Abstract
Success of HIV vaccine trials is dependent on infrastructural preparedness of the site, technical expertise of the trial team and strong Socio-political support of the local community. The processes followed and experiences gained while implementing various community based initiatives for recruitment of healthy volunteers during the three HIV vaccine trials in India are described. Major initiatives in community engagement implemented for the first time in India included establishment and involvement of Community Advisory Board and capacity building and engagement of lay community based volunteers called “peers” using “lay health promotion” model. Community education program designed for trial participants’ education, identification and enrollment was a three-tiered approach, moving from large community awareness meetings (first step) to facility-based small group meeting to provide trial specific information (second step); ending with one-to-one vaccine center based meeting with the volunteers to clear doubts, myths, and misconceptions about HIV/ AIDS, the experimental vaccine and HIV vaccine trials as well as to explain trial specific procedures (third step). It is important to focus on gender issues, locally relevant socio-cultural factors, informed consent, and post-trial care related matters during the conduct of sensitive clinical trials in socio-culturally diverse and resource limited setting like India.
Introduction
The recruitment of participants in sensitive clinical trials such as HIV vaccine trials poses significant challenges to researchers. Getting community support and ensuring community engagement is difficult due to HIV related stigma. Retention in clinical trials is another challenge as the study protocols and procedures are complicated and time intensive for the participants. Lower level of literacy, poverty, and lack of empowerment pose additional challenges,Citation1 in recruitment of volunteers in resource limited settings like India. Success of sensitive and complex clinical trials like HIV vaccine trials is dependent on effective partnerships between biomedical scientists, social scientists, and communities.
Recruitment of research participants within ethical framework is central to good research practice,Citation2 and asking potential participants to provide written informed consents is the gold standard in recruitment practice,Citation3 community education is critically important in this context. A commissioned report to the US Bioethics Advisory Council,Citation1 commented that research projects may need to adopt a :multistep consent process: to ensure that community and the potential participants are adequately educated.
Community education helps to build support for a trial, and assists in ensuring that participants are fully informed about the implications of participating in clinical trials.Citation4 This may result in community ownership of the research being conducted. Cultural barriers can create difficulties for trial participants, especially for women whose decision making is controlled by their spouses.Citation5 The need for being sensitive to cultural norms in implementing informed consent is very important where there could be conflict between ethos and ethics.Citation6
All these issues are critically important in the Indian scenario. While preparing for the upcoming vaccine trial, the vaccine trial team of National AIDS Research Institute in Pune, India studied community willingness to participate in the clinical trials and the findings of the study were used in formulating appropriate awareness and recruitment strategies.Citation7
Traditionally the first information-sharing step is the announcement of the potential clinical trial by the researchers, policy makers or program managers because of their knowledge and authority. However, this may be perceived as paternalistic attitude.Citation6 To prevent this, National AIDS Research Institute (NARI) (Indian Council of Medical Research) and its trial partners in India took several steps to inform and engage different faces of the community during the conduct of the three HIV vaccine trials in India. The institutional committees of the respective sites approved all the three HIV vaccine trials. Written informed consents were obtained from the study participants who were screened and/ or enrolled.
Following the first HIV vaccine trial in Pune trial using AAV based HIV vaccine, another trial was conducted in Chennai using Modified Vaccinia Ankara (MVA) based HIV vaccine and the third trial was conducted at both sites using prime-boost strategy using DNA-MVA candidates. The safety data of all the three trials conducted in Pune and Chennai have already been published.Citation8-Citation10 The processes followed and experiences gained while implementing various community based initiatives for recruitment of healthy volunteers during the three HIV vaccine trials in India are described.
Taking a Socio-Culturally Appropriate Approach
The investigators of the first phase 1 HIV vaccine trial in India developed a recruitment model based on multi-level participatory approach employing expanded informed consent principle,11 and employed a two-phased enrollment process using lay health promotion techniques ().
Figure 1. A conceptual framework of community involvement using lay health promotion model and using participatory approach to facilitate community based recruitment of healthy volunteers for phase 1 HIV vaccine trial in India. The structural components of recruitment plan were: (1) Community Advisory Board; (2) Community volunteers designated as peers. Peers and CAB were involved in evolving strategies of recruitment based on lessons learnt during interim assessments. Media was informed periodically.
