Abstract
Influenza affects 5–15% of the population during an epidemic. In Western Europe, vaccination of at-risk groups forms the cornerstone of influenza prevention. However, vaccination coverage of the elderly (>65 y) is often low in Central and Eastern Europe (CEE); potentially because a paucity of country-specific data limits evidence-based policy making. Therefore the medical and economic burden of influenza were estimated in elderly populations in the Czech Republic, Hungary, Kazakhstan, Poland, Romania, and Ukraine. Data covering national influenza vaccination policies, surveillance and reporting, healthcare costs, populations, and epidemiology were obtained via literature review, open-access websites and databases, and interviews with experts. A simplified model of patient treatment flow incorporating cost, population, and incidence/prevalence data was used to calculate the influenza burden per country. In the elderly, influenza represented a large burden on the assessed healthcare systems, with yearly excess hospitalization rates of ~30/100 000. Burden varied between countries and was likely influenced by population size, surveillance system, healthcare provision, and vaccine coverage. The greatest burden was found in Poland, where direct costs were over EUR 5 million. Substantial differences in data availability and quality were identified, and to fully quantify the burden of influenza in CEE, influenza reporting systems should be standardized. This study most probably underestimates the real burden of influenza, however the public health problem is recognized worldwide, and will further increase with population aging. Extending influenza vaccination of the elderly may be a cost-effective way to reduce the burden of influenza in CEE.
Introduction
Influenza is a seasonal, highly contagious infectious disease and one of the most important respiratory tract infections from a public health point of view. Influenza outbreaks have a seasonal distribution and typically peak in the northern hemisphere between December and March. The World Health Organization (WHO) estimate that each year approximately 5–15% of the population will be infected and put the annual global number of severe infections at 3–5 million.Citation1 Infection is associated with increased morbidity and mortality, often due to associated infections such as pneumonia,Citation2 and excess healthcare expenditure.
Influenza affects the whole population, but the highest rates of morbidity and mortality are observed in those aged 65 y and above, especially in those who have one or more comorbidities. Of the 250 000–500 000 influenza-related deaths each year, the elderly (>65 y old) account for around 90%.Citation1,Citation3 Within the elderly population, hospitalization and mortality rates increased with age; whereby patients aged ≥85 y were 6-times more likely to be hospitalized and 16-times more likely to die compared with patients aged 65–69 y.Citation2,Citation4,Citation5 Their median stay in hospital was also one-third longer compared with patients aged 50–64 y.Citation2,Citation5 Compared with children aged <5 y, people aged ≥85 y accounted for approximately twice the number of days in hospital due to influenza.Citation4
The costs associated with influenza and its complications can be substantial. In the United States, a study based on the 2003 US population estimated that the annual burden of influenza was 3.1 million days in hospital and 31.4 million outpatient visits.Citation6 From a societal perspective, the total economic burden (direct costs and indirect costs, including loss of earnings and loss of life) of influenza has been estimated at USD 87.1 billion annually, with direct costs accounting for more than USD 10 billion, of which 40% was spent on the treatment of patients older than 65 y of age. A large proportion of influenza-related costs derive from the elderly population,Citation7,Citation8 often because elderly populations are more prone to complications that may require costly treatment interventions.Citation9 At 2011 prices, the cost per influenza case in Italy was calculated to be EUR 1270.18 for a patient aged over 65 y and EUR 940.39 for a patient aged 18–65 y.Citation10 In recent health economics studies in elderly populations in Poland, 50% of the elderly were considered to be at high risk of influenza complications, with the costs of treatment placed at EUR 25 and EUR 1900 for outpatients and inpatients, respectively.Citation11,Citation12
While most medical and economic studies have evaluated influenza within high income countries, data regarding the impact of influenza in Central and Eastern Europe (CEE) is scarce. In this paper, the influenza burden within six CEE countries, namely the Czech Republic, Hungary, Kazakhstan, Poland, Romania, and Ukraine, which vary in their vaccination coverage, healthcare systems, reimbursement policies, and surveillance programs, is evaluated to meet this data gap. These countries were chosen based on their geographic distribution in CEE and varied healthcare development status, e.g., Kazakhstan is an emerging country with limited data availability, whereas Hungary has stable healthcare provision that can act as a reference for other countries. In all cases, data are required to support reimbursement, funding and vaccination policy decisions. Analyses focus on burden in elderly populations because research indicates that they account for the majority of infection-related complications and associated economic burden. In CEE, where Eurostat data were available, the percentage of the populations aged ≥65 y in 2010 in Poland, the Czech Republic, Romania, and Hungary were 13.5%, 15.2%, 14.9%, and 16.6%, respectively.Citation13 Moreover the proportion of people classed as elderly has risen considerably in the last decades and this trend is expected to continue throughout Europe.Citation14,Citation15
