, Streptococcus pneumoniae pneumonia, all-cause pneumonia, and AOM between the pre-PCV years and the whole vaccination period, Apulia region, Italy
Discussion
To our knowledge, this is the first study conducted in Italy assessing vaccine effectiveness of PCV vaccination program. It also provided early evidence of the vaccination impact in the transition period between the use of PCV7 and the sequential introduction of PCV13.
Our findings show a good overall vaccination effectiveness (84.3%) for the PCV7/PCV13 program against vaccine-type IPD and an overall reduction in the number of pneumococcal disease related hospitalizations in children under 5 y of age used as indicator of disease reduction—impact of the vaccination program. In a similar study in the US, the screening method produces vaccine effectiveness estimates for the ≥1-dose schedule that are consistently lower, by approximately 5%, than those using the case-control method for PCV7-serotype IPD.Citation23 In England and Wales, using the indirect cohort design the adjusted vaccine effectiveness for two routine doses is 79% (95% CI: 24–94%).Citation24 Another study in the same countries reports different vaccine effectiveness for PCV13 according to the schedule—one priming dose: 38% (95% CI: -218 to 89%); two priming doses: 78% (95% CI: -18–96%); single dose in the second year of life: 73% (95% CI: 29–90%) and 77% (95% CI: 38–91%) when including in the estimation also those who had received one or more PCV13 doses in the first year.Citation15
The high coverage for the PCV program may lead to a possible underestimation of the vaccine effectiveness as herd immunity and reduction of vaccine-type nasopharyngeal carriage, induced by the widespread PCV use, may contribute to lower attack rates in the unvaccinated population.Citation23 In our region, vaccination coverage has reached 95.1% (birth cohort 2010). Most of the children enrolled in the prospective surveillance system were fully vaccinated with either vaccine. In England and Wales the rapid achievement of 94.2% coverage in the national program for the two-dose schedule allowed the halving of IPD cases in children under 2 y due to one of the additional serotypes covered by PCV13.Citation15 Similarly, our findings show an evident effect of the PCV7/PCV13 vaccination program with halving of hospitalizations for pneumococcal pneumonia, followed by one-third reduction of IPD. This result is consistent with the greater reduction in the incidence rate of pediatric invasive pneumococcal disease requiring hospitalization observed in the Madrid region, Spain, following the change from PCV7 to PCV13 in the childhood vaccination calendar. This reduction might not be exclusively attributable to the additional serotypes in multi-valent conjugate vaccines, but probably to the overall vaccination strategy.Citation25 Among Alaska native children, the introduction of PCV13 with catch-up vaccination to age 5 y is temporally associated with a 73% decrease in overall IPD, a 65% decrease in PCV13-type IPD and a 91% decrease in non-PCV13 IPD. However, the main future concern is whether replacement IPD with non-PCV13 serotypes could affect what is the early PCV13 success, as occurred after PCV7 introduction. Although it is difficult to predict how the characteristics of the serotypes will change after the introduction of PCV13, projections based on the invasiveness of the serotypes suggest that this new vaccine will result in additional reductions in disease incidence, therefore the global effectiveness of the current vaccination strategy should not result affected.Citation11 Nevertheless, theoretical models on disease reduction are no substitute for careful ongoing and continuous surveillance of circulating serotypes to monitoring and confirm whether vaccines are having the desired effect of reducing the incidence of disease over the long-term.Citation14
In Italy, data of IPD cases in children <5 y old are available from the national laboratory-based strain-surveillance system for invasive bacterial diseases. Two aspects affect the official national pooled data: first, failure to report is common in Italy and varies in time and by geographical area; second, surveillance of pneumococcal and meningococcal diseases has been recently enhanced. Due to the lack of longitudinal data, some Italian Regions have implemented regional IPD surveillance systems.
