Abstract
Nanocarriers with various compositions and biological properties have been extensively applied for in vitro/in vivo drug and gene delivery. The family of nanocarriers includes polymeric nanoparticles, lipid-based carriers (liposomes/micelles), dendrimers, carbon nanotubes, and gold nanoparticles (nanoshells/nanocages). Among different delivery systems, polymeric carriers have several properties such as: easy to synthesize, inexpensive, biocompatible, biodegradable, non-immunogenic, non-toxic, and water soluble. In addition, cationic polymers seem to produce more stable complexes led to a more protection during cellular trafficking than cationic lipids.
Nanoparticles often show significant adjuvant effects in vaccine delivery since they may be easily taken up by antigen presenting cells (APCs). Natural polymers such as polysaccharides and synthetic polymers have demonstrated great potential to form vaccine nanoparticles. The development of new adjuvants or delivery systems for DNA and protein immunization is an expanding research field. This review describes polymeric carriers especially PLGA, chitosan, and PEI as vaccine delivery systems.
Introduction
Nanoparticles (NPs) are solid colloidal particles with diameters ranging from 1–1000 nm. They consist of macromolecular materials and can be used therapeutically as adjuvant in vaccines or as drug carriers.Citation1,Citation2 Polymers are the most common materials for constructing nanoparticle-based drug carriers. One of the earliest reports of their use for cancer therapy dates back to 1979 when adsorption of anticancer drugs to polyalkylcyanoacrylate nanoparticles was studied.Citation3 Polymers used to form nanoparticles can be both synthetic and natural polymers.Citation1 These nanocarriers have been demonstrated for a variety of applications such as drug delivery, imaging, and detection of apoptosis.Citation3
Many cationic polymers have been studied both in vitro and in vivo for gene delivery.Citation4 The DNA encapsulated in polymers may be in a condensed or non-condensed form, depending on the nature of the polymer and the method used for formulating the vector system.Citation5 Recently, researchers have focused on biodegradable carrier systems. The potential advantage of biodegradable carriers compared with their non-degradable counterparts is their reduced toxicity and the prevention of the polymer accumulation in the cells after repeated administration.Citation4 Furthermore, the degradation of the polymer can be used as a tool to release the plasmid DNA into the cytosol.Citation4 Efficient non-viral gene delivery based on cationic polymers as DNA condensing agents is dependent on a variety of factors such as complex size, complex stability, toxicity, immunogenicity, protection against DNase degradation and intracellular trafficking, and processing of the DNA.Citation6
The nanoparticles (size <1000 nm) such as virus-like particles, liposomes, the immuno-stimulating complexes (ISCOMs), polymeric, and non-degradable nanospheres have received attention as potential delivery vehicles for vaccine antigens which can both stabilize vaccine antigens and act as adjuvants. Importantly, some of these nanoparticles are able to enter antigen presenting cells (APCs) by different pathways, thereby modulating the immune response to the antigen. This may be critical for the induction of protective Th1-type immune responses to intracellular pathogens.Citation7 Different polymers were used in solid particulate vaccine delivery.Citation8 The vaccine antigen is either encapsulated within or decorated onto the surface of the NP. By encapsulating antigenic material, NPs provide a method for delivering antigens which may otherwise degrade rapidly upon injection or induce a short-lived, localized immune response. Conjugation of antigens onto NPs can allow presentation of the immunogen to the immune systems at the same way that it would be presented by the pathogen, thereby generating a similar response.Citation7
Generally, there are obstacles in manufacturing, formulation and stability of polymers, in vitro and problems of extracellular nonspecific interactions and intracellular trafficking to the nucleus, in vivo.Citation9 Recent efforts include the development of new polymers for gene delivery, the modification of traditional polycations with hydrophilic polymers for salt and serum stability and the addition of bioactive molecules to polymers for enhanced intracellular trafficking.Citation10 Herein, we describe the roles of polymeric constructs in vaccine delivery as well as gene and drug delivery in vitro and in vivo.
Polymeric Nanoparticles in Vaccine Delivery
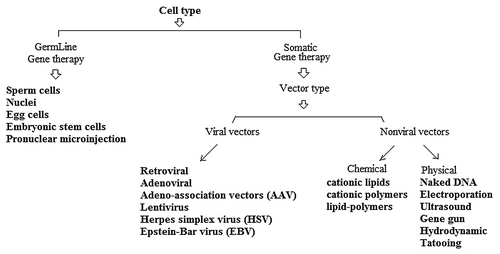
Polymeric microparticles or nanoparticles have applied to deliver genes especially in vaccine design (e.g., DNA vaccines). Moreover, gene therapy has shown an excellent potential to help patients in a variety of disease conditions.Citation11 Various strategies can be used in cancer gene therapy. Some of the gene therapy strategies to kill or slow down growth of cancer cells are included (1) Immunomodulation; (2) Prodrug activation; (3) Anti-sense/ RNAi, and (4) Induction of apoptosis.Citation12 However, the lack of suitable vectors for the delivery of nucleic acids (e.g., DNA and siRNA), especially in vaccine development, represents a major problem to their therapeutic application. Gene delivery systems include viral vectors, cationic liposomes, polycation complexes, and microencapsulated systems.Citation13 The failure of viral gene therapy in clinical trials due to toxicity, immunogenicity, and carcinogenicity strongly motivates a non-viral approach.Citation14 Synthetic vectors based on polycations are promising vectors for gene delivery as they are relatively safe and can be modified by the incorporation of ligands for targeting to specific cell types. However, the levels of gene expression mediated by synthetic vectors are low compared with viral vectors.Citation15 Several vectors have been developed in order to target genes to specific cells as shown in .
As known, a perfect gene vector includes four conditions: (1) be able to condense DNA effectively; (2) be stable in body fluid; (3) be able to target the specific cells, and (4) be able to cross membranes and release efficiently.Citation16 Strategies for overcoming some of these barriers have resulted in polymer/DNA complexes with increased stability and delivery efficiencies.Citation10 In addition, trafficking of nuclear proteins from the cytoplasm into the nucleus through nuclear pore complexes can be mediated by the presence of nuclear localization sequences (NLS) on proteins.Citation17 If the vector contains one or several NLS, either as covalently or non-covalently DNA-linked peptides, a competition may take place between the rate of dissociation of the DNA-vector complexes and the rate of loading of the complexes to the NLS-mediated nucleus importation machinery.Citation18 Moreover, since the cytosolic release of heterologous DNA is a prerequisite for nuclear translocation, entrapment, and degradation of plasmid DNA in endolysosomes constitute a major barrier to efficient gene transfer.Citation19
The polyplexes which are formed between cationic polymers and DNA through electrostatic interactions and thus known as polycation/DNA complexes are widely used as non-viral gene delivery vectors. Many factors such as molecular weight (MW), surface charge, charge density, hydrophilicity, and the structure of cationic polymers affect gene transfection efficiency of cationic polymers. Therefore, optimization of cationic polymers is necessary to improve the gene transfection efficiency.Citation20 Currently, several important cationic polymers were used for gene delivery such as Polyethylenimine (PEI), PLL, Chitosan, and PAMAM. Some strategies including PEGylation, combination, and multifunctional modification were developed in the cationic polymeric vectors.Citation20
Polyethylenimine (PEI) and DNA/ RNA transfection
PEI is a cationic polymer widely used for nucleic acid delivery.Citation21 It is particularly promising vector with relatively high level of transfection in a number of target organs. The high charge density of PEI is thought to be a key factor that contributes to its high transfection efficiency. On the other hand, the polycationic nature of PEI also appears to be the main origin of its toxicity, similar to many other polycations (e.g., polylysine). This toxicity has limited its use as a gene delivery vector in vivo.Citation1 Therefore, the success of gene transfection is dependent on the development of vectors that can efficiently deliver a gene to cells with minimum toxicity.Citation1 The studies have shown that PEI derivatives obtained by cross-linking low-molecular weight PEI with degradable materials display higher transfection efficiency and lower cytotoxicity.Citation16 For example, in order to develop new polymeric gene vectors with low cytotoxicity and high gene transfection efficiency, a cationic polymer was composed of low molecular weight PEI (MW ~600 Da) cross-linked by 2-hydroxypropyl-g-cyclodextrin (HP-g-CD) and then coupled to MC-10 oligopeptide containing a sequence of Met-Ala-Arg-Ala-Lys-Glu at a molar ratio of 1:3.3:1.2.