Vaccination is accepted to be the most effective means of protection against influenza and its complications and is recommended in most countries.Citation16,Citation17 Annual vaccination against influenza can prevent 70–90% of influenza-related illness in healthy individuals, and in elderly populations can reduce the severity of illness/complications and the risk of death by up to 60% and 80%, respectively.Citation18 To reduce the incidence of influenza and its associated morbidity and mortality, the US Centers for Disease Control and Prevention (CDC) recommended universal vaccination for all persons ≥6 mo from 2010.Citation19 In 2009, The EU Council expressed its aim that EU member states achieve vaccination coverage rate of 75% in “older age groups” and, if possible, in other high-risk groups, as early as possible and preferably by the 2014–15 winter season.Citation20 WHO recommendations have developed over time and were updated in 2012 to prioritize vaccination in pregnant women, children, the elderly, individuals with underlying health conditions, and healthcare workers.Citation21 Having previously advocated vaccine coverage of 75% in at-risk groups,Citation18 the new recommendation is that vaccination coverage goals should be determined at regional and country levels, as influenza immunization programs are dependent on country-specific capacity and resources.Citation21 In the 2007–08 season, of the 15 countries surveyed, only the Netherlands (82%) and the UK (78%) exceeded the WHO target for vaccination coverage in the elderly.Citation17 The Netherlands introduced their vaccination program for the elderly in 1996, and vaccination coverage increased from around 30% in 1993 to over 70% in 2003.Citation22 In the same period, a general reduction in influenza-related mortality was recorded, with excess deaths reduced by 35% (191 in 1993 vs. 125 in 2003).Citation17
Coverage levels in the 6 targeted CEE countries are currently very low (), and notably, where multiple years for comparison are available, the coverage of elderly populations has been decreasing since the 2008–09 season. The seasonal burden of influenza on national healthcare systems is influenced by preventative strategies undertaken. By providing clinical and epidemiological support for early warning systems, surveillance systems, such as EuroFlu, help policymakers to understand the projected seasonal burden of influenza in order to optimize resource allocation within prevention and treatment programs. There is growing need for improving the monitoring systems for influenza, especially since the 2009–10 pandemic, to provide experts with precise information on the strain, timing, and severity of influenza cases.Citation23 Such data can inform evidence-based policy decisions, which are of extreme importance in low and middle income countries with limited healthcare resources for allocation.
Table 1. Vaccination coverage rate in the elderly (aged >65 y, except Hungary in the 2008–09 and 2009–10 seasons)
The burden of influenza and the value of vaccination in Western Europe is well documented and supported by much published data, however in CEE specifically, the burden of influenza disease is heterogeneously reported. If the burden of influenza is not reported or underestimated in CEE, policymakers cannot assess the need for allocation of more resources to optimize preventative strategies to tackle this public health problem.
The objective of this study was to estimate the direct medical and economic burden of influenza in a representative set of CEE countries among the elderly (≥65 y old). These estimates are expected to benefit healthcare policymakers and payers, and be of use in evaluating the cost-effectiveness of funding different strategies of influenza vaccination compared with influenza disease management. This is the first study of its type to focus on multiple CEE countries. The available literature was supplemented by data and interpretation from local experts from each target country, allowing for both quantitative and qualitative inter-country comparisons. Such comparisons are hoped to offer insight on healthcare policy and its potential impact on management of influenza in the elderly.
Results
In this analysis, the elderly population ranged from 1.17 million in Kazakhstan to 6.98 million in Ukraine. Literature review and interviews with experts indicated that healthcare services and accessibility, in particular for the elderly, varied by target country (). Healthcare payment systems ranged from compulsory insurance (Romania) to those incorporating substantial out-of-pocket costs (Kazakhstan and Ukraine). Other systems, such as in Poland, are mostly publicly funded but specialist outpatient care is on a fee-for-service basis. The expert completed “diagnostic tool” shows that influenza vaccination of the elderly is recommended and free or fully reimbursed in the majority of target countries (). The exceptions are Kazakhstan and Ukraine, in which it is recommended that the elderly receive the influenza vaccine but the vaccine is neither free nor reimbursed.
Table 2. Summary of healthcare systems in target countries
Table 3. Summary of results from the “Diagnostic Tool” completed by country experts
There was a general paucity of data available on the incidence and prevalence of influenza in elderly populations in the target countries. The precise monitoring and reporting of influenza trends in CEE is still a work in progress. To populate the model, influenza incidence data were available from seasons 2009–10 and 2010–11; the 2010–11 data were used for modeling because the data were both recent and, unlike 2009–10, not influenced by a pandemic strain. Pertinent to this study were the definitions of influenza-like illness (ILI), acute respiratory infection (ARI), and severe ARI (SARI) that are not influenza-specific health states but can derive from other causes. In the peak weeks (51–6) of the 2010–11 influenza season, EuroFlu reported that the percentage of ILI and ARI samples testing positive for influenza was approximately 50%.Citation24
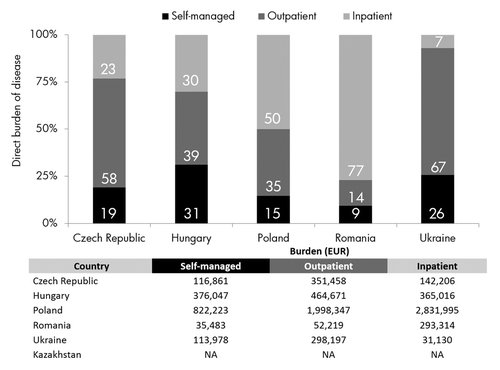
A complete overview of model health states, including costs per item, patient population and overall burden can be found in . ILI attack rates were used to calculate the proportion of the elderly population affected by influenza and attack rates were, from lowest to highest, Romania (0.3%), Ukraine (0.9%), Czech Republic (2.1%), Poland (4.4%), and Hungary (4.9%); data were not available for Kazakhstan. Taking the population size and the attack rate for non-complicated, self-managed cases into account, the largest burden of ILI infection is found in Poland, where it was estimated that 161 215 patients self-manage their infection and a further 72 092 report to their general practitioner (GP). The prevalence of SARI (influenza and pneumonia) hospitalization was 1036 cases, of which approximately 75% visited their GP after discharge. In all countries, the healthcare burden of SARI patients after hospital discharge, both numerically and economically, was <1% of total burden. The overall, regional rate of excess hospitalizations due to SARI was calculated to be ~30 per 100 000 people. It is noted that, qualitatively, the burden of influenza was influenced by: (1) vaccination coverage; (2) accessibility to healthcare services; and (3) quality of the surveillance system. In countries where these three factors were high, e.g., Poland, the data integrity is likely to be improved and better reflect the burden of influenza. Confounding this is low healthcare accessibility, whereby influenza-rates are underreported by surveillance systems because of limited access to healthcare providers and increased self-management.