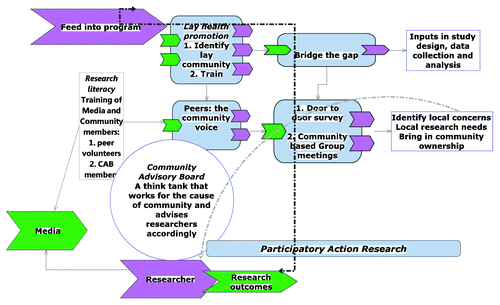
Principles of participatory action research,Citation12 were adopted to ensure adequate education of the community and potential participants about HIV, its prevention and the research process. Active and systematic involvement of Community Advisory Board (CAB) and media helped in demystifying the process of vaccine trial and making it readily understood by the lay community. Study specific education material was developed to provide information on HIV/ AIDS, purpose of the clinical trials involving human volunteers, trial procedures, study sponsors and investigators. The education material was given to any individual on request irrespective of expressed interest in trial participation to ensure wider distribution of knowledge regarding HIV vaccines and trials. A community friendly electronic power point presentation was developed with the goal of making the community understand technical issues in a simplistic, visual, and non-technical manner during group and individual meetings. The following information was imparted to the community and potential volunteers:
Principles of immunization: How vaccines work in the body, what are antibodies and how vaccine induced antibodies are different than those following natural HIV infection.
The nature and structure of the vaccine candidate
Principles and procedure of conducting preventive Vaccine Trials
Side effects, stigma, and discrimination
Ethical principles including autonomy, rights and meaning of participation, and grievance redressal mechanism.Citation13
Hearing the Voices of Community and Community Oriented Approach for Recruitment
Community based recruitment has been traditionally reported to be a challenge,Citation14 but adequate and appropriate education and information sharing with the community are known to lead to improved enrollment in clinical trials.Citation15,Citation16
As per the UNAIDS guidance on community involvement for HIV vaccine trial,Citation17 community was engaged throughout the trial process in India. The structural components of the recruitment plan included: (1) Working closely with Community Advisory Board and (2) Involvement of community volunteers designated and trained as peers ().
The Pune study information model had 3 step approach: information sharing and announcing the trial by the researchers in the community (Level 1), receiving inputs from the representatives of the community regarding locally prevalent values, norms, and opinions (Level 2) and incorporating useful knowledge in the education and programs planned to provide the information to the potential participants and the community (Level 3).Citation18
Both the institutes that conducted the three HIV vaccine trials in India worked with locally constituted Community Advisory Boards (CAB).Citation8,Citation9 Contribution of Community Advisory Board members in discussions regarding the type of population to be targeted for enrollment, in creating “community literacy” among researchers and in development of informed consent forms as well as study information material in a non-technical and community language was crucially important. During CAB consultations to discuss trial recruitment strategies, investigators in Pune had proposed approaching blood donors as potential trial participants following the Thailand model.Citation19 However the site CAB members in India strongly opposed the idea:
Blood donors are a rare commodity in the country. They would not be able to donate blood if they participated in the trial as they may test HIV antibody positive…at least for the trial period.
Eventually, despite being informed about the possibility of testing HIV positive and not being able to donate blood; one of the trial participants who participated in the vaccine trial expressed disappointment because he developed vaccine induced antibodies and was not able to donate blood on his birthdays, the practice he had followed for years.
The other group representing the community that helped in the vaccine trial recruitment process was that of “peers”. National AIDS Research Institute developed collaborative partnerships for community support, education, and field based recruitment with seven non-governmental organizations (NGO) based in Pune.Citation20 Community coordinators of NARI identified 115 volunteers from the service area of 7 partner NGO who were imparted training and empowerment to work at the grass-root level in the community. They created awareness about the trials, identified potential volunteers, and guided them to meet key investigators from the trial team to participate in the trial recruitment process. The National Institute for Research on Tuberculosis (NIRT) collaborated with Y.R. Gaitonde Centre for AIDS Research and Education (YRG CARE), a local reputed NGO to identify volunteers for the second and third HIV vaccine trials conducted in Chennai.