This new gene vector was able to target delivery of genes to HER2 positive cancer cells for gene therapy.Citation22 Furthermore, water-soluble lipopolymer (WSLP) consisting of a low molecular weight PEI and cholesterol was employed for in vivo gene therapy of cancer or ischemic myocardium. The Preformed PEI/DNA complexes were encapsulated in PEG stabilized liposomes resulting in the so-called “pre-condensed stable plasmid lipid particle” (pSPLP).Citation23
Currently, cell penetrating peptides (CPPs) have also been used to enhance the intracellular delivery of DNA by PEI and/ or dendrimers.Citation24 Our group showed that two delivery systems including PEI 25 kDa and PEI600-Tat conjugates are efficient tools for HPV16 E7 gene transfection. Although the level of transfected COS-7 cells is higher using PEI 25 kDa in comparison with PEI600-Tat, but its toxicity was obstacle in vivo.Citation25 Transfection experiments demonstrated that the use of PEI600-Tat conjugates was more effective than the two compounds without chemical conjugation. Furthermore, the newly developed conjugates maintain the desirable property of low cytotoxicity displayed by lower molecular weight PEI polymers and Tat peptides. It has been confirmed that a type of low molecular weight polymer, so-called PEI (MW <2000 Da), covalently coupled to Tat was able to improve Tat peptide mediated gene delivery as chloroquine.Citation26,Citation27
Adsorption of nucleic acid onto cationic nanoparticles is one of the approaches used for DNA or RNA delivery.Citation21 This technique facilitates the immediate release of DNA or RNA at target site.
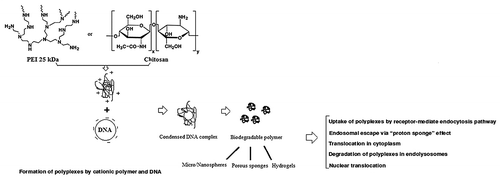
Furthermore, the preparations of polymer and DNA/RNA complexes by adsorption can avoid the chemical effects used in other approaches such as encapsulation.Citation21 For example, PEI possesses very high positive charges from amines in molecules which can form complexes with phosphate groups of nucleic acids through electrostatic interaction. The complexes can be later delivered into the cell through endocytosis.Citation21 PEI is considered to be the most effective cationic polymer due to its buffering capacity via the proton sponge effect.Citation21 Its high proton-buffering capacity results in rapid osmolysis of the endosomes and the PEI/DNA complexes escape into the cytosol and are subsequently transported into the nucleusCitation28 ().
Figure 2. Functions of two important examples of current cationic polymers (PEI and chitosan) as non-viral gene delivery vectors.
In a study, Heparin-PEI (HPEI) nanoparticles were used to deliver plasmid-expressing mouse survivin-T34A (ms-T34A) to treat C-26 carcinoma in vitro and in vivo. According to the in vitro studies, HPEI nanoparticle-mediated ms-T34A could efficiently inhibit the proliferation of C-26 cells by induction of apoptosis. Moreover, intra-tumoral injection of HPEI nanoparticle-mediated ms-T34A significantly inhibited growth of subcutaneous C-26 carcinoma in vivo by induction of apoptosis and inhibition of angiogenesis.Citation29
Chitosan and DNA transfection
Chitosan, produced by deacetylation of chitin, is a non-toxic and hydrophilic polysaccharide. Commercially, chitin and chitosan are obtained from shellfish sources such as crabs and shrimps.Citation30 Chitosan and its derivatives could accelerate wound healing by enhancing the functions of inflammatory cells and repairing cells.Citation31 Recent studies further indicated that chitosan and its derivatives are used as a carrier of DNA for gene delivery applications.Citation13 It is able to condense nucleic acid into stable complexes (100–250 nm in diameter), which protects DNA from degradation by nuclease.Citation7 The DNA/polymer complexes are taken up into the cells via endocytosis into the endosomes, following with burst release of complexes fraction in endosomes and the DNA translocates into the nucleusCitation32 (). Chitosan could be a useful oral gene carrier because of its adhesive and transport properties in the GI tract.Citation13 Although most chitosans are able to form polyplexes, the transfection efficiency of chitosans depends on structural variables such as the fraction of acetylated units, the degree of polymerization, the chain architecture and chemical modifications.Citation33 On the other hand, the researchers found that in vitro chitosan-mediated transfection depends on the cell type, serum concentration, pH, and molecular weight of chitosan.Citation1 For example, Hela cells were efficiently transfected by this system even in the presence of 10% serum. In contrast, chitosan has not been able to transfect HepG2 human hepatoma cells and BNLCL2 murine hepatocytes. The transfection efficiency was found to be higher at pH 6.9 than that at pH 7.6. Indeed, at pH < 7, amine groups of chitosan are protonated which facilitate the binding between complexes and negatively charged cell surface. Moreover, transfection efficiency mediated by chitosan of high molecular weight (MW) > 100 kDa is less than that of low MW ~15 and 52 kDa.Citation1
Although, chitosan successfully transfected cells in vitro, the transfection efficiency showed to be lower than that of other cationic polymer vehicles such as PEI.Citation1,Citation28 One of the primary causes of poor gene delivery efficiency is the insufficient release of chitosans from endosomes into the cytoplasm.Citation28 Two approaches have been developed to increase transfection efficiency of chitosan nanoparticles: (1) Enhancement of chitosan solubility and (2) Attachment of cell targeting ligands to the chitosan particles.Citation1 As known, chitosan is insoluble at physiological pH and also it lacks charge. Thus, for development of an efficient gene vector with high transfection and low cytotoxicity, amphiphilic chitosan was linked with low-molecular weight PEI.Citation16 In addition, a liver cancer-targeted specific peptide (FQHPSF sequence) was bound with chitosan-linked PEI (CP) to form a new targeted gene delivery vector called CPT (CP/peptide). The vector showed low cytotoxicity and strong targeting specificity to liver tumors in vitro. The in vivo results showed that IL-12 delivered by CPT (CPT/DNA) significantly enhanced the antitumor effects on ascites tumor bearing mice as compared with PEI 25 kDa and CP as a control.Citation28
PEI and chitosan as immune stimulators
Vaccination is cost-effective and the best prophylactic strategy against most diseases.Citation34 Vaccines are the preparations given to patients to stimulate immune responses leading to the production of humoral or cell-mediated responses that will combat infectious agents or non-infectious conditions such as tumors. Vaccines may be prophylactic (e.g., to prevent the effects of a future infection by pathogens) or therapeutic (e.g., vaccines against cancer). Attempts are being made to deliver vaccines through carriers as they control the presentation of antigens to immune system thus leading to their prolonged release and targeting.Citation8 Thus, lower doses of weak immunogens can be effectively directed to stimulate immune responses and eliminate the need for the administration of prime and booster doses as a part of conventional vaccination regimen.Citation8
The previous studies demonstrated that the linear PEI (L-PEI) is being more efficient in vivo than the branched PEI (B-PEI). The researchers have analyzed the production of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-6, IL-12/IL-23, IFN-β, and IL-1β) and hepatic enzyme levels (alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and alkaline phosphatase) in the blood serum of mice after systemic injection of DNA or siRNAs delivered with L-PEI.Citation35 The data showed no major production of pro-inflammatory cytokines or hepatic enzymes after injection of DNA or oligonucleotides active for RNA interference (siRNAs or sticky siRNAs) complexed with L-PEI. Only a slight induction of IFN-γ was detected after DNA delivery, which is probably induced by the CpG mediated response. Altogether, the results highlighted that linear PEI is a delivery reagent of choice for nucleic acid therapeutics.Citation35
Using nanoparticles to deliver antigens, the efficiency of uptake into dendritic cells is significantly increased compared with soluble antigen alone.Citation7 Particle shape and surface charge are also important physicochemical factors playing critical roles in the interaction between particles and antigen-presenting cells (APCs). Generally, cationic particles are taken up into cells much more readily than those with an overall negative surface charge due to the anionic nature of cell membranes.Citation7 When NPs as poly (amino acid) with encapsulated ovalbumin were used to immunize mice, significantly higher levels of total IgG, IgG1, and IgG2a as well as IFN-gamma (stimulator of Ig class switching to IgG2a) were induced as compared with those in soluble ovalbumin, suggesting the particles have the ability to prime humoral and cellular immune responses.Citation7 In this line, chitosan could act on tumor cells directly to interfere with cell metabolism, inhibit cell growth, and induce cell apoptosis.Citation36 Chitosan induced apoptosis of bladder tumor cells via caspase-3 activation.Citation37 In addition, it showed an anti-tumor role through improving the body’s immune function.Citation36 Indeed, hydrophilic polysaccharide polymers are also good candidates for vaccine delivery with both dextran and chitosan being chosen for preparing NPs.Citation7 On the other hand, PEI /DNA complexes (“polyplexes”) conjugated with the cell-binding ligand transferrin (Tf) or epidermal growth factor (EGF) were used to achieve receptor-mediated endocytosis. The surface charge of the complexes was masked by covalently linking PEI to polyethylene glycol (PEG). Intravenous injection of Tf–PEG-coated polyplexes resulted in gene transfer to subcutaneous neuroblastoma tumors of syngeneic A/J mice. Furthermore, EGF-PEG coated polyplexes were intravenously applied for targeting human hepatocellular carcinoma xenografts in SCID mice.Citation38 In this line, our group showed that the mixture of a DNA vaccine expressing HPV16 E7 with PEI600-Tat cojugate is immunologically more potent than E7 alone. Indeed, binding of cationic peptide (Tat)-polymer (PEI) to plasmid DNA encoding an antigen (E7) enhanced the uptake of the plasmid DNA and consequently induced both humoral and cellular immune responses in vaccinated mice. Our observations illustrated the ability of PEI-Tat conjugate to augment immune responses in vivo. Herein, the ratio of PEI600-Tat/E7DNA complex formation has significant influence on the level of protein expression and consequently immune responses in C57BL/6 mice model.Citation39