Table 4. Burden of influenza in the elderly by influenza health states (2010–11 season)
Cost items for self-management per country were generally low and had a median of EUR 4.75 (range: 2.6 in Ukraine to 6.6 in Hungary). The cost for ILI treated by a GP also varied little between countries, with a median of EUR 16.6 (range: 11 in Kazakhstan to 32 in Czech Republic). Variation in costs between countries was apparent for inpatient care, for example SARI care in an intensive care unit (ICU) had a median cost of EUR 2023 but ranged from EUR 175 in Kazakhstan to EUR 18 210 in Romania. The different costing approaches used were reflected in ICU costs, whereby micro-costing (Romania and Poland) resulted in higher costs compared with other countries evaluated. Overall, the cost per case varied with per capita GDP and total health care spending. Individual cases that correlated at the 90% level with GDP were ILI treated by a GP (r = 0.76, P = 0.08) and GP treatment after discharge from ICU (r = 0.73, P = 0.10). A full breakdown of costs is provided in .
Total annual economic burden of influenza-related direct medical costs ranged from EUR 381 016 in Romania to EUR 5 652 565 in Poland (). The highest mean cost per patient, however, was found in Romania, EUR 35, where 10 860 elderly patients were infected in the 2010–11 season. Although the elderly populations of Czech Republic and Hungary are of similar sizes, 1.67 and 1.68 million, respectively, the burden of influenza varied substantially between the two countries. At EUR 610 526, the burden of influenza in Czech Republic is almost half that of the burden in Hungary (EUR 1 205 734). Although cost per health state differences do exist between these two countries, the main driver of influenza burden was the ILI attack rate, it being 2.1 in Czech Republic and 4.9 in Hungary.
The healthcare setting that contributed most to the total economic burden of influenza in the elderly was country specific. In Poland and Romania, inpatient costs accounted for 50% and 77% () of overall burden, respectively. Furthermore, in Poland and Romania, in which a micro-costing approach was used, the burden of SARI (including death) was greater than that of ILI (including self-managed). The burden of ILI and SARI in Poland was EUR 2 799 268 and EUR 2 853 297, respectively, and the equivalent values in Romania were EUR 86 336 (ILI) and EUR 294 680 (SARI). In the other target countries the highest burden derived from ILI. In Ukraine, 25.7% of the influenza burden was accounted for by self-management while a further 66.7% came from GP consultancy (). The burden of outpatient GP consultancy was often a strong determiner of overall burden, accounting for 56.9%, 38.1%, and 35.0% of overall burden in Czech Republic, Hungary and Poland, respectively. The corresponding value in Romania was substantially lower at 13.3%. The high burden of outpatient care in Ukraine might derive from low healthcare accessibility, such that most patients remain in the community setting. Furthermore, the “diagnostic tool” indicated that hospitals in Ukraine had “influenza vaccination policies in place” that may limit or reduce the inpatient burden of influenza. Such policies were also in place in Hungary and Kazakhstan, and although burden data could not be calculated for Kazakhstan, the outpatient burden in Hungary was also relatively high.
Figure 1. Relative healthcare burden in elderly patients per setting per country. Relative healthcare burden in Euro per setting varies considerably by country. Self-managed: influenza-like illness (ILI) and acute respiratory infection (ARI) patients that do not contact a healthcare professional; Outpatient: ILI and ARI patients that visit a healthcare professional or are referred to a GP after hospital discharge; Inpatient: Patients treated in a hospital (intensive care unit (ICU) or non-ICU) setting.
