Abstract
Toxoplasma gondii infection occurs commonly in humans and other warm-blooded animals. Its serious impact on public health and livestock sectors makes the development of an effective vaccine particularly important. In the current study, we constructed a multiantigenic DNA vaccine expressing ROP16 and GRA7 of T. gondii and evaluated the protective efficacy of these two fragments with or without a plasmid encoding murine costimulatory molecule B7-2. These recombinant eukaryotic expression plasmids were termed pROP16, pGRA7, pROP16-GRA7 and pB7-2, respectively. After intramuscular immunization in Kunming mice, we assessed the immune response using cytokine and antibody determinations, T lymphocyte subsets analysis, and the survival times of mice post acute T. gondii challenge. The results showed that mice immunized with the multiantigenic DNA vaccine pROP16–GRA7 gained higher levels of IgG titers and IgG2a subclass titers, production of IFN-γ, percentage of CD8+ T cells and median survival times against the acute infection of T. gondii compared with those of mice administered with pROP16 or pGRA7 and those in control groups. Moreover, the adjuvant pB7-2 formulated with DNA vaccine boosted these humoral and cellular (Th1, CD8+ T cell) immune responses. Therefore, it might be a promising genetic adjuvant to DNA vaccine against T. gondii for further investigation.
Introduction
Toxoplasma gondii, a ubiquitous obligate intracellular parasite, is considered to be one of the most successful eukaryotic pathogens to infect warm-blooded vertebrates including humans, capable of causing toxoplasmosis.Citation1 The T. gondii infections are benign in most cases, may occasionally cause severe or lethal damages in immunosuppressive populations and congenitally infected individuals. Moreover, T. gondii infection has considerable economic importance due to abortion and neonatal loss in livestock.Citation2 Treatment of toxoplasmosis is difficult since the available drugs frequently cause adverse effects.Citation3 Under the present scenario, developing an effective vaccine against T. gondii would be of great value for controlling and preventing toxoplasmosis.
In recent years, many studies have shed light on DNA vaccines, as they showed more prominent advantages over traditional vaccines in terms of flexibility, rapid manufacture, low cost, and the ability to induce broad-spectrum immunity.Citation4 Various efforts have been done to search promising vaccine candidate antigens for T. gondii. To date, these candidates include surface antigens (SAGs), dense granule antigens (GRAs), rhoptry proteins (ROPs), and microneme proteins (MICs). Among them, ROP16, known as a specialized kinase released from rhoptries into the host cell during invasion, is considered one of the key virulence factors in the pathogenesis of T. gondii infection, for the ability to directly target to the host cell nucleus and activate both signal transducer and activator of transcription 3 and 6 (STAT3, STAT6) signaling pathways.Citation5 GRA7, a potent antigen expressed in all infectious stages of T. gondii, can trigger significant humoral and cellular immune responses against toxoplasmosis.Citation6 Accordingly, the two antigens ROP16 and GRA7 in the form of recombinant plasmids are very promising for further experiments.
Additionally, careful selection of the adjuvant is as pivotal as the selection of the candidate antigen for vaccine which may lead to an appropriate response.Citation7 Studies have demonstrated that the B7 costimulatory molecules including B7-1 (CD80) and B7-2 (CD86) play critical roles in providing costimulatory signals required for the generation and maintenance of antigen-specific immune response.Citation8-Citation10 Thus, for example, a plasmid expressing B7-1 or B7-2 coadministered with a plasmid DNA vaccine can fully costimulate vaccine-elicited specific antibodies (Ab) and CD8+ cytotoxic T lymphocyte (CTL) responses.Citation11-Citation13 Furthermore, vectors that upregulate the expression of costimulatory ligands on antigen presenting cells (APCs) will help generate a IFN-γ-dependent protective immune response.Citation14 With regards to DNA vaccination, several studies suggested that the codelivery of plasmids encoding B7-2 was more effective than that encoding B7-1 at driving cellular immune responses especially for the generation of antigen-specific CTL response.Citation15-Citation17
Accumulating evidences indicate that vaccination with stage-specific antigens leads to stage-limited protection against T. gondii.Citation3,Citation18 Therefore, developing multiantigenic DNA vaccine in connection with the different life stages of the parasite may conquer the deficiency of using a single antigen.Citation19,Citation20 In the present study, we constructed DNA vaccines expressing the ROP16/GRA7 antigens (pROP16, pGRA7, and pROP16-GRA7), and a plasmid encoding murine costimulatory molecule B7-2 (pB7-2) as a genetic adjuvant. The expression abilities of these DNA vaccines were mimicked and examined in mammalian cells in vitro and their protective efficacy with or without the genetic adjuvant pB7-2 to protect Kunming mice against acute toxoplasmosis was investigated.