Generally, interactions of cationic polymers with the immune systems are rarely studied. Agonists of toll like receptors (TLRs) are potential therapeutic reagents for cancer immunotherapy. Cationic polymers have significant immunological activity mediated by TLRs. The studies indicated that cationic polymers including PEI, polylysine, cationic dextran and cationic gelatin specifically stimulate the macrophage to secrete IL-12 which is one of the main Th1-inducing cytokines.Citation40 Cationic polymers could interact with macrophages through TLR-4 which is the receptor of LPS. The stimulation ability of cationic polymer was related with their cationic degree and molecular weight. Larger molecular weight and higher positive charge of polymers exhibited stronger stimulation ability.Citation40 Additionally, the cationic polymers such as PEI and cationic dextran could reverse tumor-associated macrophages (TAMs) polarization and promote IL-12 expression both in vitro and in vivo. Indeed, these cationic polymers exerted direct tumoricidal activity by promoting Th1 and NK cell infiltration, suppressing tumor angiogenesis, and prolonging the survival of sarcoma-bearing wild-type mice.Citation41 As known, IL-12 is a potent anti-tumor cytokine that exhibits significant clinical toxicities following systemic administration. A study showed that intra-tumoral administration of IL-12 co-formulated with the biodegradable polysaccharide chitosan could enhance the anti-tumor activity of IL-12 in mice bearing established colorectal (MC32a) and pancreatic (Panc02) tumors while limiting its systemic toxicity. Chitosan/IL-12 is a well-tolerated, effective immunotherapy with considerable potential for clinical trials.Citation42 Vaccine immunotherapy using a specific antigen, such as prostate specific antigen (PSA) led to stimulate both the innate and adaptive immune systems to destroy tumor cells in the body. An adenovirus encoding PSA (Ad-PSA), as a viral gene delivery system could stimulate anti-tumor activity.Citation43 To enhance transfection efficiency, the combination of this system with a cationic polymer such as PEI or chitosan was applied. In fact, cationic polymers could complex with the negatively charged adenovirus to form nanoparticles. To further augment immune response, CpG sequences were used as an adjuvant delivered in particulate form.Citation43 In this line, the studies demonstrated that the adenovirus encoding OVA (AdOVA) as a model antigen, coupled with PEI, increased tumor protection in vivo compared with AdOVA alone. In addition, AdOVA + CpG showed the best tumor protection in therapeutic studies.Citation43 In other set of experiments, AdOVA + chitosan + CpG represented a decrease in protective levels and antigen-specific immune responses. Indeed, the kinetic studies showed that peak levels of effector T cells were present 14 d later in AdPSA + CpG immunized mice than in AdPSA alone. This delayed effect may explain the increased levels of protection in AdPSA + CpG mice against AdPSA + chitosan + CpG. The data are useful in vaccine design concerning the timing of peak response.Citation43 Recently, cancer vaccine has become a novel modality for cancer treatment and the important role of adjuvant has been realized. Chitin, chitosan, and their derivatives are important adjuvants for immunotherapy. Based on their principal mechanisms of action, adjuvants can be generally divided into two classes: (1) vaccine delivery systems such as mineral salts, emulsions, liposomes, and virosomes; (2) immunostimulatory adjuvants including toll-like receptor (TLR) agonists (e.g., monophosphoryl lipid A), saponins and cytokines.Citation44 Chitin has the ability to activate innate immune cells and induce cytokine and chemokine production. The cell surface receptors include macrophage mannose receptor, TLR-2, C-type lectin receptor Dectin-1 and leukotriene B4 receptor (BLT1). In addition, intraperitoneal injection of chitin particles induced adaptive Th2, Th1, and Th17 immune responses. TLR-2, MyD88, and IL-17A have been proved to play important roles in the adjuvant properties of chitin and chitosan.Citation44 It is believed that chitosan enhances both humoral and cell-mediated immune responses e.g., in subcutaneous vaccination. In addition, recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) accelerates neutrophil recovery in cancer patients receiving chemotherapy. When it co-formulated with chitosan, local rGM-CSF retention at a subcutaneous injection site was increased in mice for up to 9 d. In contrast, when delivered in a saline vehicle, rGM-CSF was undetectable in 12–24 h. This indicated that chitosan helped to control the distribution of rGM-CSF.Citation44
Poly (lactic-co-glycolic acid) and immunity
Biodegradable polymers such as poly (lactic-co-glycolic acid) (PLGA) are also being developed for matrix antigen delivery. PLGA microspheres are rapidly taken up by M-cells and translocated toward the underlying lymphatic tissue within 1 h.Citation8 For instance, the loading of Hepatitis B core antigen into PLGA NPs (300 nm) induced a stronger cellular immune response as compared with Hepatitis B core antigen alone in a mouse model. Particle size plays an important role in directing the immune response. Immunization with PLGA NPs (200–600 nm) was associated with higher levels of IFN-γ production related to a Th1 response. In contrast, immunization with PLGA microparticles (2–8 μm) promoted IL-4 secretion related to a Th2 response.Citation7 The studies have indicated that both PLGA NPs and liposomes are efficiently phagocytosed by dendritic cells in culture, resulting in their intracellular localization.Citation7 However, the use of PLGA can be limited by acid hydrolytic degradation products detrimental to the entrapped protein and loss of immunogenicity on storage. Also, organic solvents used to load the antigen onto the polymer can be detrimental to the antigen.Citation8
Formulation of DNA into both liposomal and polymeric cationic nanoparticles completely blocks vaccination-induced antigen expression in mice and ex vivo human skin. Furthermore, this negative effect of cationic nanoparticle formulation is associated with a complete block in vaccine immunogenicity.Citation45 The reports showed that shielding of the surface charge of the nanoparticles by PEGylation improves in vivo antigen expression more than 55 fold. Furthermore, this shielding of cationic surface charge results in antigen-specific T cell responses similar to those induced by naked DNA for both lipoplex and polyplex DNA carrier systems. These observations suggest that charge shielding forms a useful strategy for the development of dermally vaccine formulations.Citation45
Polymeric Vaccine Delivery Systems in Clinical Trials
The considerable research on microparticle-based vaccines has generated a number of strategies based on optimizing antigen release rates to produce single dose delivery systems. For example, pulse release of antigen from biodegradable microparticles is considered advantageous for simulating the conventional, multi-dose vaccine delivery regime.Citation46 However, most microparticulate delivery systems are considered to function on the principles of efficient phagocytosis and transport to the lymph nodes and sustained antigen release over extended time periods which may present a continuous leakage of antigen to the immune system.Citation46,Citation47
Cationic polymers and DNA vaccines
Within a decade, a myriad of potential applications of DNA vaccines developed targeting infectious agents, various cancers, allergy and immune dysfunction and by 1998 early clinical trials reported induction of immune responses against HIV and malaria in humans. The only licensed and approved DNA-based vaccines are for animal use.Citation48 One targets flavivirus (West Nile virus) infection in horses and has also been used to protect wild Californian condors; the other has been used to protect commercial salmon against infectious hematopoietic necrosis virus. Surface DNA loading can be facilitated by supplementation with the cationic surfactant cetyltrimethylammonium bromide (CTAB) as well as by the incorporation of PEI either into the matrix of the microsphere or at the surface. Many other materials such as DOTAP, DEAM, and PLL have been used to absorb DNA to PLGA microparticles.Citation48 PLGA/CTAB microparticles were recently developed into stage I clinical trials by Novartis for HIV-1 DNA vaccination. Incorporating PEI into PLGA microspheres has also been developed as a method for avoiding the problems associated with internal encapsulation of plasmid DNA. PEI imparts a positive charge to the PLGA microsphere. Unlike CTAB, which by itself is not a transfection agent, PEI has intrinsic ability to form nanoparticles with DNA and increase transfection efficiency.Citation48 In addition, PLGA–PEI microspheres improved in vitro transfection and caused upregulation of co-stimulatory signals on APCs. Increased survival against a lethal dose of lymphoma tumor challenge was observed following intradermal (ID) vaccination with PLGA microspheres prepared with branched PEI on the surface. While both intramuscular (IM) and ID PLGA–PEI vaccination routes provided protection against lymphoma tumor challenge, IM vaccination was more efficient among vaccination routes.Citation48 On the other hand, PLGA can also be used to encapsulate and release pre-formed PEI–DNA nanoparticles. Microspheres release DNA in PEI–DNA nanoparticle with kinetics similar to that of simple PLGA microparticles and also efficiently transfect non-phagocytic cells as well as APCs. It was demonstrated that PLGA-PEI-DNA microspheres increase humoral responses compared with naked DNA following IM vaccination and can induce efficient CTL responses at doses lower than that obtained with naked DNA vaccination.Citation48