The Profile of HIV Vaccine Trial Participants in India
Throughout the preparatory and actual vaccine trial phase, that is between December 2003 and January 2006, community awareness was created. In a span of 6 y, a total of 94 healthy volunteers were recruited in three phase I trials in India.Citation8,Citation10,Citation18 At Pune site, 30 and 16 enrollments made were in the first (AAV) and third (DNA-MVA prime boost) HIV vaccine trials by reaching out to 8349 and 5881 individuals respectively. At the Chennai site, 32 and 16 volunteers were recruited in the second (MVA) and third (DNA-MVA prime boost) HIV vaccine trials by approaching 13 920 and 14 080 respectively.
Overall, 32 578 individuals were contacted from 5 different sources viz. people residing in the service area of partner NGO, health care providers, people working in the academia, industrial workers, and the general community in the three Indian HIV vaccine trials ().
Table 1. Number of volunteers and their referral sources contacted during community mobilization program for three phase1 HIV vaccine trials in India
Of the individuals reached in the first level meetings, 53% represented the general community including the civil society and 30% represented professional groups like academia. Nearly half of the enrollment was from NGO service areas (53%; 50/94) and nearly one third from the community and civil society (31%; 29/94) (). Nine (10%) health care providers participated in these trials in India. Academia and industries contributed very little to enrollment of volunteers (4.2%, 4/94).
Figure 2. Proportion of volunteers enrolled from each referral source in 3 phase 1 vaccine trials conducted at Pune and Chennai in India.
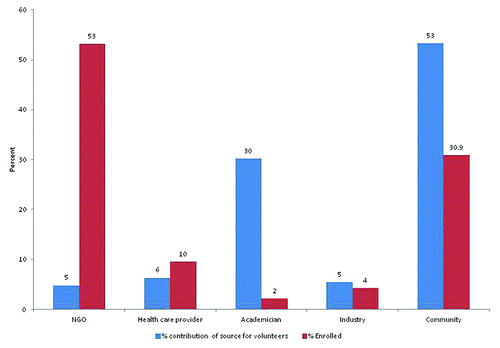
It has been reported that individuals might participate in clinical studies for assured access to medical care or perceived personal benefits.Citation21,Citation22 It was anticipated that people from academia, industrial workers or health care professions with a better educational profile and better understanding of the technical process, might be more enthusiastic about participating in HIV vaccine trials; however, this was not the case. Differential motivation to participate or not to participate in HIV vaccine trials among different sects of the Indian society with differences in educational status, awareness, and income is an area that needs exploration. The Indian trial experience supported the prevalent view that civil society organizations have significantly impacted health research and served a pivotal stakeholder role.Citation23
With the prevalent HIV related stigma,Citation24-Citation26 during that period, ensuring “research literacy” in the community and among the potential participants was essential. The site investigators ensured that each volunteer participated after acquiring adequate information and with full commitment. A two-step informed consent process for screening and enrollment was designed for HIV vaccine trials in India.
Gender Issues
Women have traditionally been under-represented in clinical trials of any phase.Citation27-Citation29 Various strategies have been used to include women in clinical research which could benefit them, for example, cervical cancer screening,Citation20,Citation30 but barriers have still remained. Considering women's greater vulnerability to HIV due to a complex mix of biological, economic, and social variables unique to India, the vaccine trial teams in India took special efforts to inform women, ensured adequate enrollment of women and addressed some of the gender issues. Advanced gender training was conducted for the HIV vaccine trial teams in Pune and Chennai. It was realized that time needs to be invested in educating potential women volunteers as well as their spouses and families because women are unable to exercise autonomy in India. The existing gender norms in the country could render women vulnerable to coercion or they were likely to be misled into participation. A case has been described below an important gender issue (Box 1).