Results
Identification of the recombinant plasmids
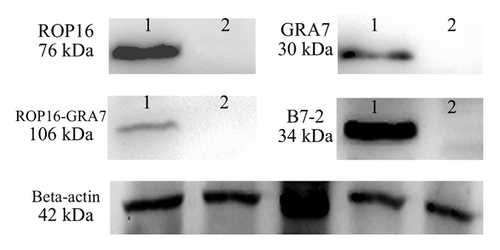
Using molecular biological clone-oriented method and gene combination technique, truncated DNA fragments ROP16, GRA7, and B7-2 were inserted into the eukaryotic expression plasmid pEGFP-C1. The recombinant plasmid pROP16, pGRA7, pROP16-GRA7, and pB7-2 were successfully constructed which made it possible to establish a stable and highly effective expression system in vitro and carry out the research of DNA vaccination against T. gondii infection. To determine whether the recombinant plasmids could be expressed in mammalian cells, the plasmids were transfected into HEK293T cells and the expressed proteins were determined by SDS-PAGE and western blotting. As shown in , the green fluorescence was observed in HEK293T cells transfected with pROP16, pGRA7, pROP16-GRA7, pB7-2, or pEGFP, whereas no fluorescence was observed in the untransfected cells. Western blotting demonstrated that the expressed proteins (lane 1) were reacted with anti-STAg mouse sera or anti-B7-2 antibody, whereas the control empty plasmid-transfected cells did not show any band (lane 2) upon incubation with the same antibodies ().
Figure 1. Fluorescence microscopy images of cells. HEK293T cells were transfected with pROP16 (A), pGRA7 (B), pROP16-GRA7 (C), pB7-2 (D), or empty plasmid pEGFP (E). None transfected HEK293T cells (F).
Figure 2. Western blotting analysis of the expression protein in vitro using anti-STAg mouse sera or anti-B7-2 antibody as primary antibody. β-actin serves as a loading control and from the left side there were lysates of HEK293T cells transfected with pEGFP, pROP16, pGRA7, pROP16-GRA7 or pB7-2 recombinant plasmid incubated with the same anti-β-actin antibody.
Determination of T. gondii-specific IgG and IgG subclass titers
As shown in , significantly high levels of total IgG antibodies were detected in all the treatment groups as compared with the control group on the second, fourth, and eighth weeks post the first immunization. The group immunized with pROP16-GRA7 combined with genetic adjuvant pB7-2 showed higher total IgG antibody response than any other vaccination strategy, especially on the eighth week after the first immunization (P < 0.01). Moreover, groups immunized with either pROP16 or pGRA7 combined with pB7-2 or the multiantigenic vaccine pROP16-GRA7 also had preponderance of IgG compared with mice immunized with the single-gene vaccine, and the differences between them were statistically significant (P < 0.05).
Figure 3. Determination of T. gondii-specific IgGs and IgG subclass titers. (A) Anti-T. gondii IgG titers in sera (diluted 1:100) of mice immunized with pEGFP, pB7-2, pEGFP+pB7-2, pROP16, pGRA7, pROP16-GRA7, pROP16+pB7-2, pGRA7+pB7-2, and pROP16-GRA7+pB7-2. Immune sera were collected at each time point. (B) Anti-T. gondii IgG1 and IgG2a titers in the sera (diluted 1:100) of mice injected with pEGFP, pB7-2, pEGFP+pB7-2, pROP16, pGRA7, pROP16-GRA7, pROP16+pB7-2, pGRA7+ pB7-2, and pROP16-GRA7+pB7-2. Sera were collected at the fourth week post the final injection. Results were expressed as means ± SD for three determinations.
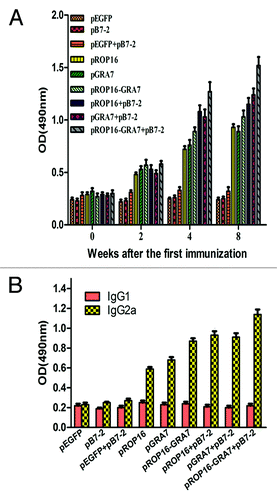
In order to determine whether a Th1 or Th2 response was elicited in immunized mice, T. gondii-specific IgG1 and IgG2a titers of immunized mice sera (4 weeks after the final immunization) were detected by ELISA (). IgG2a titers were significantly higher than IgG1 titers in all the treatment groups, especially for the pROP16-GRA7 codelivery with pB7-2 group which obtained the highest IgG2a/IgG1 level, while there was no significant difference in control groups. Additionally, pB7-2 enhanced this preponderance when used with single-gene vaccine.
T lymphocyte subsets analysis
As shown in , CD4+ and CD8+ T lymphocyte subsets from the immunized mice were assayed by flow cytometry. The percentage of CD8+ T cells in treatment groups was significantly increased compared with that in the control groups. In addition, coimmunization of pB7-2, along with plasmid DNA encoding for T. gondii antigens ROP16 or/and GRA7, resulted in a dramatically greater increase in the CD8+ T cells induction. Interestingly, the percentage of CD4+ T cells in pROP16-GRA7, pROP16+pB7-2, pGRA7+pB7-2, and pROP16-GRA7+pB7-2 groups also showed some statistically change compared with that in the control groups.
Table 1. Percentages of T lymphocyte subsets from the immunized micea by flow cytometry
Cytokine production by spleen cells
To determine whether single- or multiple-gene immunization augments the Th1 or Th2 cytokine response, culture supernatants of splenocytes were obtained from immunized mice four weeks after the final immunization. As shown in , the levels of IFN-γ in mice immunized with single- or multiple-gene vaccines were markedly higher than those of mice immunized with controls (pEGFP, pB7-2, or pEGFP+pB7-2). Additionally, the production of IFN-γ in mice immunized with pROP16-GRA7 was further enhanced by pB7-2 coadministration. However, levels of IL-4 or IL-10 in all groups failed to show significant differences.