Almost 100 Phase I and II clinical trials have confirmed the safety of DNA vaccines in humans.Citation49 PLGA is one of the most widely studied polymers of interest in the vaccine field.Citation50 For instance, to increase the efficacy of DNA-based vaccines, DNA encoding hepatitis B surface antigen (HBsAg)-encapsulated formulation of PLGA nanoparticles could induce enhanced immunity in mice.Citation51 In addition, PLG encapsulated DNA encoding human papillomavirus antigen has been tested in phase I and II human clinical trials.Citation52 PLGA microspheres containing DNA encoding for the E6 and E7 genes of human papillomavirus virus (HPV) 16 and 18 have been developed into clinical trials by MGI Pharma, Inc. to treat advanced pre-cancerous cervical intraepithelial neoplasia (CIN) by inducing CTL-mediated responses to HPV-infected pre-cancerous epithelial cells. A phase I trial of PLGA microparticles encapsulating plasmid DNA encoding only HPV-16 E7 antigen (ZYC101, Eisai Pharmaceuticals) established T-cell immunologic responses in 11/15 participants and complete clinical response in 5/15 patients following three intramuscular (IM) vaccinations. In a phase II study, patients with CIN grade 2/3 were injected IM with DNA encoding for both the E6 and E7 antigens in PLGA microspheres (ZYC101a/Amolimogene). These two trials demonstrate the clinical potential of DNA vaccines delivered by PLGA microspheres ().Citation53-Citation70 A phase II/III trial of ZYC101a is currently underway.Citation52 Tumor antigen (ZYC300)-encoding plasmid DNA encapsulated in biodegradable polymer microparticles was evaluated in cancer patients and was shown to induce detectable immune responses and clinical improvement.Citation49 PLG microparticles with adsorbed DNA encoding HIV antigens have recently entered human clinical trials in healthy volunteers.Citation52
Table 1. DNA vaccine delivery system
Moreover, PEI is also under clinical study for DNA vaccine delivery. Mannose–PEI was originally used for the ex vivo transfection of dendritic cells (DCs), which successfully generated effector and memory CTL responses following subcutaneous (SC) injection in non-human primates mediated by transfection of Langerhans cells in vivo. Interestingly, these robust cell-mediated responses were not accompanied by antibody production.Citation48 DermaVir is an intradermal administration of linear PEI conjugated to mannose for the purpose of generating HIV immunity and is currently in phase I/II studiesCitation70 [].
Poloxamers (Pluronics) are a well-studied group of copolymers used as surfactants in a variety of pharmaceutical applications including vaccine delivery and as adjuvants for DNA vaccines. Poloxamers are thought to act as adjuvants by recruiting and activating APCs. Poloxamers consist of blocks of poly (ethylene oxide) (PEO) flanking a central poly(propylene oxide) (POP) core, and CRL1005 is a triblock copolymer that has a POP core of 12 kDa flanked with 350 Da PEO.Citation48 CRL1005 forms microparticles spontaneously above a phase transition temperature, though formulation with the cationic surfactant benzalkonium chloride (BAK) reduces particle sizes into the nanometer range (200–300 nm). Without BAK, DNA does not associate with CRL1005. However, adding plasmid DNA to BAK-CRL1005 particles increases the size to 300 nm, indicating CRL1005-BAK-DNA particle formation, and these ternary nanoparticles were shown to increase cell-mediated immune responses in non-human primates.Citation48
While the mechanism of CRL1005 without BAK as a DNA vaccine adjuvant is poorly understood, there is some evidence that CRL1005 enhances delivery of DNA in vivo. CRL1005 has also been shown to be safe and practical. In pre-clinical trials, CRL1005-BAK-DNA nanoparticles increased cell-mediated and humoral responses to cytomegalovirus (CMV) antigens.Citation48 In addition, CRL1005-BAK-DNA induced cell-mediated and humoral CMV responses in humans in phase I clinical trials, and this formulation is now in phase II clinical trials. Poloxamer was also mixed with PLGA to form nanoparticles for nasal delivery of DNA to elicit a strong humoral response.Citation48
Since the beginning of year 2000, several phase I clinical trials investigating DNA vaccination against cancer have evaluated DNA delivery to patients with colorectal carcinoma, HPV16-associated anal dysplasia, B-cell lymphoma, metastatic melanoma, and prostate cancer.Citation71 All studies demonstrated that repetitive DNA administration is well tolerated, with no dose-limiting toxicities even at doses of 2 mg per injection.Citation71 The first study of Prostate-Specific Antigen (PSA)/DNA vaccine demonstrated that PSA-specific cytotoxic T lymphocytes could be induced in mice. When two cytokine adjuvants, granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin-2 (IL-2), were co-delivered with the DNA vaccine, 80% of the mice were protected against a syngenic challenge with PSA-expressing tumor cells. Then, the safety, feasibility, and Biological efficacy of PSA/DNA vaccine was evaluated in a phase I clinical trial in patients with hormone-refractory PC. No adverse effects (WHO grade > 2) were observed in any patients.Citation71 PSA-specific cellular responses and an increase in anti-PSA antibodies were detected after vaccination with the highest vaccine dose (900 µg). However, new adjuvants and/or delivery systems need to be explored to enhance the anti-tumor immune responses activated by DNA vaccines in humans.Citation71,Citation72
Polymeric nanoparticles in clinical trials
Nowadays, the use of polymeric materials to elicit DNA vaccine responses seems promise.Citation47 The use of available polymers such as PLGA, chitosan, and PEI has shown much promise in pre-clinical and clinical studies. Polymers used for gene delivery including POEs, PAMAMs, and PBAEs have only recently emerged as promising strategies for DNA vaccine delivery.Citation48 The improvement of oral bioavailability of several other therapeutic peptides by encapsulation in polymeric nanoparticles was also studied. Immunization with DNA encoding HLA-A2-restricted epitopes from the HPV16 E7 protein, encapsulated in biodegradable polymer microparticles, could induce HPV-specific T-cell responses in 10/12 patients which were still elevated after 6 mo.Citation71 Currently, vaccines are certainly the most promising applications for orally delivered nanoparticulate systems. Indeed, immunological stimulation does not require a dose as high as those required obtaining a pharmacologic effect and control of time release profile could be less critical.Citation73 In addition, several nanoparticle-siRNA therapies are in human clinical trials to assess their safety and efficiency. Since the initial discovery of RNAi, there have been over 30 clinical trials assessing the potential of siRNA as a novel therapeutic.Citation74
Alternatives of Natural or Synthetic Polymers
Natural polymers such as chitosan, albumin, and heparin have been used for the delivery of oligonucleotides, DNA, and protein, as well as drugs. An albumin-paclitaxel nanoconjugate has been studied in the treatment of metastatic breast cancer during phase III clinical trials.Citation75 Different in vitro and in vivo research studies have focused on the use of conjugated polymeric nanoparticles with chemotherapeutic drugs to reduce the damaging effects of the free drug administration. Currently, more than 20 nano-particle therapeutics, are in clinical use, validating the ability of nanoparticles to improve the therapeutic index of drugs.Citation75
Anticancer drugs often have poor solubility in water and thus need to use organic solvents or detergents for clinical applications, resulting in undesirable side effects such as venous inflammation and respiratory distress. Therefore, designing a distinct carrier system that encapsulates a large quantity of drugs and specially targets tumor cells is essential for successful cancer therapy.Citation76 To date, at least 12 polymer-drug conjugates have entered Phase I and II clinical trials and are especially useful for targeting blood vessels in tumors. Examples include anti-endothelial immune-conjugates, fusion proteins, and caplostatin, the first polymer- angiogenesis inhibitor conjugates.Citation3 Recently, water-soluble polymers have been proposed due to simple preparation methods without the use of organic solvent.Citation1
Polymeric NPs have attracted much attention for their ability to deliver drugs as well as being biodegradable.Citation7 Among cationic water-soluble polymers available, chitosan is one of the most extensively studied polymers.Citation1 Recently, further studies have been focused on using nanoparticles in cell culture.Citation30 Chitosan showed significantly lower toxicity than poly-l-lysine and PEI.Citation1 For therapeutic applications, drugs can either be integrated in the matrix of the particle or attached to the particle surface. A drug targeting system should be able to control the fate of a drug entering the biological environment.Citation77 Chitosan microsphere have several applications in novel drug delivery systems such as GI-delivery systems, colon and intestinal drug delivery, opthalmic drug delivery, oral, buccal and sublingual drug delivery, nasal and transdermal drug delivery, and vaginal drug delivery.Citation13