The ad hoc implementation, in the shifting period between PCV7 and PCV13, of a laboratory based prospective surveillance system, together with standardized serotyping identification of vaccine-contained strains, allowed the confirmation of IPD cases and the ascertainment of their vaccination status. In another Italian region, Lombardy, data from a similar surveillance system suggests that the 7- and the 13-valent pneumococcal conjugate vaccines covered respectively 30.8 and 84.6% of typified IPD cases.Citation26 Our study allowed also to value a reference laboratory for IPD and other invasive bacterial diseases in our region.
Our study could have some limitations due to the selected methodology to assess vaccine effectiveness, the small number of IPD cases occurred in the study period, and the use of the hospital discharge diagnoses as source of information on hospitalized children. The screening method has advantages and disadvantages compared with other methods for estimating vaccine effectiveness post licensure such as case-control or cohort design methods. In this method, instead of choosing one or more controls per case, the entire population is used as a reference group. We used the screening method as the 3 data points needed from our population were available, e.g., (1) the number of IPD cases from the ad hoc pneumococcal surveillance, (2) the number of cases vaccinated also from the ad hoc pneumococcal surveillance, and (3) the proportion of the population vaccinated computed from the regional vaccination registry. Moreover, it is a rapid method for the preliminary assessment of vaccine effectiveness that can be used for monitoring purposes both during the first years after the introduction of a vaccination program and when high coverage levels are reached. Such a high vaccine coverage as in Puglia among infants and children (95.1%), explains the small number of IPD confirmed cases occurred in the study population; consequently, the solidity of our estimate of vaccine effectiveness might be affected. The use of hospital discharge diagnoses might either under or over estimate the number of pneumococcal outcomes as it relies on the review of the discharge forms (where i.e., laboratory confirmation, risk factors, vaccination status, etc are not reported) and not on the review of the medical records of the children hospitalized for diseases caused by or related to S. pneumoniae.
Data exploring overall PCV program show promising findings in terms of both PCV13 effectiveness and its impact in reducing the number of IPD in children under 5 y of age.
Materials and Methods
Vaccination program effectiveness
We estimated the overall PCV (PCV7/PCV13) vaccination program effectiveness with 95% confidence interval (CI) using the screening methodCitation27 as follows: where, PPV (proportion of the population vaccinated) was the average coverage for PCV7 or PCV13 in children ≤60 mo (birth cohorts 2006–2011) and PCV was the proportion of cases vaccinated (the proportion of laboratory confirmed IPD cases caused by vaccine serotypes and adequately vaccinated).
IPD cases were detected through an ad hoc prospective laboratory-confirmed surveillance of IPD cases among hospitalized children 0–60 mo conducted between May 2010 and January 2013 in Apulia region (southern Italy; approximately 4 000 000 inhabitants). The surveillance included 28 pediatric wards of all hospitals in the region (admitting about 60 000 children aged ≤60 mo per year) and enrolled children residing in the monitored area with a suspected IPD. Children admitted to the participating pediatric wards and meeting the clinical case definition were enrolled as suspected cases. Clinical case definition included: meningitis, pneumonia with bacteremia, sepsis, occult bacteraemia, and bacterial pneumonia defined as at least two of the following symptoms: fever or hypothermia, sweating, cough, appearance of dyspnea, chest pain, or compatible radiological findings.
For each enrolled child, information on demographics, risk factors, co-morbidities, clinical symptoms and immunization history for PCV7 or PCV13 (number of doses, date of vaccination) were collected by the physicians participating in the surveillance network using eCRF. Data were checked for completeness and quality, cleaned and managed by the Principal Investigator of the study and collaborators. Information on vaccination was validated with the regional immunization registry. According to vaccine received (PCV7 and/or PCV13), number of doses and age at enrolment, children were classified in 4 groups: (1) fully PCV7 vaccinated; (2) fully PCV13 vaccinated (complete schedule, sequential schedule PCV7/PCV13, catch up with one or more PCV13 doses); (3) partially PCV7 or PCV13 vaccinated (subjects who did not complete the schedule); (4) not vaccinated.