Table 2. Cytokine concentrations of culture supernatants of the splenocytes from the immunized micea by ELISA
Protection against challenge with T. gondii RH strain in mice
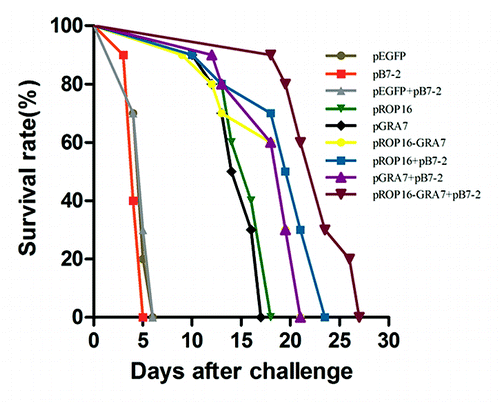
Survival curves of the nine groups are shown in . All control mice (pEGFP, pB7-2, and pEGFP+pB7-2 group) died within 6 d postinfection. Survival times were notably prolonged in all the treatment groups compared with the controls (P < 0.01) and the group of pROP16-GRA7 with pB7-2 as a genetic adjuvant obtained the most extended mean survival time.
Figure 4. Survival curves of immunized Kunming mice after T. gondii challenge infections. Ten mice per group were challenged with 1000 tachyzoites of the virulent T. gondii RH strain on the 4th week after the final immunization. Mortality was monitored daily for 27 d post-challenge with the parasites.
Discussion
Using DNA vaccines with the ability to express encoded antigens in the host cells has been considered an effective approach to help the host control T. gondii infection.Citation21 Previous studies have suggested that ROP16 and GRA7 are promising candidates for the development of DNA vaccines against T. gondii. Vaccination of mice with a plasmid encoding ROP16 could induce both strong humoral and cellular immune responses, which resulted in partial protective immunity against T. gondii infection.Citation22 Other studies have showed that the GRA7 antigen was more effective in eliciting a significant immunity against T. gondii compared with GRA1, GRA4, GRA6, ROP2 when used alone or in a cocktail DNA vaccine.Citation23,Citation24 Similar investigation in our previous study revealed that the DNA prime-protein boost vaccination based on GRA7 could induce both humoral and cellular immune responses and effective protection against lethal T. gondii infection.Citation25 To further evaluate the protective efficacy of these two antigens, we constructed a multiantigenic plasmid pROP16-GRA7 and tested whether it could be a potent vaccine against the challenge with highly virulent T. gondii RH strain.
In the present study, we evaluated the immunogenic and protective potential of multiantigenic recombinant DNA vaccines encoding ROP16 and GRA7 of T. gondii in a murine model of acute toxoplasmosis. Since stage-specific antigens could lead to stage-limited protection, the vaccines contained antigens characteristic for various life stages of the parasite. Our data showed that Kunming mice immunized intramuscularly with different DNA vaccine produced high levels of IgG and IgG2a, production of IFN-γ and percentage of CD8+ T cells against T. gondii infection, and protective immune responses were effectively elicited. Moreover, immunization with the multiantigenic gene plasmid pROP16-GRA7 induced greater humoral and cellular immune responses than that immunization with the single-antigen gene plasmid pROP16 or pGRA7. This protective immunity was further enhanced by the coinoculation of pB7-2 as a genetic adjuvant.
As one of the robust protective immune responses against pathogen infection, humoral immunity resulting in IgG antibodies seems to be important in controlling T. gondii chronic infection and preventing reactivation. In this study, Kunming mice in the treatment group exhibited higher specific IgG antibody titers than control ones, especially after the third injection of DNA vaccine. Furthermore, coexpression of adjuvant pB7-2 with DNA vaccines exhibited higher specific IgG antibodies than DNA vaccines used alone. This further demonstrated that pB7-2 was successful in costimulating vaccine-elicited specific antibody responses.
Besides the humoral immune response, it has been suggested that adaptive cellular immunity comprising T helper type 1 (Thl) and CD8+ T-lymphocyte responses is specifically required for coping with infections caused by intracellular parasites.Citation26-Citation28 Generally, the production of IFN-γ from T cells and IgG2a isotype titers in sera favors Thl responses. Especially, IFN-γ with the capacity to mediate intracellular killing, is critical for the control of T. gondii. In this study, all treatment groups gained high levels of IFN-γ or IgG2a, Especially for the multiantigenic vaccine group, indicating that the fusion DNA vaccine induced a more efficient Th1 response than the single-gene one. Meanwhile, no markedly change was detected in the secretion of IL-4, IL-10, or IgG1 titers which characterizes Th2 responses. What merits our attention is that B7-2 molecular used in this study showed some ability on optimizing functional Thl responses as DNA vaccines coadministration with pB7-2 induced remarkably higher levels of IFN-γ and IgG2a compared with groups immunization with DNA vaccines alone. This may indicate that B7-2 molecule plays a key role of driving T cell differentiation into the Th1 pathway in the model of T. gondii infection. However, it should be considered most plasmids for DNA vaccination containing CpGs immunostimulatory sequences, which favor a Th1 response.Citation29 With regard to the subclass of T lymphocyte response, a great increase of the CD8+ T lymphocytes subsets percentage was observed in immunized groups especially for that codelivery with pB7-2. It indicated that recombinant plasmids expressing ROP16 or/and GRA7 using as DNA vaccines predominantly induce a strong CD8+ T-cell mediated immune response and the stimulation of B7-2 molecule enhanced this protective immunity.