Chitosan and its derivatives can be covalently cross-linked to prepare nano-sized particles as the drug carriers.Citation78 The chemical cross-linkers that have been widely used for chitosan include bifunctional agents such as PEG dicarboxylic acid, glutaraldehyde or mono-functional agents such as epichlorohydrin.Citation76 The release kinetics of loaded drugs from polymeric NPs can be controlled by compositional changes to the copolymer. This class of NP can be prepared from a range of polymers including poly (α-hydroxy acids), poly (amino acids), or polysaccharides to create a vesicle which can either accommodate or display antigens.Citation7
Chitosan can be formulated in a variety of forms such as powder, film, sphere, gel, and fiber.Citation30 Chitosan nanoparticles showed selectivity for tumor cells.Citation36 Studies have indicated significant differences in antitumor activity of nanoparticles prepared by chitosan from different producers.Citation36 Nanogels are nanosized hydrogel particles formed by physical or chemical cross-linked polymer networks.Citation79 The materials used for the preparation of nanogels ranged from natural polymers like ovalbumin, pullulan, hyaluronic acid, methacrylated chondroitin sulfate and chitosan, to synthetic polymers like poly (N-isopropylacrylamide), poly (N-isopropylacrylamide-co-acrylic acid), and poly (ethylene glycol)-b-poly (methacrylic acid).Citation79
The mechanism of nano-particles formation is based on electrostatic interaction between amine group of chitosan and negatively charge group of polyanion such as tripolyphosphate. This technique offers a simple preparation method in the aqueous environment.Citation1 In order to improve targeting and bioavailability of chitosan nano-particles, an increasing number of studies are focusing on modification of chitosan. Modified chitosan nano-particles are characterized by pH sensitivity, thermosensitivity, and targeting accuracy.Citation36
Generally, chitosan possesses some ideal properties of polymeric carriers for nanoparticles such as biocompatible, biodegradable, nontoxic, and inexpensive. Furthermore, it possesses positively charge and exhibits absorption enhancing effect.Citation1 The reports mentioned the preparation of pH-responsive chitosan-based microgels (<200 nm diameter) by ionically cross-linking N-[(2-hydroxy-3-trimethylammonium) propyl] chitosan chloride in the presence of tripolyphosphate. These microgels were loaded with methotrexate and conjugated to apo-transferrin.Citation76 The authors demonstrated that the conjugated microgels exhibited a significant increase in mortality of Hela cells, compared with non-conjugated microgels. This was ascribed not only to receptor-mediated endocytosis of the conjugated microgels, but also to pH-mediated release of methotrexate from the microgels by their swelling at the intracellular level.Citation76
The benefits of chitosan-based vectors include their availability, ease of modification, and unique biological properties related to their polycationic nature.Citation33 Chitosan nano-particles are capable of passing through biological barriers in vivo (e.g., the blood-brain barrier) and delivering drugs to the lesion site due to their small size.Citation36 Evidence has shown that chitosan nanoparticles may exert differential bactericidal and pharmacological effects on prokaryotic and eukaryotic cells in culture.Citation30 In vitro anti-tumor testing of chitosan nano-particles indicated that inhibition rate of 500 mg/L chitosan nano-particles was 27% on Hela cells of cervical cancer, 23% on liver SMMC-7721 cells, 29% on gastric cancer BGC-823 cells, and 55% on breast cancer MCF-7 cells.Citation30
The studies have shown that cancer treatments consisting of a combination of chemotherapy and immunotherapy have been exploited to further improve the efficacy of cancer therapies. In a study, a chitosan hydrogel (CH) system loaded with GM-CSF and a cancer drug was utilized as a chemo-immunotherapeutic agent in an effort to assess the anti-tumor effects in mice model.Citation80
The growth of TC-1 tumors was significantly reduced in mice treated with a CH harboring a cancer drug (doxorubicin: DOX), cisplatin (CDDP) or cyclophosphamide (CTX), and GM-CSF (CH-a cancer drug + GM-CSF), as compared with other groups that were treated with CH containing only a cancer drug (CH-a cancer drug) or GM-CSF (CH-GM-CSF).Citation80
Curcumin, a polyphenolic compound found in the spice turmeric, has been found to exert preventive and therapeutic effects in various cancers.Citation81 It is able to inhibit the growth of breast cancer cell lines in a dose dependent manner and induces an increase in the percentage of cells in sub-G0 phase, representing the apoptotic cell population.Citation82 Curcumin is being applied to a number of patients with breast cancer, rheumatoid arthritis, Alzheimer disease, colorectal cancer, and psoriatic.Citation83 Various basic and clinical studies elucidated curcumin's limited efficacy due to its low solubility, high rate of metabolism, poor bioavailability, and pharmacokinetics.Citation84
Recently, the polymeric nanoparticle encapsulated curcumin (nanocurcumin) is under development for cancer therapy and also to overcome these challenges.Citation85 In addition, curcumin loaded biodegradable self-assembled polymeric micelles have been developed to overcome poor water solubility of curcumin and to meet the requirement of intravenous administration.Citation86 In an experiment, our group tested tumor inhibition rates of a chitosan hydrogel system loaded with curcumin (nanocurcumin) on breast cancer MCF-7 cells. Cytotoxicity study showed that the encapsulated curcumin remained its potent anti-tumor effect. IC-50 was calculated 23% and 44% after 48 h and 72 h, respectively (unpublished data, 2013). The studies showed that curcumin treatment could display anti-proliferative and pro-apoptotic activities and induce cell cycle arrest at G2/M phase.Citation87 Briefly, polymeric nano-particles have long been chosen as carriers for systemic and targeted drug delivery. The ability of these particles to circulate in the bloodstream for a prolonged period of time is often a prerequisite for successful targeted delivery.Citation88 The present results suggest that a combinational coating of PEG and chitosan may represent a significant step in the development of long-circulating drug delivery carriers for tumor drug delivery.Citation88
PLGA nanoparticles are widely used for the delivery of various chemotherapeutic agents (especially hydrophobic drugs) to the target site.Citation88 However, rapid opsonization by cells of the phagocytic system is a major limitation for achieving effective drug targeting to the site of action by PLGA nanoparticles. Thus, to maximize the therapeutic benefits of drug loaded nanoparticles, they should be able to evade the reticuloendothelial system (RES). This can be done through the use of various surface coatings of hydrophilic polymers, as opsonization of hydrophobic nanoparticles may occur more quickly in comparison to hydrophilic nanoparticles due to the enhanced adsorption of opsonins on their surfaces.Citation88
Perspectives: Myths and Facts
Different NP delivery systems have been described, each offering advantages over current methods of vaccine delivery. Nanotechnology platforms are being investigated as vaccine carriers, adjuvants, and drug delivery systems to target inflammatory and inflammation-associated disorders. Recently, researchers started to understand the effects of particle size, surface characteristics, and material interactions with the innate immune system. Investigating of the underlying biological mechanisms of DNA vaccination requires strategies that can isolate one polymer function from another, such as DNA release kinetics and transfection efficiency. Future development of polymeric and other synthetic materials must focus on these considerations for DNA vaccination.
Rather than conventional vaccines which use whole microbes (live or killed), this new generation of vaccines use components of microbes to elicit an immune response and mimic the way in which these antigens would be delivered during a natural infection. Often these antigens are poor immunogens on their own and thus require an adjuvant to boost the immune response. NPs provide an alternate method for antigen delivery which not only activates different elements of the immune system but also have good biocompatibility. Delivering antigens in different ways also has a profound effect on the resulting immune response, whether the antigen is decorated on the NP surface for presentation to antigen-presenting cells or encapsulated for slow release and prolonged exposure to the immune system. Recently, the enhancement of vaccine potency through the use of different delivery systems (e.g., NPs) is underway. However, this novel and promising approach (polymeric NPs) should be improved as an efficient delivery system for gene, drug, and vaccine in future as well as focusing on the modification of their structures to reduce toxicity and overcome in vivo barriers. Recent preclinical and clinical studies reflect the effects of immunotherapy in combination with chemotherapy as a potential approach to specifically target cancer leaving normal cells safe. Furthermore, the immunosuppressive microenvironment of tumor should be blocked by inhibitors using different delivery systems, as a promising application in cancer immunotherapy.