Box 1. Gender training is critical for the research team to understand gender relevant issues in the trials
Need for gender training-a case at Pune site
Mrs. Surbhi*, a middle-aged social worker showed keen interest in contributing to the social cause by participating in the vaccine trial. The issue of family involvement and support was repeatedly discussed with her, but she kept ignoring the matter. She insisted that it was perfectly within her rights to decide about participating in the vaccine trial. Mrs. Surbhi was screened and found to be medically eligible to participate in the vaccine trial. She visited the clinic with her spouse, prior to the enrollment visit. Mrs. Surbhi reiterated that she was looking forward to participate in the trial, but suggested that the trial team should talk with her spouse. Mr. Sam*, her spouse, mentioned that he was very enthusiastic and supportive of her participation in the vaccine trial. On enquiry he talked about his casual affairs and it appeared that he had a hope that the trial vaccine might protect his spouse from AIDS. In a separate discussion, Mrs. Surbhi shared her suspicions about her spouse’s fidelity and she explained that she wanted to win her husband back by complying with his wish to participate in the trial. A case of “coercion” by the spouse was noticed and Mrs. Surbhi’s participation was forestalled. The woman was informed about “false sense of security” following the use of experimental vaccine. (*Names are fictitious)
Other challenges to successful recruitment of women into vaccine research also emerged. A crucial requirement for childcare emerged as the study specific procedures were time-consuming. Insistence of women participants to hasten and complete study related procedures during the visit (who used to be worried about their young children) put pressure on the trial team. Such child care needs have been reported in other trials also.Citation31 A nurse and a counselor offered childcare help to the women clinical trial participants who brought their children along with them. Hence, provision of childcare for optimal and hassle-free participation of women in vaccine trials is recommended. Confidentiality remained an important and critical issue for women participants who were worried about harmful consequences including violence resulting from possible disclosure of their vaccine trial participation. Some women participants also found it difficult to comply with protocol specific contraceptive usage or procedural requirement of urine pregnancy test (UPT) as some of them were widows or had utilized permanent method of family planning. CAB objected to the need for UPT among widowed and unmarried women. The community education material was further modified to include the reasons for UPT and contraception in the trials. Gender training was found to be an important component for the researchers involved in HIV vaccine trials.
A number of potential women participants withdrew after qualifying in the screening eligibility tests. Reasons for non-participation of “medically eligible” women from HIV vaccine trials even after initial commitment need to be investigated.Citation32
Psychological Fitness to Participate in Difficult Clinical Trials and Personality Types
Ethics committees in India raised concerns about ability of potential participants to bear the “stress of HIV vaccine trial participation.” A recent mental model study to understand perceptions about vaccine induced antibodies among men having sex with men in India has reported the mental fear of HIV related stigma resulting from vaccine trial participation.Citation33 A case in the third trial at one of the two HIV vaccine trial sites is described (Box 2).
Box 2. Need for assessment of preparedness of potential volunteers
Participant: “I am becoming weaker. My wife also feels that. You talk with her.”
Wife: “I feel he is becoming weaker…you take this out of his mind that he is …[/sick/]”
And one day, the same volunteer came and informed: “My wife has left”.
Even now, nearly 7 years after HIV vaccine trial participation, the volunteer contacts the trial team: “I have got myself tested. Please see my reports. Am I Ok?”
The participant has a feeling that he has become weak due to trial participation and getting HIV infected. He does not get re-assured despite counseling and serological evidence of absence of HIV infection. The fact that his wife left him must also have made a strong impact on him. It is unclear if the reason for this is related to vaccine trial participation because both of them had jointly taken the decision to participate in the HIV vaccine trial
Following the first HIV vaccine trial in India, the specially constituted National Advisory Board (NAB) for HIV vaccine trials in India had advised that potential participants be assessed for their psychological fitness for participation in such sensitive trials. Following recommendations of NAB and NARI Ethics Committee, during the third HIV vaccine trial in India, a voluntary psychological and personality assessment test of the potential volunteers was introduced. Among those who opted for the assessment, it was observed that those with lower scores never returned for participation despite early expression of motivation to participate. Assessment of personality has some ethical angles. There can be a possible controversy about the appropriateness of the selected instrument to assess personality and the assumption that the personalities so detected with some deficiencies would face problems in bearing the trial related stress. World over, the ethicists and the researchers need to build some consensus to address the issue of psychological vulnerability of potential trial volunteers.