For protective potency evaluation, intraperitoneal challenge with the highly virulent RH strain of T. gondii at lethal doses of 1 × 103 tachyzoites was performed in Kunming mice which are genetically susceptible to infection with T. gondii. Those in the DNA vaccine groups experienced significantly extended median survival times when compared with control groups. However, none of these vaccine regimens provided an ideal protection against T. gondii RH strain, since all mice died within 27 d. Under lack of systematic preliminary study, we used 50 μg of the recombinant DNA vaccine in each immunization. Future studies should be considered to optimize the inoculation doses and ratios of each regimen.
Several studies have shown that multiantigenic DNA vaccines were superior to a single-gene DNA vaccine. A multiantigenic DNA vaccine encoding MIC3 and ROP18 of T. gondii immunized in ICR mice showed that mice immunized with pROP18-MIC3 elicited stronger humoral and Th1 cellular immune responses, and prolonged survival time against toxoplasmosis in comparison to those using pMIC3 or pROP18 single-gene plasmids.Citation30 Another multiantigenic DNA vaccine expressing GRA7 and ROP1 of T. gondii in BALB/c mice obtained higher IgG2a titers, production of IFN-γ and TNF-α, survival time, and cyst reduction rate compared with those of mice with either pGRA7 or pROP1 alone.Citation20 Our previous study showed that BALB/c mice with pSAG1-GRA2 was more effective in stimulating host protective immune responses than separately injected single-gene plasmid pSAG1 or pGRA2.Citation31 In the current study, although the mortality of mice was 100% in the group vaccinated with pROP16-GRA7, the protective immunity of mice was greatly increased compared with those in single-gene vaccine groups, demonstrating that T. gondii ROP16 and GRA7 multiantigenic DNA vaccine could induce a higher level of protective immune efficacy than their separate use.
Correspondingly, it has been suggested that B7-2 molecule plays a central role in the Ag-specific induction of CD8+ CTL response when delivered as a vaccine adjuvant. Importantly, muscle cells are the major producer cells of xenoantigens in intramuscular immunization mode. However, muscle cells are non-professional antigen presenting cells (APCs) which cannot active T cells effectively because of low expression of MHC class I genes and lacking of the costimulatory molecules such as B7.Citation32-Citation34 This may be the limitation of using DNA vaccine only. In this case, intramuscular immunization with DNA vaccine coinjection with B7-2 made muscle cells express the costimulatory molecule B7-2 along with expressing MHC-antigen peptides simultaneously, which would be beneficial for the activation of T cells (primarily MHC class I-restricted CD8+ T cells), and thus cause an effective T-cell response. Interestingly, the percentages of CD4+ T cells in single-gene vaccine groups were mildly enhanced by coinjection of pB7-2. As both T cells are important sources of IFN-γ, it resulted in the high levels of IFN-γ production in these groups.
It is yet to determined whether the immune responses induced by ROP16 and GRA7 of T. gondii RH strain (type I) could be effective against chronic challenge with tissue cyst-forming strains of type II T. gondii strains. Since T. gondii strains corresponding to different genotypes are responsible for different toxoplasmosis in humans and animals,Citation35 protective immunity should be demonstrated using different T. gondii genotypes in further studies. Actually, this current study had its limitations probably due to an inappropriate immunization protocol and an insufficiency evaluation criterion. Many parameters such as the vaccine construct, the parasite strain, the dose of inoculum, the inoculation route and the mouse strain might influence the assessment of protective immunity. Future studies should address all these aspects and optimize immunization protocol to correctly evaluate the DNA vaccine and the genetic adjuvant. Additionally, to approach more informative data, the histological observation in immunized muscle cells following the fluorescence detection should be considered in our future study.
It is of special noted that we selected the empty plasmid as the control and not to include the PBS control in the present study. This was in consideration of avoiding unnecessary use of animals. Furthermore, previous vaccination studies using PBS and empty plasmid as control groups have shown that there were no statistically significant differences between these two controls.Citation22,Citation25,Citation30,Citation31 The practice of eliminating PBS as the control was also reported in other vaccine studies.Citation19,Citation20,Citation24,Citation36 However, a control of PBS will make the vaccination experiments more rigorous. Therefore, a lack of PBS control may be a shortcoming in the experimental design of this study.