A confirmed IPD case was defined as a child with isolation of S. pneumoniae by PCR positive sample from a normally sterile body site, such as blood, pleural or cerebrospinal fluid.Citation28 In each of the participating hospitals, from all specimens taken for routine diagnostic ascertainment within 24 h from enrolment of a suspected case, an aliquot was stored at −20 °C and sent to the Regional Reference Laboratory for Invasive Bacterial Diseases for standardized testing of S. pneumoniae.
S. pneumoniae was isolated by PCR and multiplex sequential PCR. Bacterial genomic DNA was extracted from 200 μl of biological samples (blood or cerebrospinal fluid) using the QIAamp Dneasy Blood and Tissue kit (Qiagen), according to the manufacturer’s instructions. Detection of S. pneumoniae was performed using a commercial multiplex assay (Pneumobacter ACE Detection for blood and Meningitis ACE Detection for CSF, Seegene; Sensitivity: detection limit of the Seeplex Pneumobacter Ace Detection = 10 copy/reaction − 10 copy/3 µl DNA). S. pneumoniae serotyping was performed on PCR positive samples through a sequential multiplex PCR.Citation29 Twenty-nine primer pairs were designed to target serotypes 1, 3, 4, 5, 6 A/B, 7F, 7C, 8, 9V, 10A, 11A, 12F, 14, 15A, 15 B/C, 16F, 17F, 18, 19A, 19F, 20, 22F, 23F, 31, 33F, 34, 35B, 35F, and 38. A primer pair (primers cpsA-f and cpsA-r) was also included as an internal control targeting the cpsA (pneumococcal capsular polysaccharide synthesis gene) locus found in all pneumococci.Citation30 The amplified products, ranging from 250 bp to 988 bp, were analyzed by means of electrophoresis on a 2% agarose gel (Life Technologies) and visualization under UV light.
To assess for potential differences among enrolled children by vaccination status, chi-square or Fisher exact test were used as appropriate. P value was set at <0.05. Stata MP 10.1 (Stata Corp. LP) was used for the analysis.
Ethics statement
The study was conducted in accordance with Guideline for Good Clinical Practice and the ethical principles that have their origins in the Declaration of Helsinki. The protocol was approved by the ethical committees of the Apulian Local Health Units. For each enrolled child involved in the study, written informed consent was obtained from the parents or legal guardians. No incentive was provided to encourage study participation.
Impact of the vaccination program
In order to assess the impact of the vaccination program, we monitored the disease burden on the population of children under 5 years of age and we chose to follow the indicators “hospitalization rates for pneumococcal diseases” before and after the introduction of the vaccination program. We extracted the number of hospitalizations in children aged ≤60 mo for diseases caused by or related to S. pneumoniae from the Apulian regional discharge registry for the period 2001–2011. We defined IPD as ICD9-CM code 320.1 (pneumococcal meningitis) or 038.2 (pneumococcal septicemia) or as each of the following codes: 320.8 (other specified meningitis), 790.7 (bacteremia), or 038.9 (unspecified septicemia) if associated with 041.2 (bacterial infection in conditions classified elsewhere and of unspecified site—pneumococcus); pneumonia with diagnosed S. pneumoniae infection as ICD9-CM code 481.xx (Streptococcus pneumoniae pneumonia); all-cause pneumonia as ICD9-CM codes 480.xx–486.xx without mention of a diagnosis of IPD as defined above;Citation5 unspecified AOM as ICD9-CM codes 382.xx. We scanned across discharge diagnoses in each child record for any mention of these disease codes.
For each outcome, we constructed time series using outcome-specific Poisson regression models: the hospitalization rate in pre-PCV era (average of annual disease rates between 2001 and 2005), and the hospitalization risk ratios (RRs) estimates with 95% CIs for both the PCV7 vaccination (mean of annual hospitalization rates from 2006 to 2010) and PCV7/PCV13 transition era (hospitalization rate in 2011). Moreover, we calculated the hospitalization RR with 95% CIs comparing the pre-PCV years (2001–2005) with the whole vaccination period (2006–2011).