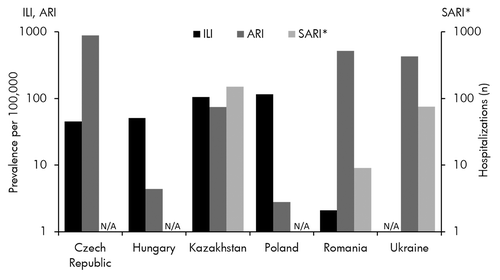
The study demonstrated that the burden of influenza was generally high in the six targeted CEE countries. Poland had the second largest elderly population (5.25 million) and second highest attack rate (4.4%) in this study, which resulted in the highest burden (EUR 5.65 million). As evidenced by Poland, total burden was mainly driven by the size of the elderly population and the influenza attack rate, highlighting the importance of surveillance in evaluating the burden of influenza and its prevention. Due to current limitations of the EuroFlu surveillance system, results presented here are likely to represent an under estimate of the influenza burden. The WHO sentinel surveillance network, EuroFlu, reported rates for ILI and ARI in target countries were low and might indicate substantial under reporting. Data from the 2011–12 season (week 15) showed ILI/ARI rates of 45.0/882.9 (Czech Republic), 50.6/NA (Hungary), 104.9/74.4 (Kazakhstan), 115.7/NA (Poland), and 2.1/518.4 (Romania) per 100 000 people ().Citation25 In Ukraine, 3.7% of total outpatient visits were due to ILI/ARI and the ARI rate was 427.0 per 100 000 people.Citation25 The comparative rates of ILI/ARI in Belgium, Italy, Netherlands, and Scotland were 39.0/1198.0, 84.7/NA, 21.9/NA, and 12.3/474.3 cases, respectively, per 100 000 people.Citation25
Figure 2. Influenza‐related infections and hospitalizations in target countries, week 15, Season 2011–12. Source: EuroFlu weekly bulletinCitation25. ILI, Influenza-like illness; ARI, Acute Respiratory Infection; SARI, Severe Acute Respiratory Infection; N/A, Not Available. *SARI values are estimates from EuroFlu graphs and are the count of hospitalizations in participating institutions.
Literature review found that the vaccination coverage in the six targeted CEE countries was far below recommendations from The EU Council and the WHO (75% for the years in question) and was in fact decreasing (). In Romania, vaccination coverage of the elderly reached almost 50% in 2008–09, but dropped in successive years to 28.5% (2009–10) and 19.5% (2010–11).Citation26,Citation27 The highest rate, in 2009, might have been influenced by the pandemic influenza strain and the international efforts made to restrict its spread. The study by Mereckiene et al. (2008) reported that coverage in the elderly was <10% in Poland, <20% in Romania, <30% in the Czech Republic, and <40% in Hungary.Citation14 Expert interviews and completion of the “diagnostic tool” identified that in no instance does a “national vaccination industry group” evaluate vaccine coverage or performance and that only Poland and Ukraine have annual targets for vaccination coverage that aim to increase coverage toward those recommended by the WHO. Results from the “diagnostic tool” also highlighted that only in Romania and Kazakhstan do healthcare professionals (HCPs) systematically get invited to receive the flu vaccination themselves. Only in Ukraine is a Continuing Medical Education (CME) program on influenza provided.
Discussion
This is the first multi-national study to explore the burden of influenza in elderly populations in CEE. Although significant differences in the availability and quality of epidemiological data were identified between countries included in this analysis, the medical burden of influenza in the elderly in CEE was found to be large. The total associated economic burden varied by country and differences were largely driven by the population size, the surveillance system, the healthcare system, and the current influenza vaccine coverage rate.
The medical burden of influenza in the elderly in the six targeted CEE countries may, importantly, also be underestimated in this study. In the analysis, the rate of excess hospitalizations due to ILI: ~30 per 100 000 people, is far below (at least 5 times fewer than) that reported in elderly populations in three Western European countries: 165, 189, and 219 per 100 000 population in Germany, France, and England and Wales, respectively.Citation28 It is, thus, likely that SARI cases are generally underrepresented in this study of influenza burden. Low healthcare accessibility and limited surveillance centers might be exacerbating underreporting. In target countries, ILI and/or ARI rates were low compared with western European countries such as Belgium, Netherlands, and Scotland,Citation25 countries in which surveillance systems are well established and healthcare accessibility is high. It is possible that the burden of influenza in the elderly in CEE remains hidden by this population’s inability (physical or economic) or reluctance to access medical facilities. The burden of influenza appears to have higher visibility in other populations, for example among healthcare workers in Ukraine there were 60 000 ARI cases and 42 deaths reported during the 2009–10 flu season,Citation29 during this season the Ukrainian Ministry of Health had confirmed a total of 265 deaths by week 44.Citation30
Based on this study there are significant differences in the availability and quality of epidemiological data between countries included in this analysis. The influenza surveillance systems and their reporting should be improved and standardized. As broached by Ercole et al. in regard to the 2009 H1N1 pandemic,Citation31 there is a need for more publicly available raw data to facilitate further accurate and informative analysis in the future. The weaknesses in current surveillance and reporting, which lead to data gaps, may result in underestimation influenza burden and potentially limit the implementation of prevention and management programs. To complement traditional surveillance methodologies both the UK and Netherlands are using internet-based surveillanceCitation32-Citation35 and results correlate well with Sentinel data.Citation32,Citation34,Citation35 Compared with sentinel reporting, internet surveillance might provide an earlier warning of potential influenza burden but overestimate the burden due to dependence on self-reporting.Citation34,Citation35 Given the high proportion of patients that self-manage their influenza, a higher attack rate might be acceptable; internet use, however, often excludes the elderly. Internet surveillance might be of benefit in CEE to produce data on incidence and prevalence rates of influenza that are, at this time, poorly documented.