To conclude, this study showed that multiantigenic DNA vaccine encoding ROP16 and GRA7 was capable of triggering further augmented humoral and cellular immune responses, as well as eliciting more efficient immunity protection against the lethal challenge with T. gondii RH strain in Kunming mice, compared with the single-gene vaccine. In addition, the formulation of pB7-2 with either a multiantigenic DNA vaccine (pROP16-GRA7) or a single-gene vaccine (pROP16 or pGRA7), all resulted in dramatically enhanced antibody titers, both Thl and CD8+ T cell mediated immune responses, therefore, it might be a feasible method of boosting protective immunity induced by a recombinant DNA vaccine.
Materials and Methods
Animals
Specific-pathogen-free (SPF)-grade female congenic Kunming mice, aged 6 weeks, were purchased from the Animal Centre of Shandong University, China. All experimental protocols were in accordance with the approval from the Institutional Animal Care and Use Committee of Shandong University under Contract 2011-0015.
Parasites and preparation of soluble tachyzoite antigens (STAg)
Tachyzoites of T. gondii RH strain were preserved in our laboratory (Laboratory of Parasitology, Shandong University School of Medicine) and maintained by serial intraperitoneal passage in Kunming mice. The tachyzoites were collected from the peritoneal cavity of infected Kunming mice 4 d after intraperitoneal (i.p.) injection and then infected into HEK293T cells (parasite/cell ratio, 4:1). After incubated at 37 °C with 5% CO2 for 3 d, the cultures were collected and centrifuged at 2000 × g for 10 min. The final sediment was suspended in cold phosphate-buffered saline (PBS), and then passed through a 3.0-µm-pore-size filter (Millipore). Purified tachyzoites were used for genomic DNA extraction, challenge for immunized mice, and preparation of STAg.
For preparation of STAg, the purified tachyzoites were disrupted by three cycles of freezing and thawing, and then sonicated on ice at 150 W/s. The lysate was centrifuged at 10 000 × g for 20 min and supernatants were pooled. After sterile filtering and concentration determinacy, STAg was aliquoted and stored at –70 °C until use.
Construction of DNA vaccine candidates
The eukaryotic expression plasmid pEGFP-C1 was used as a DNA vaccine vector. To construct the ROP16-GRA7 fusion expression plasmid, the ROP16 gene (GenBank No. DQ116422.2, 124 bp from sequence positions 1 to 2124) and GRA7 gene (GenBank No. DQ459443, 708 bp from sequence positions 25–732) were amplified by PCR from genomic DNA of T. gondii RH strain using the following primers: ROP16 5′-GAAGATCTAT GAAAGTGACC ACGAAAGA-3′ (forward) and 5′-CGAGCTCCAT CCGATGTGAA GAAAG-3′ (reverse) containing the BglII and SacI sequences (underlined); GRA7 5′-CGAGCTCATG GCCCGACACG CAATT-3′ (forward) and 5′-CGGAATTCCT GGCGGGCATC CTC-3′ (reverse) containing the SacI and EcoRI sequences (underlined). To construct the murine B7-2 expression plasmid, the B7-2 gene (GenBank No. NM019388.3, 930bp from sequence positions 117–1046) was amplified by PCR from cDNA of murine spleens with a pair of oligonucleotide primers: 5′-CGGAATTCAT GGACCCCAGA TGCA-3′ (forward), and 5′-GCTCTAGACT ACTCTCACTG CCTTCACTC-3′) (reverse) contained the EcoRI and XbaI sequences (underlined). PCR products were cloned into the pEASY-T1 Easy vector (TransGen Biotech) and sequenced in both directions to ensure fidelity. Then they were digested with the appropriate restriction enzymes, and purified from agarose gels. ROP16, GRA7, and B7-2 gene fragments were subcloned into the eukaryotic expression vector pEGFP-C1 (Clontech) respectively, generating pEGFP-C1-ROP16 (pROP16), pEGFP-C1-GRA7 (pGRA7), and pEGFP-C1-B7-2 (pB7-2). The SacI/EcoRI fragment encoding GRA7 was excised and cloned into the SacI/EcoRI sites of the pROP16 vector to produce pEGFP-C1-ROP16-GRA7 (pROP16-GRA7). These recombinant plasmids were transformed into the JM110 bacterial strain (TransGen Biotech) and cultured in Luria-Bertani (LB) broth containing kanamycin (50 μg/ml). The plasmids were verified by restriction enzyme digestion, PCR, and sequencing the complete insert, respectively. Large-scale plasmid preparation and purification were performed using Endotoxin-Free Plasmid Maxi Kits (CoWin Biotech) according to the manufacturer’s instructions. DNA concentration and purity were determined by measuring the optical density, and the A260/A280 ratios for purified DNA were 1.8–2.0. Plasmids were finally resuspended in sterile phosphate-buffered saline (PBS) at a concentration of 500 μg/ml for vaccination.