Disclosure of Potential Conflicts of Interest
The ad hoc implementation of laboratory-confirmed surveillance of IPD was sponsored by Wyeth (Protocol Number 0887X1-4608), which was acquired by Pfizer Inc. in October 2009.
R.P. has received honorarium, grant and travel expenses by Pfizer to take part to advisory boards and expert meetings, to be a speaker in conferences, and to act as principal investigator in the IPD surveillance. No honorarium, or any other form of payment was provided to the other authors, with the exception of funding needed for the conduct of the surveillance. The authors have no financial relationships relevant to this article to disclose. R.P.’s Institution took charge of conceiving and designing the study, analyzing and interpreting data, and drafting the manuscript.
Acknowledgments
We thank Dr Alessandro Zollo and Dr Paolo Reggio (Medical Department Pfizer Italia S.r.l., Rome, Italy), and Dr Antonella Bonamano (Project Manager Senior Harrison Clinical Research Italia S.r.l.) who supported us with the ad hoc IPD surveillance implementation.
Apulian Group for the surveillance of pediatric IPD
Valerio Aprile, Patrizia Barcaglioni, Tatiana Battista, Maria S. Cappelletta, Enrico Caputo, Vincenza Carbone, Fabio Cardinale, Francesco Carella, M. Susanna Coccioli, Michele Conversano, Lucia Rita De Lallo, Attilio De Santis, Marcello De Simone, Cesare Di Bari, Angela d'Onofrio, Angela Dileo, Alberto Fedele, Giovanni Iannucci, Paola Lanzilotto, Ignazio Lofù, Domenico Lagravinese, Angela Loiodice, Pasquale Pio Maccarone, Mariano Manzionna, Vito Martucci, Luigi Mastronuzzi, Giuseppe Merico, Giuseppina Moffa, Fulvio Moramarco, Maria Nesta, Giuseppe Palamà, Pasquale Pedote, Luigi Ratclif, Vincenza Ruberti, Maria Lazzarina Russo, Michele Sacco, Stefano Termite, Vincenzo Tota, Giuseppina Turco, Lucia Vincenti, Michele Vitacco
References
- Prato R, Tafuri S, Fortunato F, Martinelli D. Why it is still important that countries know the burden of pneumococcal disease. Hum Vaccin 2010; 6:918 - 21; http://dx.doi.org/10.4161/hv.6.11.13352; PMID: 21045538
- Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998-2005. MMWR Morb Mortal Wkly Rep 2008; 57:144 - 8; PMID: 18272956
- Weil-Olivier C, van der Linden M, de Schutter I, Dagan R, Mantovani L. Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective. BMC Infect Dis 2012; 12:207; http://dx.doi.org/10.1186/1471-2334-12-207; PMID: 22954038
- Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine - United States, 2007. MMWR Morb Mortal Wkly Rep 2010; 59:253 - 7; PMID: 20224541
- Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio 2011; 2:e00309 - 10; http://dx.doi.org/10.1128/mBio.00309-10; PMID: 21264063
- Ingels H, Rasmussen J, Andersen PH, Harboe ZB, Glismann S, Konradsen H, Hoffmann S, Valentiner-Branth P, Lambertsen L, Danish Pneumococcal Surveillance Collaboration Group 2009-2010. Impact of pneumococcal vaccination in Denmark during the first 3 years after PCV introduction in the childhood immunization programme. Vaccine 2012; 30:3944 - 50; http://dx.doi.org/10.1016/j.vaccine.2012.03.060; PMID: 22504662
- Durando P, Alicino C, De Florentiis D, Martini M, Icardi G. Improving the protection against Streptococcus pneumoniae with the new generation 13-valent pneumococcal conjugate vaccine. J Prev Med Hyg 2012; 53:68 - 77; PMID: 23240163