Reasons for Vaccine Trial Participation
There was almost equal participation of men and women representing various socio-economic strata. Altruism has been reported to be the main motivator for participation in HIV vaccine trials both in the OECD (Organizations for Economic Cooperation and Development) and the non-OECD countries the world over.Citation7,Citation34,Citation35 Altruism was one of the major reasons for participation in Indian HIV vaccine trials as well,Citation7 and no regional differences were noted among participants for volunteering in clinical trials. Knowing a person with HIV infection was another motivator both at Pune and Chennai sites.
Some examples from the trial sites are quoted below:
When I took care of a neighbor who had AIDS, I understood the agony and pain associated with this killer disease. When she passed away I was looking forward to getting involved in fight against this disease, and what better opportunity than to volunteer for this preventive vaccine trial? [A volunteer from Chennai site]
She wants to do something for preventing HIV/AIDS as some of her relatives (brother, his son, and sister’s son) died of HIV/AIDS. [A peer sharing what a volunteer had told her …from Pune]
Participation of social workers, especially community level peers in the vaccine trial is an example of true ownership of the trial signifying successful participatory research model.
I am a peer, I want to set example for the community …If I participate, I can set an example for others. [Peer who had volunteered for trial participation at Pune site]
I read in the newspaper some time back the gesture of Bill Gates, who had donated a huge sum for AIDS research. I thought to myself that even if I take 10 more births I would not be able to donate even a fraction of the same. But when I read about this trial in the paper I was thrilled of the prospect of involving myself in a worthy noble cause like this [A volunteer from Chennai site]
I am a very ordinary person and I did not have the opportunity for higher education. But my dream was to contribute to research and development and I have fulfilled my dream by volunteering in this International Phase I Vaccine Trial [A volunteer from Chennai site]
Shared Decision making is cultural norm: A debate about autonomy and non maleficence
The stepwise recruitment approach helped in carefully guided and shared decision making in HIV vaccine trials in India. Family involvement; especially that of the spouse, was observed to be useful at both sites in all the three trials. We experienced more instances of failure of enrollment following lack of family involvement in the decision making process. At Chennai site, examples of family involvement and support for this noble cause were noted (Box 3).
Box 3. Family involvement in Indian vaccine trials
Chennai site
Volunteers D0012636 & 37: A couple decided to participate together primarily for doing good for the society.
Volunteers D0012654 & 55: A pair of brothers-in-laws participated. They were accompanied by their families to know more about the trial and it was jointly decided that men in the families would participate.
Volunteers D0012639 & 40: A pair of sisters who learnt about the trial came in with their grown up children and spouses to participate in the trial.
Despite major emphasis on autonomy in medical decision making in the 20th century, the medical community and the public have been increasingly embracing shared decision making in the United States and elsewhere in the 21st century.Citation4,Citation36,Citation37,Citation38,Citation39 On one hand autonomy signifies capacity to make decisions,Citation40 on the other hand it has been criticized for promoting a pernicious model of human individuality that overlooks the importance of social relationships and dependency.Citation41 In Indian trials, we pursued both; with majority preferring the shared decision making approach.
Key Messages and Lessons Learned from the HIV Vaccine Trials in India
The trials demonstrated that community engagement and ownership is possible even in resource-limited settings. Recruitment process was participatory and culturally relevant. Coordinated efforts of the medical, social science, and field teams helped to resolve participant’ problems and issues. Participants are a representative mix of both genders, different educational backgrounds as well as socio-economic strata. The gap between intention to participate and actual participation exists and to bridge that the reasons for stepping back need to be explored. Although the community based recruitment approach is labor intensive, it is rewarding and capable of providing committed volunteers.
References
- NBAC. (2010). National Bioethics Advisory Commission (US) Report:Ethical and Policy Issues in International Research: Clinical Trials in Developing Countries. Bethesda, MD: NBAC 1.