It was previously noted that ILI and ARI rates were low compared with other countries, and the magnitude of potential underestimation might be demonstrated by the rate of excess hospitalizations identified (~30 per 100 000 people), which is over 5 times fewer than were reported in Western Europe. The economic burden of influenza in the elderly in target countries calculated might be also an underestimate because assumptions in the model were conservative so not to adversely influence the economic findings toward higher costs. The costs presented here are direct-healthcare-only costs definitely linked to influenza. Although frequent in the elderly, the complications and costs associated with influenza-related exacerbation of chronic diseases (e.g., chronic obstructive pulmonary disease) and other acute medical events (e.g., acute coronary syndrome) have not been broached in this research.
Self-management data utilized in this model were extracted from a Dutch study and may prove an underestimate for CEE because self-management of disease is generally thought to be more common if access to healthcare services is limited. This is of particular relevance in low and middle income countries and elderly populations, who are in general less mobile and more house bound. It is, therefore, possible that the community-based burden of self-management is higher than reflected in the estimates presented here. Indeed, self-management of influenza or ILI in the elderly is likely a misnomer because their care often requires intervention by a family member or assisted living care assistant. Such care commitments can result in absence from work or reduced workplace productivity, which can generate indirect costs (not considered in this study) that are not negligible. In addition, the elderly often have an important societal role in the care of young family members, e.g., grandchildren. The presence of influenza or ILI in the elderly is expected to impinge on their availability to provide care and may result in increased absenteeism and decreased productivity of other family members. With data unavailable on this issue for target countries, it is difficult to estimate the economic burden imposed. It is, however, expected to be large. Compared with the time period of April to November, during the peak flu season (December to March) the number of full time workers in the US that reduced their hours to cover family/personal obligations increased by 18.7% in 2009–2010.Citation36 A 2004 report in the US found that absenteeism due to problems associated with the breakdown of childcare or requirement for care of the elderly cost US businesses more than USD 3 billion annually.Citation37 Furthermore, the study by Molinari et al. found that loss of earnings increased the economic burden of influenza in the elderly by 4700% for those who were not medically attended (self-managed their influenza), by 200% in those that received outpatient care and by 16% in those that were hospitalized.Citation6 Paucity of data regarding self-management in the elderly and its associated economic and societal costs in target countries is expected to result in further underestimation of the influenza burden in this analysis.
The burden of influenza is associated with the size of the elderly population and the influenza attack rate. Given that populations are aging worldwide, the burden of influenza in the elderly is likely to increase in the coming years if no prevention strategies are put in place. Where relevant Eurostat data were available, the elderly population was projected to approximately double from 2010 to 2060, going from 15.2 to 30.7% in the Czech Republic, 16.6 to 32.1% in Hungary, 13.5 to 34.5% in Poland, and 14.9 to 34.8% in Romania.Citation13 Without adjusting for inflation, this will mean that the healthcare burden will reach EUR 1.2 million, EUR 2.3 million, EUR 14.4 million, and EUR 0.9 million in the Czech Republic, Hungary, Poland, and Romania, respectively, in 2060.
Although vaccination is currently the most effective means of preventing influenza infection, the seasonal variation in influenza virulence and few placebo-controlled randomized clinical trials (RCTs) of influenza vaccine efficacy in elderly populations have led some to question the effectiveness of vaccines in preventing hospitalizations and deaths.Citation38 In a recent meta-analysis of influenza in the elderly, mean (95% CI) vaccine efficacy was estimated to be 58% (34–73%) against laboratory confirmed influenza (LCI) and 41% (27–53%) against ILI.Citation39 Kissling et al. found that vaccine effectiveness was 59% (95% CI: 15–80%) against LCI in adults aged ≥65 y.Citation40 In a matched, case-control study of elderly patients in Genoa, Italy, during the 2010–11 influenza season, the vaccine effectiveness was found to be 94.8% (95% CI: 77.1–98.8%) in preventing hospitalization for influenza.Citation41 In the most recent influenza season (2012–13), vaccine effectiveness against LCI was calculated to be 33% in the elderly.Citation42 A recent observational study conducted in Spain found that vaccine effectiveness in preventing LCI-related hospitalizations was 59% (16–79%) for those aged 60 y and over.Citation43 Vaccine effectiveness has resulted in a measurable difference in influenza burden, for example: when comparing disease burden before and after introduction of a mass vaccination campaigns in the elderly in Brazil, researchers found a 26% decrease in influenza-related mortality.Citation44
The benefits of synergistic vaccination and surveillance programs are demonstrated by the UK and the Netherlands. In 1957 the first international influenza center was founded in London, and currently there is twice-weekly reporting in England and Wales provided by 100 sentinel practices serving a patient population of 900 000.Citation45,Citation46 Implementation of prevention strategies in the UK was associated with significant reduction in ILI incidence from 1400 per 100 000 people in 1969 to around 400 per 100 000 people in 1999.Citation46 In the Netherlands, there are ca. 65 nationally representative Sentinel practices.Citation47 After introduction of a specific vaccination program for the elderly, a reduction in influenza-related mortality has been recorded, with excess deaths reduced by 35%.Citation22