Future and Alternative Directions
Nanoparticles can be engineered to either avoid immune system recognition or specifically inhibit or enhance the immune responses. Some formulations are already in clinical trials, whereas many others are in various phases of preclinical development. Although in recent years, our understanding of nanoparticle interaction with components of the immune system has improved; many questions still require being clear. Further mechanistic studies investigating particle immunomodulatory effects (immunostimulatory and immunosuppression) are required to improve our understanding of the physicochemical parameters of nanoparticles that define their effects on the immune system.
Development of delivery system remains a critical area for future research. Important areas for future research include modifying viral vectors to reduce toxicity and immunogenicity, increasing the transduction efficiency of non-viral vectors, enhancing vector targeting and specificity, regulating gene expression, and identifying synergies between gene-based agents and other cancer therapeutics. As known, DNA vaccination indicates great potential for combating a variety of diseases. Initial results are promising and some technologies have advanced to clinical trials. However, safe and efficient delivery of plasmid DNA to initiate immune responses remains a major barrier in bringing DNA vaccination into human medicine. Development of novel nonviral delivery strategies for DNA vaccines must continue to serve as both methods of biological insight and clinically relevant outcomes. Specific concerns include the observed difficulty in transfecting DCs, methods to target APC uptake and lymph node trafficking, and providing strong danger signals without sacrificing biocompatibility.
In addition, self-assembling synthetic vectors for DNA delivery are designed to perform several biological functions. They must be able to deliver their genetic load specifically to the target tissue in a site-specific manner, while protecting the genetic material from degradation by metabolic or immune pathways. Furthermore, they must exhibit minimal toxicity and be proven safe enough for therapeutic use. Ultimately, they must have the capability to express a therapeutic gene for a limited period of time in an appropriate fashion. The whole process presents many barriers at both tissue and cellular levels. Overcoming these hurdles is the principal objective for efficient polymer-based DNA therapeutics.
Many nanoparticles appear to show some toxicity in various cell types. Regarding to the use of nanoparticles in pharmaceutical and other biomedical applications, the putative cytotoxicity of such particles should be eliminated. As the active antitumor components of plant drugs are being constantly discovered, development of targeted polymeric carriers (e.g., chitosan) for controlled release plant drugs is also an area of future studies.
Briefly, cationic polymers are the subject of critical research as non-viral gene delivery systems, due to their flexible properties, simple synthesis, and proven gene delivery efficiency. However, low transfection efficiency and undesirable cytotoxicity remain the most challenging aspects of these cationic polymers. To overcome the disadvantages, various modifications have been made to improve their gene and vaccine delivery efficacy. Among them, hydrophobic modifications of the cationic polymers are receiving more attention.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Tiyaboonchai W. Chitosan nanoparticles: A promising system for drug delivery. Naresuan University Journal 2003; 11:51 - 66
- Termsarasab U, Cho HJ, Kim DH, Chong S, Chung SJ, Shim CK, Moon HT, Kim DD. Chitosan oligosaccharide-arachidic acid-based nanoparticles for anti-cancer drug delivery. Int J Pharm 2013; 441:373 - 80; http://dx.doi.org/10.1016/j.ijpharm.2012.11.018; PMID: 23174411
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007; 2:751 - 60; http://dx.doi.org/10.1038/nnano.2007.387; PMID: 18654426
- Luten J, van Nostrum CF, De Smedt SC, Hennink WE. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J Control Release 2008; 126:97 - 110; http://dx.doi.org/10.1016/j.jconrel.2007.10.028; PMID: 18201788
- Shenoy DB, Amiji MM. An overview of condensing and noncondensing polymeric systems for gene delivery. CSH Protoc 2007.
- Borchard G. Chitosans for gene delivery. Adv Drug Deliv Rev 2001; 52:145 - 50; http://dx.doi.org/10.1016/S0169-409X(01)00198-3; PMID: 11718938
- Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol 2013; 3:13; http://dx.doi.org/10.3389/fcimb.2013.00013; PMID: 23532930
- Saroja Ch, Lakshmi PK, Bhaskaran S. Recent trends in vaccine delivery systems: A review. Int J Pharm Investig 2011; 1:64 - 74; http://dx.doi.org/10.4103/2230-973X.82384; PMID: 23071924
- Davis ME. Non-viral gene delivery systems. Curr Opin Biotechnol 2002; 13:128 - 31; http://dx.doi.org/10.1016/S0958-1669(02)00294-X; PMID: 11950563
- Hwang SJ, Davis ME. Cationic polymers for gene delivery: designs for overcoming barriers to systemic administration. Curr Opin Mol Ther 2001; 3:183 - 91; PMID: 11338932
- Xu J, Ganesh S, Amiji M. Non-condensing polymeric nanoparticles for targeted gene and siRNA delivery. Int J Pharm 2012; 427:21 - 34; http://dx.doi.org/10.1016/j.ijpharm.2011.05.036; PMID: 21621597
- Mulherkar R. Gene therapy for cancer: Is there light at the end of the tunnel?. J Indian Inst Sci 2012; 92:1 - 6
- Mitra A, Dey B. Chitosan microspheres in novel drug delivery systems. Indian J Pharm Sci 2011; 73:355 - 66; PMID: 22707817
- Jamieson GJ, Langer RS. Enhanced polymeric nanoparticles for gene delivery. Massachusetts Institute of Technology 2007, http://hdl.handle.net
- Read ML, Logan A, Seymour LW. Barriers to gene delivery using synthetic vectors. Adv Genet 2005; 53:19 - 46; http://dx.doi.org/10.1016/S0065-2660(05)53002-5; PMID: 16240989
- Liu C, Zhu Q, Wu W, Xu X, Wang X, Gao S, Liu K. Degradable copolymer based on amphiphilic N-octyl-N-quatenary chitosan and low-molecular weight polyethylenimine for gene delivery. Int J Nanomedicine 2012; 7:5339 - 50; PMID: 23071395
- Escriou V, Carrière M, Scherman D, Wils P. NLS bioconjugates for targeting therapeutic genes to the nucleus. Adv Drug Deliv Rev 2003; 55:295 - 306; http://dx.doi.org/10.1016/S0169-409X(02)00184-9; PMID: 12564982
- Hébert E. Improvement of exogenous DNA nuclear importation by nuclear localization signal-bearing vectors: a promising way for non-viral gene therapy?. Biol Cell 2003; 95:59 - 68; http://dx.doi.org/10.1016/S0248-4900(03)00007-8; PMID: 12799061
- Lechardeur D, Lukacs GL. Intracellular barriers to non-viral gene transfer. Curr Gene Ther 2002; 2:183 - 94; http://dx.doi.org/10.2174/1566523024605609; PMID: 12109215
- Sun X, Zhang N. Cationic polymer optimization for efficient gene delivery. Mini Rev Med Chem 2010; 10:108 - 25; http://dx.doi.org/10.2174/138955710791185109; PMID: 20408796
- Saengkrit N, Sanitrum P, Woramongkolchai N, Saesoo S, Pimpha N, Chaleawlert-Umpon S, Tencomnao T, Puttipipatkhachorn S. The PEI-introduced CS shell/PMMA core nanoparticle for silencing the expression of E6/E7 oncogenes in human cervical cells. Carbohydr Polym 2012; 90:1323 - 9; http://dx.doi.org/10.1016/j.carbpol.2012.06.079; PMID: 22939347
- Huang H, Yu H, Tang G, Wang Q, Li J. Low molecular weight polyethylenimine cross-linked by 2-hydroxypropyl-gamma-cyclodextrin coupled to peptide targeting HER2 as a gene delivery vector. Biomaterials 2010; 31:1830 - 8; http://dx.doi.org/10.1016/j.biomaterials.2009.11.012; PMID: 19942284