- Duke S, Bennett H. Review: a narrative review of the published ethical debates in palliative care research and an assessment of their adequacy to inform research governance. Palliat Med 2010; 24:111 - 26; http://dx.doi.org/10.1177/0269216309352714; PMID: 19965950
- Vellinga A, Cormican M, Hanahoe B, Bennett K, Murphy AW. Opt-out as an acceptable method of obtaining consent in medical research: a short report. BMC Med Res Methodol 2011; 11:40; http://dx.doi.org/10.1186/1471-2288-11-40; PMID: 21470399
- Murray E, Pollack L, White M, Lo B. Clinical decision-making: Patients’ preferences and experiences. Patient Educ Couns 2007; 65:189 - 96; http://dx.doi.org/10.1016/j.pec.2006.07.007; PMID: 16956742
- Kaleebu P. (2005) HIV vaccine trials in Uganda: Personal experiences as an investigator. In: P. Kahn(ed.) AIDS Vaccine Handbook: Global Perspectives New York. AIDS Vaccine Action Coalition, pp. 145–151, http://www.avac.org/ht/d/sp/i/2311/pid/2311
- Lindegger G, Richter LM. HIV vaccine trials: critical issues in informed consent. S Afr J Sci 2000; 96:313 - 7; PMID: 15739286
- Sahay S, Mehendale S, Sane S, Brahme R, Brown A, Charron K, Beyrer C, Bollinger R, Paranjape R. Correlates of HIV vaccine trial participation: an Indian perspective. Vaccine 2005; 23:1351 - 8; http://dx.doi.org/10.1016/j.vaccine.2004.09.032; PMID: 15661383
- Ramanathan VD, Kumar M, Mahalingam J, Sathyamoorthy P, Narayanan PR, Solomon S, Panicali D, Chakrabarty S, Cox J, Sayeed E, et al. A Phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C-modified vaccinia Ankara virus vaccine candidate in Indian volunteers. AIDS Res Hum Retroviruses 2009; 25:1107 - 16; http://dx.doi.org/10.1089/aid.2009.0096; PMID: 19943789
- Mehendale S, Sahay S, Thakar M, Sahasrabuddhe S, Kakade M, Shete A, Shrotri A, Spentzou A, Tarragona T, Stevens G, et al. Safety & immunogenicity of tgAAC09, a recombinant adeno-associated virus type 2 HIV-1 subtype C vaccine in India. Indian J Med Res 2010; 132:168 - 75; PMID: 20716817
- Mehendale S, Thakar M, Sahay S, Kumar M, Shete A, Sathyamurthi P, Verma A, Kurle S, Shrotri A, Gilmour J, et al. Safety and immunogenicity of DNA and MVA HIV-1 subtype C vaccine prime-boost regimens: a phase I randomised Trial in HIV-uninfected Indian volunteers. PLoS One 2013; 8:e55831; http://dx.doi.org/10.1371/journal.pone.0055831; PMID: 23418465
- Woodsong C, Macqueen K, Namey E, Sahay S, Morar N, Mlingo M, Mehendale S. Women’s Autonomy and Informed Consent in Microbicides Clinical Trials. J Empir Res Hum Res Ethics 2006; 1:11 - 26; http://dx.doi.org/10.1525/jer.2006.1.3.11; PMID: 19385819
- Freire P. (1983). Pedagogy of the Oppressed, New York: Continuum.
- Mehendale S. Acquired immune deficiency syndrome vaccine trials: Managing ethical issues before, during and after the trials. Perspect Clin Res 2013; 4:84 - 8; http://dx.doi.org/10.4103/2229-3485.106401; PMID: 23533989
- Moutsiakis DL, Chin PN. Why blacks do not take part in HIV vaccine trials. J Natl Med Assoc 2007; 99:254 - 7; PMID: 17393949
- Harro CD, Judson FN, Gorse GJ, Mayer KH, Kostman JR, Brown SJ, Koblin B, Marmor M, Bartholow BN, Popovic V, VAX004 Study Group. Recruitment and baseline epidemiologic profile of participants in the first phase 3 HIV vaccine efficacy trial. J Acquir Immune Defic Syndr 2004; 37:1385 - 92; http://dx.doi.org/10.1097/01.qai.0000122983.87519.b5; PMID: 15483468
- Luzi AM, Gallo P, Colucci A, Marcotullio S, Bellino S, Longo O, Ensoli B. Communication, recruitment and enrolment in the preventative and therapeutic phase I clinical trial against HIV/AIDS based on the recombinant HIV-1 Tat protein. AIDS Care 2011; 23:939 - 46; http://dx.doi.org/10.1080/09540121.2010.542124; PMID: 21390884
- UNAIDS. (2000). “Ethical considerations in HIV preventive vaccine research: UNAIDS guidance document.” 3rd Reprint April 2004. Retrieved 11 September, 2013, from http://www.who.int/rpc/research_ethics/jc072-ethicalcons_en_pdf.pdf.