Expression of recombinant genes in vitro
HEK293T cells were transfected with pROP16, pGRA7, pROP16-GRA7, pB7-2, or pEGFP-C1 (control) using Lipofectamine™ 2000 reagent (Invitrogen) according to the manufacturer’s instructions. After incubation for 24 h at 37 °C in a 5% CO2 incubator, protein expression was detected for specific green fluorescence on HEK293T cells under a fluorescence microscope (Carl Zeiss). Then the cells were lysed with Nonident P40 lysis buffer (Sigma) and centrifuged at 12 000 × g for 10 min, 24 h and 48 h after transfection. The products were identified by the sodium dodecyl sulfate-PAGE (SDS-PAGE) and western blotting. Protein productions from HEK293T cells were checked by western blotting as following described: Cells were solubilized in lysis buffer (1 × 108 cells/ml). The whole cell extracts were mixed in Laemmli loading buffer, boiled for 5 min, and then subjected to SDS-PAGE. After electrophoresis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes(Immobilon-Psq) and blocked with 5% non-fat milk for at least 1 h followed by an overnight blotting against anti-β-actin (Santa cruz), anti-B7-2 (Biosynthesis Biotech), and anti-STAg mouse serum at a dilution of 1:500 at 4 °C. After washing with TBST three times, the membranes were incubated with horseradish peroxidase conjugated second antibody for 1 h. Before detected with an enhanced chemiluminescence system (Pierce, Rockford), membranes were washed with TBST extensively. The bands were examined by ChemiDoc XRS+ with Image Lab software (Version 4.0.0, Alpha Innotech Corp.).
Immunization and infection of mice
Nine groups of Kunming mice (n = 13 each) were individually vaccinated 3 times at a 2-week interval with pEGFP-C1, pB7-2, pEGFP-C1 with pB7-2 (pEGFP+pB7-2), pROP16, pGRA7, pROP16-GRA7, pROP16 with pB7-2 (pROP16+pB7-2), pGRA7 with pB7-2 (pGRA7+pB7-2), or pROP16-GRA7 with pB7-2 (pROP16-GRA7+pB7-2). Mice were inoculated with an injection of 100 μl DNA vaccine (500 μg/ml) in each thigh skeletal muscle. Four weeks after the final immunization, mice were challenged intraperitoneally with 100 μl PBS containing 1000 tachyzoites of the virulent T. gondii RH strain to determine the number of days of survival of mice.
Antibody titers and isotypes determination
Blood samples were obtained from the mice orbital sinus via heparinized capillary tubes (Fisher Scientific) before each immunization and at the 4th week post the final immunization. Sera were separated and stored at –20 °C until measurement of IgG, IgG1, and IgG2a titers. Antibodies against T. gondii were analyzed by enzyme-linked immunosorbent assay (ELISA) as previously described.Citation25 Briefly, microtiter plates were coated with STAg (10 μg/ml) in 50 mM carbonate buffer (pH 9.6). After incubation overnight at 4 °C, the plates were blocked with PBS containing 1% bovine serum albumin at 37 °C for 1 h. Serum samples diluted in PBS were added to the wells and incubated at 37 °C for an additional hour. Horseradish peroxidase (HRP) conjugated goat anti-mouse IgG, IgG1, or IgG2a was used as the secondary antibody. Binding was visualized by incubating with 50 μl substrate solution (10 mg of 3′, 3′, 5′, 5′-tetramethylbenzidine dissolved in 0.025 M phosphate–citrate buffer) for 20 min. The absorbance was read at 490 nm using an ELISA reader (Bio-TekEL × 800). All samples were performed in triplicate.
Flow cytometry analysis of T lymphocyte subsets
For analysis of percentages of T lymphocyte subsets, Splenocytes were harvested and prepared from three mice per group four weeks after the final immunization. Cells adjusted to 1 × 106 in 200 µL PBS were stained with FITC-conjugated anti-mouse CD4+ monoclonal antibody (mAb), PE-conjugated anti-mouse CD3+ mAb and Cy5.5-conjugated anti-mouse CD8+ mAb (eBioscience). After incubation with mAbs for 1 h at 4 °C in the dark, the cultures were washed with 1 ml PBS, then centrifuged for 5 min at 650 × g and re-suspended in 200 µl PBS. The samples were measured of fluorescence profiles using a BD FACS Calibur (BD Biosciences).
Cytokine assays
For analysis of cytokine levels, splenocytes prepared as described above were plated in 96-well microtiter plates at an optimal density of 5 × 105 cells per well. Thereafter, the cultures were stimulated with STAg (10 μg/ml), concanavalin A (ConA; 5 μg/ml; Sigma) or medium alone (negative control) respectively, at 37 °C in 5% CO2 condition. Cell-free supernatants were harvested and assayed for interleukin-4 (IL-4) activities at 24 h, for interleukin-10 (IL-10) activity at 72 h, and for IFN-γ activity at 96 h. The IL-4, IL-10, and IFN-γ concentrations were evaluated using a commercial ELISA kit according to the manufacturer’s instructions (KYM). All assays were performed in triplicate.
Statistical analysis
Data, such as antibody responses, percentage of T-cell isotypes and cytokine levels, were compared between the different groups by one-way analysis of variance (ANOVA). Survival rate was checked by Kaplan–Meier method and compared with the log-rank test. Differences among the various groups were considered significant when P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Outstanding Young Scientists Research Award Project of Shandong, China (BS2009SW008), the Fund Project of Medicine and Health Development Plan of Shandong, China (2005HZ028), and the Science and Technology Development Program of Shandong, China (2006GG3202045).