The European Council urged members to achieve 75% vaccination coverage in the elderly before the 2014–15 seasonCitation20 and many EU members have monitoring in place to track progress.Citation14 Coverage in CEE was, however, far below recommended levels and was found to be decreasing. Mereckiene et al. reported in 2008 that vaccination coverage of the elderly in target countries did not exceed 40%.Citation14 In 2006, Ryan et al. analyzed the healthcare costs of increased vaccination across France, Germany, Italy, Spain and the UK. Based on the mean vaccination rate of 39.7% in these countries, the cost (EUR 1.52 billion) of increasing vaccination coverage to 100% in at-risk populations would be offset by savings of EUR 39.45 million in reduced primary care visits and EUR 1.59 billion in reduced hospitalizations.Citation48
The reasons for low vaccination rates in CEE are likely to be at least partially country-specific. For example, interviews with experts identified that in Czech Republic and Kazakhstan there is no awareness campaign dedicated to influenza or vaccination. Only in Poland, Hungary, Romania, and Ukraine do “national health authorities survey and communicate their own statistics of flu vaccine coverage by target group each year.” Furthermore, only Poland and Romania provide adverts or mailing direct to the healthcare workers. Other psychological and social reasons will, however, be universal. The main reasons for non-vaccination in Poland were found to be “feeling healthy,” “lack of belief in vaccination effectiveness,” “high cost,” and “no opportunity to receive vaccination.”Citation49 Evaluating ways to increase vaccination coverage in the elderly, found that distribution of personal letters and vouchers for free vaccination were particularly pertinent for elderly people.Citation17 Other factors identified by the study that could increase vaccination uptake were (in order of importance): monitoring of vaccine coverage rate; national vaccination coverage objectives per year; reimbursement (90–100%) of vaccination costs; healthcare professional (HCP) objectives for at-risk groups; financial incentives for HCPs; awareness campaigns on radio or television; and awareness campaigns via flyers in medical rooms, press adverts or websites.
Expert completion of the “diagnostic tool” allowed a number of these items to be evaluated in target countries and results indicated that all target countries except Hungary and Kazakhstan had adopted the previous WHO recommendation of 75% vaccination coverage of the elderly; but only Poland and Ukraine had implemented incremental, annual targets via which to reach the 75% goal (). Vaccination of the elderly was recommended and free or fully reimbursed in the majority of target countries; however the cost of vaccination was not reimbursed in Kazakhstan and Ukraine. HCPs received financial incentives for vaccination of target groups in Czech Republic and Romania. All target countries run radio, television, and press awareness campaigns, but in the Czech Republic these only last for a couple of days. Personal letters have been highlighted as an important factor in increasing vaccination in elderly populationsCitation17, but only in Hungary are patients sent a personal letter or voucher from the health authority. That acknowledged, the experts noted that some GPs in Czech Republic, Hungary, Romania, and Kazakhstan do send out personal letters to at-risk patients.
Reviewing vaccination programs in the European Union and European Economic Area, Kanitz et al. found that although adult vaccination recommendations do exist in most countries, very few of these countries document their programs and only 4 of 25 countries collected coverage data for at-risk groups.Citation50 They surmised that, whereas childhood vaccination was a pillar of public health provision, adult vaccination was often overlooked. Data presented here underline the large healthcare burden of influenza in the elderly. The present findings reflect those of Blank et al.Citation17 and Kanitz et al.,Citation50 providing evidence that vaccination should be accompanied by a monitoring strategy.
In general, the evidence is growing that well-designed vaccination programs based on surveillance data produce both public health and economic benefits. Clearly, monitoring and reporting systems and vaccination programs must be optimized on a national level to maximize benefit and, with increasing surveillance in CEE, there may be advantage to be gained from introducing prevention schemes in these countries. Comparing the current situations, we find that though vaccination recommendations in CEE reflect those in the UK and Netherlands, their implementation is not as successful, with coverage rates maximally reaching 50% but usually being in the 10–30% range. In this way, much of the influenza burden in CEE is not managed as effectively as in Western Europe where reductions in hospitalizations and mortality have been reported. It must be acknowledged that implementation of vaccination programs is not simple and there are challenges to be overcome in order to recognize the public health benefits reported in the literature. These include the accessibility of vaccination schemes to less mobile elderly populations and the appropriate health education and promotion that is required to overcome psychological, social, and physical barriers to participation.Citation51-Citation54
This analysis identifies a high direct economic burden of influenza in the elderly in target CEE countries and is likely to underestimate the true burden. It is expected that the direct costs of influenza and ILI in the elderly has been underestimated due to a paucity of published data for target countries, with excess hospitalization rates in particular seemingly too low in comparison to other countries with extensive influenza prevention programs in place. Furthermore, due to data limitations at the time of study design it was decided to focus on direct medical costs. For this reason, the quantification of indirect costs is not broached in this study does, though it still represents substantial burden. As data become available we encourage researchers to extend the work presented here to facilitate a full overview of the healthcare burden associated with influenza in CEE. In saying this, the direct cost of influenza in the elderly estimated here is substantial in itself and indicates the need for healthcare policy makers and payers to make data-informed decisions on how best to limit the impact of influenza on healthcare systems and societies in general.