- Mehendale S, Sahay S, Thakar M, Sahasrabuddhe S, Kakade M, Shete A, Shrotri A, Spentzou A, Tarragona T, Stevens G, et al. Safety & immunogenicity of tgAAC09, a recombinant adeno-associated virus type 2 HIV-1 subtype C vaccine in India. Indian J Med Res 2010; 132:168 - 75; PMID: 20716817
- Jenkins RA, Chinaworapong S, Morgan PA, Ruangyuttikarn C, Sontirat A, Chiu J, Michael RA, Nitayaphan S, Khamboonruang C. Motivation, recruitment, and screening of volunteers for a phase I/II HIV preventive vaccine trial in Thailand. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 18:171 - 7; http://dx.doi.org/10.1097/00042560-199806010-00009; PMID: 9637582
- Sahay S, Mehendale S. Engaging community to support HIV prevention research. East J Med 2011; 16:168 - 77; PMID: 23807866
- Woodsong C, Alleman P, Musara P, Chandipwisa A, Chirenje M, Martinson F, Hoffman I. Preventive misconception as a motivation for participation and adherence in microbicide trials: evidence from female participants and male partners in Malawi and Zimbabwe. AIDS Behav 2012; 16:785 - 90; http://dx.doi.org/10.1007/s10461-011-0027-7; PMID: 21863339
- Nappo SA, Iafrate GB, Sanchez ZM. Motives for participating in a clinical research trial: a pilot study in Brazil. BMC Public Health 2013; 13:19; http://dx.doi.org/10.1186/1471-2458-13-19; PMID: 23302375
- Koen J, Essack Z, et al. (2012). “'It Looks Like You Just Want Them When Things Get Rough': Civil Society Perspectives on Negative Trial Results and Stakeholder Engagement in HIV Prevention Trials.” Dev World Bioeth. doi: http://dx.doi.org/10.1111/j.1471-8847.2012.00338.x. [Epub ahead of print].
- Bharat S, Aggleton P. Facing the challenge: household responses to HIV/AIDS in Mumbai, India. AIDS Care 1999; 11:31 - 44; http://dx.doi.org/10.1080/09540129948180; PMID: 10434981
- Mawar N, Saha S, Pandit A, Mahajan U. The third phase of HIV pandemic: social consequences of HIV/AIDS stigma & discrimination & future needs. Indian J Med Res 2005; 122:471 - 84; PMID: 16517997
- Steward WT, Herek GM, Ramakrishna J, Bharat S, Chandy S, Wrubel J, Ekstrand ML. HIV-related stigma: adapting a theoretical framework for use in India. Soc Sci Med 2008; 67:1225 - 35; http://dx.doi.org/10.1016/j.socscimed.2008.05.032; PMID: 18599171
- Frew PM, del Rio C, Lu L, Clifton S, Mulligan MJ. Understanding differences in enrollment outcomes among high-risk populations recruited to a phase IIb HIV vaccine trial. J Acquir Immune Defic Syndr 2009; 50:314 - 9; http://dx.doi.org/10.1097/QAI.0b013e3181945eec; PMID: 19194310
- Pinnow E, Sharma P, Parekh A, Gevorkian N, Uhl K. Increasing participation of women in early phase clinical trials approved by the FDA. Womens Health Issues 2009; 19:89 - 93; http://dx.doi.org/10.1016/j.whi.2008.09.009; PMID: 19272558
- Asiki G, Abaasa A, Ruzagira E, Kibengo F, Bahemuka U, Mulondo J, Seeley J, Bekker LG, Delany S, Kaleebu P, et al. Willingness to participate in HIV vaccine efficacy trials among high risk men and women from fishing communities along Lake Victoria in Uganda. Vaccine 2013; 31:5055 - 61; http://dx.doi.org/10.1016/j.vaccine.2013.08.080; PMID: 24021306