- Ko YT, Kale A, Hartner WC, Papahadjopoulos-Sternberg B, Torchilin VP. Self-assembling micelle-like nanoparticles based on phospholipid-polyethyleneimine conjugates for systemic gene delivery. J Control Release 2009; 133:132 - 8; http://dx.doi.org/10.1016/j.jconrel.2008.09.079; PMID: 18929605
- Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers 2008; 90:604 - 10; http://dx.doi.org/10.1002/bip.20989; PMID: 18381624
- Bolhasani A, Taghikhani M, Ghasemi N, Soleimanjahi H, Rafati S. Comparison of two delivery systems efficiency by using PEI for plasmid HPV16E7 DNA transfection into COS-7 cells. Modares Journal of Medical Sciences 2008; 11:15 - 9
- Alexis F, Lo SL, Wang S. Covalent attachment of low molecular weight poly (ethyleneimine) improves Tat peptide mediated gene delivery. Adv Mater 2006; 18:2174 - 8; http://dx.doi.org/10.1002/adma.200502173
- Wang S. Tat peptide conjugates of low molecular weight PEI as effective non-viral gene delivery vectors. Mol Ther 2006; 13:S76; http://dx.doi.org/10.1016/j.ymthe.2006.08.219
- Zhao QQ, Hu YL, Zhou Y, Li N, Han M, Tang GP, Qiu F, Tabata Y, Gao JQ. Gene-carried hepatoma targeting complex induced high gene transfection efficiency with low toxicity and significant antitumor activity. Int J Nanomedicine 2012; 7:3191 - 202; PMID: 22811604
- Zhang L, Gao X, Men K, Wang B, Zhang S, Qiu J, Huang M, Gou M, Huang N, Qian Z, et al. Gene therapy for C-26 colon cancer using heparin-polyethyleneimine nanoparticle-mediated survivin T34A. Int J Nanomedicine 2011; 6:2419 - 27; PMID: 22072877
- Gao W, Lai JC, Leung SW. Functional enhancement of chitosan and nanoparticles in cell culture, tissue engineering, and pharmaceutical applications. Front Physiol 2012; 3:321; http://dx.doi.org/10.3389/fphys.2012.00321; PMID: 22934070
- Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res 2006; 133:185 - 92; http://dx.doi.org/10.1016/j.jss.2005.12.013; PMID: 16458923
- Kuang Y, Yuan T, Zhang Z, Li M, Yang Y.. Application of ferriferous oxide modified by chitosan in gene delivery. J Drug Deliv. 2012; 2012:920764
- Garaiova Z, Strand SP, Reitan NK, Lélu S, Størset SØ, Berg K, Malmo J, Folasire O, Bjørkøy A, Davies CdeL. Cellular uptake of DNA-chitosan nanoparticles: the role of clathrin- and caveolae-mediated pathways. Int J Biol Macromol 2012; 51:1043 - 51; http://dx.doi.org/10.1016/j.ijbiomac.2012.08.016; PMID: 22947453
- Islam MA, Firdous J, Choi YJ, Yun CH, Cho CS. Design and application of chitosan microspheres as oral and nasal vaccine carriers: an updated review. Int J Nanomedicine 2012; 7:6077 - 93; PMID: 23271909
- Bonnet ME, Erbacher P, Bolcato-Bellemin AL. Systemic delivery of DNA or siRNA mediated by linear polyethylenimine (L-PEI) does not induce an inflammatory response. Pharm Res 2008; 25:2972 - 82; http://dx.doi.org/10.1007/s11095-008-9693-1; PMID: 18709489
- Wang JJ, Zeng ZW, Xiao RZ, Xie T, Zhou GL, Zhan XR, Wang SL. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine 2011; 6:765 - 74; PMID: 21589644
- Muzzarelli RAA. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar Drugs 2010; 8:292 - 312; http://dx.doi.org/10.3390/md8020292; PMID: 20390107
- Ogris M, Walker G, Blessing T, Kircheis R, Wolschek M, Wagner E. Tumor-targeted gene therapy: strategies for the preparation of ligand-polyethylene glycol-polyethylenimine/DNA complexes. J Control Release 2003; 91:173 - 81; http://dx.doi.org/10.1016/S0168-3659(03)00230-X; PMID: 12932649
- Bolhassani A, Ghasemi N, Servis C, Taghikhani M, Rafati S. The efficiency of a novel delivery system (PEI600-Tat) in development of potent DNA vaccine using HPV16 E7 as a model antigen. Drug Deliv 2009; 16:196 - 204; http://dx.doi.org/10.1080/10717540902757721; PMID: 19514980
- Chen H, Li P, Yin Y, Cai X, Huang Z, Chen J, Dong L, Zhang J. The promotion of type 1 T helper cell responses to cationic polymers in vivo via toll-like receptor-4 mediated IL-12 secretion. Biomaterials 2010; 31:8172 - 80; http://dx.doi.org/10.1016/j.biomaterials.2010.07.056; PMID: 20692033
- Huang Z, Yang Y, Jiang Y, Shao J, Sun X, Chen J, Dong L, Zhang J. Anti-tumor immune responses of tumor-associated macrophages via toll-like receptor 4 triggered by cationic polymers. Biomaterials 2013; 34:746 - 55; http://dx.doi.org/10.1016/j.biomaterials.2012.09.062; PMID: 23107297
- Zaharoff DA, Hance KW, Rogers CJ, Schlom J, Greiner JW. Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J Immunother 2010; 33:697 - 705; http://dx.doi.org/10.1097/CJI.0b013e3181eb826d; PMID: 20664357
- Graham JB. Co-delivery of cationic polymers and adenovirus in immunotherapy of prostate cancer. Chemical and Biochemical Engineering, thesis 2010.
- Li X, Min M, Du N, Gu Y, Hode T, Naylor M, Chen D, Nordquist RE, Chen WR. Chitin, chitosan, and glycated chitosan regulate immune responses: the novel adjuvants for cancer vaccine. Clin Dev Immunol 2013; 2013:387023; http://dx.doi.org/10.1155/2013/387023; PMID: 23533454
- van den Berg JH, Oosterhuis K, Hennink WE, Storm G, van der Aa LJ, Engbersen JF, Haanen JB, Beijnen JH, Schumacher TN, Nuijen B. Shielding the cationic charge of nanoparticle-formulated dermal DNA vaccines is essential for antigen expression and immunogenicity. J Control Release 2010; 141:234 - 40; http://dx.doi.org/10.1016/j.jconrel.2009.09.005; PMID: 19751778
- Coombes AGA, Lavelle EC, Jenkins PG, Davis SS. Single dose, polymeric, microparticle-based vaccines: the influence of formulation conditions on the magnitude and duration of the immune response to a protein antigen. Vaccine 1996; 14:1429 - 38; http://dx.doi.org/10.1016/S0264-410X(96)00077-1; PMID: 8994318
- Zolnik BS, González-Fernández A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology 2010; 151:458 - 65; http://dx.doi.org/10.1210/en.2009-1082; PMID: 20016026
- Nguyen DN, Green JJ, Chan JM, Langer R, Anderson DG. Polymeric materials for gene delivery and DNA vaccination. Adv Mater 2008; 20:1 - 21
- Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines 2012; 11:189 - 209; http://dx.doi.org/10.1586/erv.11.188; PMID: 22309668
- Lü JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, Chen C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn 2009; 9:325 - 41; http://dx.doi.org/10.1586/erm.09.15; PMID: 19435455
- Nandedkar TD. Nanovaccines: recent developments in vaccination. J Biosci 2009; 34:995 - 1003; http://dx.doi.org/10.1007/s12038-009-0114-3; PMID: 20093753
- Espuelas S, Irache JM, Gamazo C. Synthetic particulate antigen delivery systems for vaccination. Immunología 2005; 24:208 - 23
- Otten GR, Schaefer M, Doe B, Liu H, Srivastava I, Megede Jz, Kazzaz J, Lian Y, Singh M, Ugozzoli M, et al. Enhanced potency of plasmid DNA microparticle human immunodeficiency virus vaccines in rhesus macaques by using a priming-boosting regimen with recombinant proteins. J Virol 2005; 79:8189 - 200; http://dx.doi.org/10.1128/JVI.79.13.8189-8200.2005; PMID: 15956564
- McKeever U, Barman S, Hao T, Chambers P, Song S, Lunsford L, Hsu YY, Roy K, Hedley ML. Protective immune responses elicited in mice by immunization with formulations of poly(lactide-co-glycolide) microparticles. Vaccine 2002; 20:1524 - 31; http://dx.doi.org/10.1016/S0264-410X(01)00509-6; PMID: 11858858
- Luby TM, Cole G, Baker L, Kornher JS, Ramstedt U, Hedley ML. Repeated immunization with plasmid DNA formulated in poly(lactide-co-glycolide) microparticles is well tolerated and stimulates durable T cell responses to the tumor-associated antigen cytochrome P450 1B1. Clin Immunol 2004; 112:45 - 53; http://dx.doi.org/10.1016/j.clim.2004.04.002; PMID: 15207781
- Garcia F, Petry KU, Muderspach L, Gold MA, Braly P, Crum CP, Magill M, Silverman M, Urban RG, Hedley ML, et al. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol 2004; 103:317 - 26; http://dx.doi.org/10.1097/01.AOG.0000110246.93627.17; PMID: 14754702