The economic burden of influenza in elderly populations is recognized worldwide, and to decrease it many countries in the world have implemented vaccination programs. The funding of influenza vaccination program by governments was also based on the proven cost-effectiveness of this prevention strategy. Indeed the majority of cost–effectiveness studies and reviews worldwide have shown that influenza vaccination programs for the elderly people are highly cost-effective in reducing influenza-related deaths or gaining life years.Citation55-Citation58 The cost-effectiveness of influenza vaccination in Poland, one of the target countries of this analysis, has also already been shown in two separate studies.Citation11,Citation12 From a public health perspective, financing influenza immunization for the elderly in Poland would be very cost effective (ICER = PLN 26 118 per QALY).Citation11 A 2002 study in Western Europe that compared different strategies for the management of influenza in the elderly population (vaccination, chemoprophylaxis, and no intervention) in three countries (France, Germany, and the UK), found that vaccination strategies were the most cost-effective.Citation28 The recognition of influenza vaccination cost effectiveness and value for money has led to sustained vaccination campaigns in the UK and Netherlands. At this time, there is sufficient evidence of disease burden to consider extension of influenza vaccination programs of the elderly in CEE.
The elderly population of Europe is large and continues to grow.Citation13 In this population, influenza-related complications are common and may negatively impact quality of life.Citation9 Healthy Aging, the optimization of physical, social, and mental health in the elderly,Citation59 is a priority for both the EU and the WHO. The European Innovation Partnership reported that an important part of this optimization process is the combating of illnesses that affect the elderly through cost effective preventive strategies.Citation60 The Partnership’s suggestions aim to improve the health status and quality of life of the elderly, and support the long-term sustainability and efficiency of health and social care systems.Citation60 Target countries surveyed here may benefit from introducing preventative strategy action plans. Benefit can be increased by building on knowledge, good practice, and experience from other countries.Citation59 Results from the UK, Netherlands and other countriesCitation22,Citation28,Citation46,Citation47 indicate that, in terms of influenza prevention, vaccination should be the priority in target countries. Removing public procurement barriers, easing elderly access to health services, and enhancing the business environment to improve competitivenessCitation60 may all be of benefit in reducing the burden of influenza and improving quality of life in the elderly.
Methods
Many sources have been used in this research to collect data in terms of number of influenza cases, healthcare costs, and influenza vaccination coverage by country. The primary sources were literature review, open-access websites, and open-access databases (see Appendix). Secondary data was provided by country experts that were recruited via chain (snowball) sampling, a non-random, non-probabilistic sampling technique in which the original expert approached recommended a colleague if they themselves could not provide the data requested to a satisfactory degree of certainty. Interviews with the final set of country experts (see list in Appendix) supported primary data collection, resolved queries, provided explanations to the interpretation of results, and approved final conclusions. No approval from ethical committees was required because no individual patient-level data were collected.
The literature search was undertaken in PubMed using title and abstract search strings, the key terms included “Czech,” “Hungary,” “Kazakhstan,” “Poland,” “Romania,” “Ukraine,” “Influenza,” “Elderly/Aged/65,” “Costs/Expense,” “Vaccination,” “Policy,” “Burden,” and all common derivations thereof. Non-systematic, by hand searches of other databases were undertaken to identify any further publications of relevance. In all cases, searches were restricted to English-language publications from 2007 to 30 June 2012. Country experts supplemented the literature search through consideration of local language publications and regional journals not represented on international databases. In total 51 publications were screened for data pertinent to the research objective. Data collected was assessed and, as required, updated by country experts. Where pricing information was unavailable in the public domain these data were provided or mean costs estimated by experts. As a final step, country experts also completed a “Vaccination policy questionnaire” that was an extended version of that provided Blank et al.Citation17
In order to estimate the economic burden of influenza in a given time period: (1) the number of patients and (2) health care costs per patient have to be quantified. And, as treatment costs differ case by case, patients should be stratified into relatively homogeneous groups. Influenza infection rates from published literature were supplemented by data available via the WHO sentinel reporting system in Europe: EuroFlu. The WHO Sentinel surveillance program incorporates an international network of institutions that routinely and consistently collect epidemiologic information and laboratory specimens from patients presenting with an illness consistent with influenza. The WHO global Sentinel network covers 85 countries, involves 114 laboratories and four WHO centers.Citation16 Influenza surveillance in target countries is summarized in and, as seen in , data are not always available (e.g., SARI data in Czech Republic, Hungary, and Poland) or may be non-standard (as per ILI/ARI reporting in Ukraine). Notably, there were not always sufficient data to utilize laboratory confirmed influenza (LCI) cases and, in spite of its public health importance, mortality attributable to influenza is not routine part of surveillance systems. Furthermore, country experts indicated that the majority of influenza patients do not seek medical support, rather self-medicating in the home or remaining asymptomatic carriers of infection. There are, thus, thought to be many medically undetected cases of influenza in CEE, and for this reasons the reported rates of ILI and SARI, rather than rates of LCI, were used to populate the model. Health states in the model were constructed from WHO-defined influenza entities due to the quite heterogeneous and sparse quantity and quality of epidemiological data on influenza and related diseases in the target countries.