- Decker KM, Turner D, Demers AA, Martens PJ, Lambert P, Chateau D. Evaluating the effectiveness of cervical cancer screening invitation letters. J Womens Health (Larchmt) 2013; 22:687 - 93; http://dx.doi.org/10.1089/jwh.2012.4203; PMID: 23915107
- Mills EN, Nixon S, Singh S, Dolma S, Nayyar A, Kapoor S. Enrolling women into HIV preventive vaccine trials: an ethical imperative but a logistical challenge. PLoS Med 2006; 3:e94; http://dx.doi.org/10.1371/journal.pmed.0030094; PMID: 16478295
- Hadidi N, Lindquist R, Treat-Jacobson D, Swanson P. Participant withdrawal: challenges and practical solutions for recruitment and retention in clinical trials. Creat Nurs 2013; 19:37 - 41; http://dx.doi.org/10.1891/1078-4535.19.1.37; PMID: 23600026
- Chakrapani V, Newman PA, Singhal N, Nelson R, Shunmugam M. “If It’s Not Working, Why Would They Be Testing It?”: mental models of HIV vaccine trials and preventive misconception among men who have sex with men in India. BMC Public Health 2013; 13:731; http://dx.doi.org/10.1186/1471-2458-13-731; PMID: 23919283
- Colfax G, Buchbinder S, Vamshidar G, Celum C, McKirnan D, Neidig J, Koblin B, Gurwith M, Bartholow B. Motivations for participating in an HIV vaccine efficacy trial. J Acquir Immune Defic Syndr 2005; 39:359 - 64; http://dx.doi.org/10.1097/01.qai.0000152039.88422.ec; PMID: 15980699
- Dhalla S, Poole G. Motivators of enrolment in HIV vaccine trials: a review of HIV vaccine preparedness studies. AIDS Care 2011; 23:1430 - 47; http://dx.doi.org/10.1080/09540121.2011.555750; PMID: 21722022
- Carlet J, Thijs LG, Antonelli M, Cassell J, Cox P, Hill N, Hinds C, Pimentel JM, Reinhart K, Thompson BT. Challenges in end-of-life care in the ICU. Statement of the 5th International Consensus Conference in Critical Care: Brussels, Belgium, April 2003. Intensive Care Med 2004; 30:770 - 84; http://dx.doi.org/10.1007/s00134-004-2241-5; PMID: 15098087
- Davidson JE, Powers K, Hedayat KM, Tieszen M, Kon AA, Shepard E, Spuhler V, Todres ID, Levy M, Barr J, et al, American College of Critical Care Medicine Task Force 2004-2005, Society of Critical Care Medicine. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004-2005. Crit Care Med 2007; 35:605 - 22; http://dx.doi.org/10.1097/01.CCM.0000254067.14607.EB; PMID: 17205007
- Mercurio MR, Forman EN, Ladd RE, Maxwell MA, Ross LF, Silber TJ, American Academy of Pediatrics Section on Bioethics. American academy of pediatrics policy statements on bioethics: summaries and commentaries: part 3. Pediatr Rev 2008; 29:e28 - 34; http://dx.doi.org/10.1542/pir.29-5-e28; PMID: 18450835
- Kon AA. The shared decision-making continuum. JAMA 2010; 304:903 - 4; http://dx.doi.org/10.1001/jama.2010.1208; PMID: 20736477
- Beauchamp TL, Childress JF. (2001). Principles of Biomedical Ethics. Oxford and New York, Oxford University Press.
- Donchin A. (2000). “Autonomy and Interdependence: Quandaries in Genetic Decision Making.” In Relational Autonomy: Feminist Perspectives on Autonomy, Agency, and the Social Self Oxford, Oxford University Press.