References
- Cenci-Goga BT, Rossitto PV, Sechi P, McCrindle CM, Cullor JS. Toxoplasma in animals, food, and humans: an old parasite of new concern. Foodborne Pathog Dis 2011; 8:751 - 62; http://dx.doi.org/10.1089/fpd.2010.0795; PMID: 21486145
- Buxton D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet Res 1998; 29:289 - 310; PMID: 9689743
- Stanford MR, Gilbert RE. Treating ocular toxoplasmosis: current evidence. Mem Inst Oswaldo Cruz 2009; 104:312 - 5; http://dx.doi.org/10.1590/S0074-02762009000200027; PMID: 19430659
- Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev 2011; 239:62 - 84; http://dx.doi.org/10.1111/j.1600-065X.2010.00980.x; PMID: 21198665
- Denkers EY, Bzik DJ, Fox BA, Butcher BA. An inside job: hacking into Janus kinase/signal transducer and activator of transcription signaling cascades by the intracellular protozoan Toxoplasma gondii.. Infect Immun 2012; 80:476 - 82; http://dx.doi.org/10.1128/IAI.05974-11; PMID: 22104110
- Ferguson DJ, Jacobs D, Saman E, Dubremetz JF, Wright SE. In vivo expression and distribution of dense granule protein 7 (GRA7) in the exoenteric (tachyzoite, bradyzoite) and enteric (coccidian) forms of Toxoplasma gondii. Parasitology 1999; 119:259 - 65; http://dx.doi.org/10.1017/S0031182099004692; PMID: 10503251
- Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines 2012; 11:189 - 209; http://dx.doi.org/10.1586/erv.11.188; PMID: 22309668
- Zhang P, Martin M, Yang QB, Michalek SM, Katz J. Role of B7 costimulatory molecules in immune responses and T-helper cell differentiation in response to recombinant HagB from Porphyromonas gingivalis.. Infect Immun 2004; 72:637 - 44; http://dx.doi.org/10.1128/IAI.72.2.637-644.2004; PMID: 14742503
- Garidou L, Heydari S, Truong P, Brooks DG, McGavern DB. Therapeutic memory T cells require costimulation for effective clearance of a persistent viral infection. J Virol 2009; 83:8905 - 15; http://dx.doi.org/10.1128/JVI.00027-09; PMID: 19553326
- Boesteanu AC, Katsikis PD. Memory T cells need CD28 costimulation to remember. Semin Immunol 2009; 21:69 - 77; http://dx.doi.org/10.1016/j.smim.2009.02.005; PMID: 19268606
- Santra S, Barouch DH, Jackson SS, Kuroda MJ, Schmitz JE, Lifton MA, Sharpe AH, Letvin NL. Functional equivalency of B7-1 and B7-2 for costimulating plasmid DNA vaccine-elicited CTL responses. J Immunol 2000; 165:6791 - 5; PMID: 11120800
- Maue AC, Waters WR, Palmer MV, Whipple DL, Minion FC, Brown WC, Estes DM. CD80 and CD86, but not CD154, augment DNA vaccine-induced protection in experimental bovine tuberculosis. Vaccine 2004; 23:769 - 79; http://dx.doi.org/10.1016/j.vaccine.2004.07.019; PMID: 15542201
- Duttagupta PA, Boesteanu AC, Katsikis PD. Costimulation signals for memory CD8+ T cells during viral infections. Crit Rev Immunol 2009; 29:469 - 86; http://dx.doi.org/10.1615/CritRevImmunol.v29.i6.20; PMID: 20121696
- Subauste CS, de Waal Malefyt R, Fuh F. Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. J Immunol 1998; 160:1831 - 40; PMID: 9469444
- Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon M, Boyer JD, Wang B, Weiner DB. Development of a multicomponent candidate vaccine for HIV-1. Vaccine 1997; 15:879 - 83; http://dx.doi.org/10.1016/S0264-410X(96)00260-5; PMID: 9234538
- Iwasaki A, Stiernholm BJ, Chan AK, Berinstein NL, Barber BH. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol 1997; 158:4591 - 601; PMID: 9144471
- Agadjanyan MG, Kim JJ, Trivedi N, Wilson DM, Monzavi-Karbassi B, Morrison LD, Nottingham LK, Dentchev T, Tsai A, Dang K, et al. CD86 (B7-2) can function to drive MHC-restricted antigen-specific CTL responses in vivo. J Immunol 1999; 162:3417 - 27; PMID: 10092797
- Verma R, Khanna P. Development of Toxoplasma gondii vaccine: A global challenge. Hum Vaccin Immunother 2012; 9:291 - 3; http://dx.doi.org/10.4161/hv.22474; PMID: 23111123
- Mévélec MN, Bout D, Desolme B, Marchand H, Magné R, Bruneel O, Buzoni-Gatel D. Evaluation of protective effect of DNA vaccination with genes encoding antigens GRA4 and SAG1 associated with GM-CSF plasmid, against acute, chronical and congenital toxoplasmosis in mice. Vaccine 2005; 23:4489 - 99; http://dx.doi.org/10.1016/j.vaccine.2005.04.025; PMID: 15935521