- Pichon B, Ladhani SN, Slack MP, Segonds-Pichon A, Andrews NJ, Waight PA, Miller E, George R. Changes in molecular epidemiology of streptococcus pneumoniae causing meningitis following introduction of pneumococcal conjugate vaccination in England and Wales. J Clin Microbiol 2013; 51:820 - 7; http://dx.doi.org/10.1128/JCM.01917-12; PMID: 23269742
- Mokaddas E, Albert MJ. Impact of pneumococcal conjugate vaccines on burden of invasive pneumococcal disease and serotype distribution of Streptococcus pneumoniae isolates: an overview from Kuwait. Vaccine 2012; 30:Suppl 6 G37 - 40; http://dx.doi.org/10.1016/j.vaccine.2012.10.061; PMID: 23228356
- Harboe ZB, Valentiner-Branth P, Ingels H, Rasmussen JN, Andersen PH, Bjerre CC, Goldblatt D, Ashton L, Haston M, Konradsen HB, et al, Danish Pneumococcal Surveillance Collaborating Group. Pediatric invasive pneumococcal disease caused by vaccine serotypes following the introduction of conjugate vaccination in Denmark. PLoS One 2013; 8:e51460; http://dx.doi.org/10.1371/journal.pone.0051460; PMID: 23365635
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962 - 73; http://dx.doi.org/10.1016/S0140-6736(10)62225-8; PMID: 21492929
- DiNubile MJ. Serotype replacement after pneumococcal vaccination. Lancet 2012; 379:1388 - , author reply 1388-9; http://dx.doi.org/10.1016/S0140-6736(12)60590-X; PMID: 22500870
- World Health Organization. WHO Technical Report Series, No. 927, Annex 2 Recommendations for the production and control of pneumococcal conjugate vaccines. 2005. Available at: http://www.who.int/biologicals/publications/trs/areas/vaccines/pneumo/en/ Accessed 15 May 2013.
- Singleton R, Wenger J, Klejka JA, Bulkow LR, Thompson A, Sarkozy D, Emini EA, Gruber WC, Scott DA. The 13-valent pneumococcal conjugate vaccine for invasive pneumococcal disease in Alaska native children: results of a clinical trial. Pediatr Infect Dis J 2013; 32:257 - 63; PMID: 23001026
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine 2011; 29:9127 - 31; http://dx.doi.org/10.1016/j.vaccine.2011.09.112; PMID: 21983361
- Cohen R, Levy C, Bingen E, Koskas M, Nave I, Varon E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J 2012; 31:297 - 301; http://dx.doi.org/10.1097/INF.0b013e318247ef84; PMID: 22330166
- Alfonsi V, D’Ancona F, Giambi C, Nacca G, Rota MC, Regional Coordinators for Infectious Diseases and Vaccinations. Current immunization policies for pneumococcal, meningococcal C, varicella and rotavirus vaccinations in Italy. Health Policy 2011; 103:176 - 83; http://dx.doi.org/10.1016/j.healthpol.2011.10.002; PMID: 22030308
- Ministero della Salute. Italia (2010). Indicazioni in merito alla somministrazione del vaccino antipneumococcico Prevenar 13 in età pediatrica. Circolare del 27 maggio 2010. prot.n. 111432/72AF del 03/06/2010. Available at: http://www.fimpcalabria.org/public/vaccinazioni/indicazioni%20in%20merito%20alla%20somministrazione%20del%20vaccino%20antipneumococcico%20prevenar%2013%20in%20et%C3%A0%20pediatrica%20%282%29.pdf. Accessed 9 October 2013.
- Ministero della Salute. Italia (2012). Piano della Prevenzione Vaccinale 2012-2014. Intesa Stato-Regioni del 22 febbraio 2012. G.U. Serie Generale, n. 60 del 12 marzo 2012. Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1721_allegato.pdf. Accessed 15 May 2013.