- Sheets EE, Urban RG, Crum CP, Hedley ML, Politch JA, Gold MA, Muderspach LI, Cole GA, Crowley-Nowick PA. Immunotherapy of human cervical high-grade cervical intraepithelial neoplasia with microparticle-delivered human papillomavirus 16 E7 plasmid DNA. Am J Obstet Gynecol 2003; 188:916 - 26; http://dx.doi.org/10.1067/mob.2003.256; PMID: 12712086
- Klencke B, Matijevic M, Urban RG, Lathey JL, Hedley ML, Berry M, Thatcher J, Weinberg V, Wilson J, Darragh T, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: a Phase I study of ZYC101. Clin Cancer Res 2002; 8:1028 - 37; PMID: 12006515
- Pan CH, Nair N, Adams RJ, Zink MC, Lee EY, Polack FP, Singh M, O’Hagan DT, Griffin DE. Dose-dependent protection against or exacerbation of disease by a polylactide glycolide microparticle-adsorbed, alphavirus-based measles virus DNA vaccine in rhesus macaques. Clin Vaccine Immunol 2008; 15:697 - 706; http://dx.doi.org/10.1128/CVI.00045-08; PMID: 18287579
- Niborski V, Li Y, Brennan F, Lane M, Torché AM, Remond M, Bonneau M, Riffault S, Stirling C, Hutchings G, et al. Efficacy of particle-based DNA delivery for vaccination of sheep against FMDV. Vaccine 2006; 24:7204 - 13; http://dx.doi.org/10.1016/j.vaccine.2006.06.048; PMID: 16949709
- O’Hagan DT, Singh M, Dong C, Ugozzoli M, Berger K, Glazer E, Selby M, Wininger M, Ng P, Crawford K, et al. Cationic microparticles are a potent delivery system for a HCV DNA vaccine. Vaccine 2004; 23:672 - 80; http://dx.doi.org/10.1016/j.vaccine.2004.06.037; PMID: 15542189
- Howard KA, Li XW, Somavarapu S, Singh J, Green N, Atuah KN, Ozsoy Y, Seymour LW, Alpar HO. Formulation of a microparticle carrier for oral polyplex-based DNA vaccines. Biochim Biophys Acta 2004; 1674:149 - 57; PMID: 15374619
- Zhou X, Liu B, Yu X, Zha X, Zhang X, Wang X, Jin Y, Wu Y, Chen Y, Shan Y, et al. Controlled release of PEI/DNA complexes from PLGA microspheres as a potent delivery system to enhance immune response to HIV vaccine DNA prime/MVA boost regime. Eur J Pharm Biopharm 2008; 68:589 - 95; http://dx.doi.org/10.1016/j.ejpb.2007.09.006; PMID: 17950586
- Kasturi SP, Sachaphibulkij K, Roy K. Covalent conjugation of polyethyleneimine on biodegradable microparticles for delivery of plasmid DNA vaccines. Biomaterials 2005; 26:6375 - 85; http://dx.doi.org/10.1016/j.biomaterials.2005.03.043; PMID: 15913771
- Pai Kasturi S, Qin H, Thomson KS, El-Bereir S, Cha SC, Neelapu S, Kwak LW, Roy K. Prophylactic anti-tumor effects in a B cell lymphoma model with DNA vaccines delivered on polyethylenimine (PEI) functionalized PLGA microparticles. J Control Release 2006; 113:261 - 70; http://dx.doi.org/10.1016/j.jconrel.2006.04.006; PMID: 16793161
- Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, Chen J, Eisen HN, Langer R. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci U S A 2004; 101:9534 - 9; http://dx.doi.org/10.1073/pnas.0403549101; PMID: 15210954
- Bivas-Benita M, van Meijgaarden KE, Franken KL, Junginger HE, Borchard G, Ottenhoff TH, Geluk A. Pulmonary delivery of chitosan-DNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A*0201-restricted T-cell epitopes of Mycobacterium tuberculosis. Vaccine 2004; 22:1609 - 15; http://dx.doi.org/10.1016/j.vaccine.2003.09.044; PMID: 15068842
- Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med 1999; 5:387 - 91; http://dx.doi.org/10.1038/7385; PMID: 10202926
- Kumar M, Behera AK, Lockey RF, Zhang J, Bhullar G, De La Cruz CP, Chen LC, Leong KW, Huang SK, Mohapatra SS. Intranasal gene transfer by chitosan-DNA nanospheres protects BALB/c mice against acute respiratory syncytial virus infection. Hum Gene Ther 2002; 13:1415 - 25; http://dx.doi.org/10.1089/10430340260185058; PMID: 12215263
- Lisziewicz J, Trocio J, Whitman L, Varga G, Xu J, Bakare N, Erbacher P, Fox C, Woodward R, Markham P, et al. DermaVir: a novel topical vaccine for HIV/AIDS. J Invest Dermatol 2005; 124:160 - 9; http://dx.doi.org/10.1111/j.0022-202X.2004.23535.x; PMID: 15654970
- Roos AK. Delivery of DNA vaccines against cancer. 2006, ISBN 91-7140-895-9, Karolinska Institute, Stockholm, Sweden.
- Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol 2007; 2:469 - 78; http://dx.doi.org/10.1038/nnano.2007.223; PMID: 18654343
- des Rieux A, Fievez V, Garinot M, Schneider YJ, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release 2006; 116:1 - 27; http://dx.doi.org/10.1016/j.jconrel.2006.08.013; PMID: 17050027
- McCarroll J, Kavallaris M. Nanoparticle delivery of siRNA as a novel therapeutic for human disease. Australian Biochemist 2012; 43:9 - 20
- Moreno-Vega AI, Gomez-Quintero T, Nunez-Anita RE, Acosta-Torres LS, Castano V. Polymeric and ceramic nanoparticles in biomedical applications. Hindawi Publishing Corporation. J Nanotechnol 2012; 2012:1 - 10; http://dx.doi.org/10.1155/2012/936041
- Patel MP, Patel RR, Patel JK. Chitosan mediated targeted drug delivery system: a review. J Pharm Pharm Sci 2010; 13:536 - 57; PMID: 21486530
- Suri SS, Fenniri H, Singh B. Nanotechnology-based drug delivery systems. J Occup Med Toxicol 2007; 2:16; http://dx.doi.org/10.1186/1745-6673-2-16; PMID: 18053152
- Prego C, Paolicelli P, Díaz B, Vicente S, Sánchez A, González-Fernández A, Alonso MJ. Chitosan-based nanoparticles for improving immunization against hepatitis B infection. Vaccine 2010; 28:2607 - 14; http://dx.doi.org/10.1016/j.vaccine.2010.01.011; PMID: 20096389
- Maya S, Sarmento B, Nair A, Rejnold NS, Nair SV, Jayakumar R. Smart stimuli sensitive nanogels in cancer drug delivery and imaging: A Review. Curr Pharm Des 2013; Forthcoming PMID: 23489200
- Seo SH, Han HD, Noh KH, Kim TW, Son SW. Chitosan hydrogel containing GM-CSF and a cancer drug exerts synergistic anti-tumor effects via the induction of CD8+ T cell-mediated antitumor immunity. Clin Exp Metastasis 2006; 26:179 - 87; http://dx.doi.org/10.1007/s10585-008-9228-5
- Sinha D, Biswas J, Sung B, Aggarwal BB, Bishayee A. Chemopreventive and chemotherapeutic potential of curcumin in breast cancer. Curr Drug Targets 2012; 13:1799 - 819; http://dx.doi.org/10.2174/138945012804545632; PMID: 23140290
- Masuelli L, Benvenuto M, Fantini M, Marzocchella L, Sacchetti P, Di Stefano E, Tresoldi I, Izzi V, Bernardini R, Palumbo C, et al. Curcumin induces apoptosis in breast cancer cell lines and delays the growth of mammary tumors in neu transgenic mice. J Biol Regul Homeost Agents 2013; 27:105 - 19; PMID: 23489691
- Yang C, Su X, Liu A, Zhang L, Yu A, Xi Y, Zhai G. Advances in clinical study of curcumin. Curr Pharm Des 2013; 19:1966 - 73; PMID: 23116307
- Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanomedicine: a road to cancer therapeutics. Curr Pharm Des 2013; 19:1994 - 2010; PMID: 23116309
- Zou P, Helson L, Maitra A, Stern ST, McNeil SE. Polymeric curcumin nanoparticle pharmacokinetics and metabolism in bile duct cannulated rats. Mol Pharm 2013; 10:1977 - 87; http://dx.doi.org/10.1021/mp4000019; PMID: 23534919
- Liu L, Sun L, Wu Q, Guo W, Li L, Chen Y, Li Y, Gong C, Qian Z, Wei Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int J Pharm 2013; 443:175 - 82; http://dx.doi.org/10.1016/j.ijpharm.2012.12.032; PMID: 23287774
- Jiang M, Huang O, Zhang X, Xie Z, Shen A, Liu H, Geng M, Shen K. Curcumin induces cell death and restores tamoxifen sensitivity in the antiestrogen-resistant breast cancer cell lines MCF-7/LCC2 and MCF-7/LCC9. Molecules 2013; 18:701 - 20; http://dx.doi.org/10.3390/molecules18010701; PMID: 23299550
- Parveen S, Sahoo SK. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur J Pharmacol 2011; 670:372 - 83; http://dx.doi.org/10.1016/j.ejphar.2011.09.023; PMID: 21951969