- Quan JH, Chu JQ, Ismail HA, Zhou W, Jo EK, Cha GH, Lee YH. Induction of protective immune responses by a multiantigenic DNA vaccine encoding GRA7 and ROP1 of Toxoplasma gondii.. Clin Vaccine Immunol 2012; 19:666 - 74; http://dx.doi.org/10.1128/CVI.05385-11; PMID: 22419676
- Liu Q, Singla LD, Zhou H. Vaccines against Toxoplasma gondii: status, challenges and future directions. Hum Vaccin Immunother 2012; 8:1305 - 8; http://dx.doi.org/10.4161/hv.21006; PMID: 22906945
- Yuan ZG, Zhang XX, He XH, Petersen E, Zhou DH, He Y, Lin RQ, Li XZ, Chen XL, Shi XR, et al. Protective immunity induced by Toxoplasma gondii rhoptry protein 16 against toxoplasmosis in mice. Clin Vaccine Immunol 2011; 18:119 - 24; http://dx.doi.org/10.1128/CVI.00312-10; PMID: 21106780
- Hiszczyńska-Sawicka E, Olędzka G, Holec-Gąsior L, Li H, Xu JB, Sedcole R, Kur J, Bickerstaffe R, Stankiewicz M. Evaluation of immune responses in sheep induced by DNA immunization with genes encoding GRA1, GRA4, GRA6 and GRA7 antigens of Toxoplasma gondii. Vet Parasitol 2011; 177:281 - 9; http://dx.doi.org/10.1016/j.vetpar.2010.11.047; PMID: 21251760
- Jongert E, de Craeye S, Dewit J, Huygen K. GRA7 provides protective immunity in cocktail DNA vaccines against Toxoplasma gondii. Parasite Immunol 2007; 29:445 - 53; http://dx.doi.org/10.1111/j.1365-3024.2007.00961.x; PMID: 17727568
- Min J, Qu D, Li C, Song X, Zhao Q, Li XA, Yang Y, Liu Q, He S, Zhou H. Enhancement of protective immune responses induced by Toxoplasma gondii dense granule antigen 7 (GRA7) against toxoplasmosis in mice using a prime-boost vaccination strategy. Vaccine 2012; 30:5631 - 6; http://dx.doi.org/10.1016/j.vaccine.2012.06.081; PMID: 22789504
- Ismail N, Olano JP, Feng HM, Walker DH. Current status of immune mechanisms of killing of intracellular microorganisms. FEMS Microbiol Lett 2002; 207:111 - 20; http://dx.doi.org/10.1111/j.1574-6968.2002.tb11038.x; PMID: 11958927
- Foulds KE, Wu CY, Seder RA. Th1 memory: implications for vaccine development. Immunol Rev 2006; 211:58 - 66; http://dx.doi.org/10.1111/j.0105-2896.2006.00400.x; PMID: 16824117
- Jongert E, Lemiere A, Van Ginderachter J, De Craeye S, Huygen K, D’Souza S. Functional characterization of in vivo effector CD4(+) and CD8(+) T cell responses in acute Toxoplasmosis: an interplay of IFN-gamma and cytolytic T cells. Vaccine 2010; 28:2556 - 64; http://dx.doi.org/10.1016/j.vaccine.2010.01.031; PMID: 20117266
- Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today 1998; 19:89 - 97; http://dx.doi.org/10.1016/S0167-5699(97)01201-2; PMID: 9509764
- Qu D, Han J, Du A. Evaluation of protective effect of multiantigenic DNA vaccine encoding MIC3 and ROP18 antigen segments of Toxoplasma gondii in mice. Parasitol Res 2013; 112:2593 - 9; http://dx.doi.org/10.1007/s00436-013-3425-0; PMID: 23591483
- Zhou H, Min J, Zhao Q, Gu Q, Cong H, Li Y, He S. Protective immune response against Toxoplasma gondii elicited by a recombinant DNA vaccine with a novel genetic adjuvant. Vaccine 2012; 30:1800 - 6; http://dx.doi.org/10.1016/j.vaccine.2012.01.004; PMID: 22240340
- Nagaraju K. Immunological capabilities of skeletal muscle cells. Acta Physiol Scand 2001; 171:215 - 23; http://dx.doi.org/10.1046/j.1365-201x.2001.00823.x; PMID: 11412133
- Wiendl H, Hohlfeld R, Kieseier BC. Immunobiology of muscle: advances in understanding an immunological microenvironment. Trends Immunol 2005; 26:373 - 80; http://dx.doi.org/10.1016/j.it.2005.05.003; PMID: 15922662
- Shirota H, Petrenko L, Hong C, Klinman DM. Potential of transfected muscle cells to contribute to DNA vaccine immunogenicity. J Immunol 2007; 179:329 - 36; PMID: 17579053
- Sibley LD, Ajioka JW. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annu Rev Microbiol 2008; 62:329 - 51; http://dx.doi.org/10.1146/annurev.micro.62.081307.162925; PMID: 18544039
- Rashid I, Hedhli D, Moiré N, Pierre J, Debierre-Grockiego F, Dimier-Poisson I, Mévélec MN. Immunological responses induced by a DNA vaccine expressing RON4 and by immunogenic recombinant protein RON4 failed to protect mice against chronic toxoplasmosis. Vaccine 2011; 29:8838 - 46; http://dx.doi.org/10.1016/j.vaccine.2011.09.099; PMID: 21983362