- WHO Publication. Pneumococcal vaccines WHO position paper - 2012 - recommendations. Vaccine 2012; 30:4717 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.04.093; PMID: 22621828
- Commissione tecnico-scientifica regionale vaccini, Apulia Region, Italy (2005). Piano Regionale della Prevenzione 2005-2007. Approvazione del Piano regionale Vaccini triennio 2005-2007. Deliberazione della Giunta Regionale 30 dicembre 2005, n. 2037. Bollettino Ufficiale della Regione Puglia - n. 12 del 24-1-2006. pp: 932-953. Available at: http://www.regione.puglia.it/web/files/sanita/DELVAC.pdf and http://www.regione.puglia.it/web/files/sanita/PIANOVAC.pdf. Accessed 15 May 2013.
- Commissione tecnico-scientifica regionale vaccini, Apulia Region, Italy (2010). Modifica del Calendario Vaccinale della Regione Puglia per l’anno 2009 approvato con D.G.R. n.1286/09. Adozione del Calendario Vaccinale della Regione Puglia per l’anno 2010. Deliberazione della Giunta Regionale 28 settembre 2010, n. 2068. Bollettino Ufficiale della Regione Puglia - n. 159 del 19-10-2010. Pp: 28621-28631. Available at: http://www.fimp.org/latuaregione/documenti/PugliaBURR8945Adozione%20Piano%20Vaccini.pdf. Accessed 15 May 2013.
- Cohen AL, Taylor T Jr., Farley MM, Schaffner W, Lesher LJ, Gershman KA, Bennett NM, Reingold A, Thomas A, Baumbach J, et al. An assessment of the screening method to evaluate vaccine effectiveness: the case of 7-valent pneumococcal conjugate vaccine in the United States. PLoS One 2012; 7:e41785; http://dx.doi.org/10.1371/journal.pone.0041785; PMID: 22870248
- Andrews N, Waight PA, Borrow R, Ladhani S, George RC, Slack MP, Miller E. Using the indirect cohort design to estimate the effectiveness of the seven valent pneumococcal conjugate vaccine in England and Wales. PLoS One 2011; 6:e28435; http://dx.doi.org/10.1371/journal.pone.0028435; PMID: 22164292
- Picazo J, Ruiz-Contreras J, Casado-Flores J, Giangaspro E, García-de-Miguel MJ, Hernández-Sampelayo T, Otheo E, Méndez C, Heracles Study Group. Impact of introduction of conjugate vaccines in the vaccination schedule on the incidence of pediatric invasive pneumococcal disease requiring hospitalization in Madrid 2007 to 2011. Pediatr Infect Dis J 2013; 32:656 - 61; http://dx.doi.org/10.1097/INF.0b013e31827e8594; PMID: 23249906
- Riva E, Salvini F, Garlaschi ML, Radaelli G, Giovannini M. The status of invasive pneumococcal disease among children younger than 5 years of age in north-west Lombardy, Italy. BMC Infect Dis 2012; 12:106; http://dx.doi.org/10.1186/1471-2334-12-106; PMID: 22554011
- Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, Sirotkin B. Field evaluation of vaccine efficacy. Bull World Health Organ 1985; 63:1055 - 68; PMID: 3879673
- Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep 1997; 46:RR-10 1 - 55; PMID: 9148133
- Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol 2006; 44:124 - 31; http://dx.doi.org/10.1128/JCM.44.1.124-131.2006; PMID: 16390959
- Mavroidi A, Godoy D, Aanensen DM, Robinson DA, Hollingshead SK, Spratt BG. Evolutionary genetics of the capsular locus of serogroup 6 pneumococci. J Bacteriol 2004; 186:8181 - 92; http://dx.doi.org/10.1128/JB.186.24.8181-8192.2004; PMID: 15576766