Table 5. Summary of surveillance systems in target countries
Accordingly, a simplified influenza treatment algorithm was modeled () and incorporated the following health states: (1) Self-managed influenza; (2) GP-managed ILI; (3) Hospital-managed, non-ICU SARI; (4) ICU-managed SARI; (5) GP-management after discharge; (6) Death (either in hospital or ICU). Within the model, and based on ILI attack rates, a proportion of the healthy population per country succumbs to influenza infection. Of the infected population, a proportion is defined to be self-managed and the others populate the remaining health states within the model on a per country basis. The distribution of patients between health states is based on country-specific prevalence and incidence data were available, or from local-expert derived or inferred data. Due to a lack of data on inter-health-state transitions, it is assumed that patients in one health state have progressed through the previous health states. For this reason, patients within the hospital setting are considered to have used outpatient services prior to hospitalization. Likewise, a proportion of hospitalized patients will visit the GP after discharge.
Figure 3. Simplified influenza treatment algorithm used to model the disease burden. Health states in the model are mutually exclusive. Transitions between states are identified with an arrow, a solid line indicates a reversible transition and unidirectional movement is shown via a dotted line. Health states are classified as Outpatient or Inpatient as shown in in the “Setting” row. Cost items are summarized below the setting. The diagnoses considered to be influenza-related per health state are listed below the diagram. ILI, Influenza-like Illness; ARI, Acute Respiratory Infection; SARI, Severe Acute Respiratory Infections; OTC, Over The Counter; GP, General Practitioner; ICU, Intensive Care Unit.
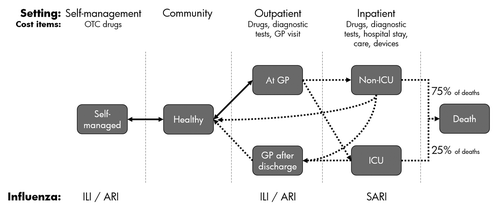
The model was populated with epidemiological and economic data identified during literature review and interviews with country experts. Given data availability a number of assumptions were made in the model, these were: (1) that no outpatient was managed by a specialist, this was due to a lack of relevant data; (2) patients were referred to a GP for continued care (one visit) after hospital discharge; (3) all influenza-related deaths occur in hospital, whereby 25% of total deaths came from ICU and 75% from non-ICU treated patients. This predefined 25:75 split was validated by country experts and did not influence the total number of influenza deaths per country, which was assigned from the literature where available. Assumptions were conservative and should not adversely influence model outcomes, having been validated by country experts to preferentially be cost neutral or cost saving.
Due to observed data gaps and inconsistencies in local epidemiological data (see Appendix) the following country-expert-validated assumptions were used to estimate the number of cases per health state: (1) equal influenza incidences were assumed in the 60+ and in the 65+ population in Czech Republic and Hungary, where data were reported only in the 60+ age cohort; (2) the ARI in total population:ILI in 65+ population ratio from the Czech Republic (1:58.1) was used to estimate the ILI incidence in Ukraine; (3) the SARI:ILI ratio in Hungary (1:63.7) was used to estimate SARI incidences in Poland, Czech Republic, and Ukraine; (4) the SARI-ICU:ILI ratio in Hungary (1:268.9) was used to estimate SARI-ICU incidences in Poland, Czech Republic, Romania, and Ukraine; (5) the influenza-death:ILI ratio in Hungary (1:751.4) was used to estimate influenza-related deaths in Poland, Czech Republic, Romania, and Ukraine; (6) the rate of self-management was taken from a Dutch publicationCitation33 and so in all countries 69% of ILI patients are self-managed.
The economic analysis considered the direct medical cost of influenza in the elderly. The potential impact of co-morbidities and the long-term chronic consequences of influenza related complications were not analyzed. Moreover, direct non-medical costs were not included in this analysis because they were not available in the literature and difficult to obtain via the expert interviews only. Indirect costs were considered to be outside the scope of the analysis due to a paucity of data. Estimating the economic consequences of absence from work and loss of productivity depends on the proportion of elderly patients active in labor market and/or supporting active family members through caregiving. Although this loss may make a substantial contribution to the burden of influenza in the elderly, the magnitude of productivity loss is expected to be lower for elderly patients compared with active-age cohorts. Information on family support is highly limited in target countries and as such data could not be included in the analysis. This means that only the direct costs to healthcare policymakers (public payers) or patients themselves were estimated and our cost estimation is therefore highly conservative.
The methodology of cost per case estimates differed between countries: a micro-costing approach was used in some countries, while a gross costing approach based on outpatient and hospital financing were used in other countries (full details in Appendix). For the micro-costing approach, the resource use of each cost item was multiplied by its unit cost and then aggregated cost items were used to calculate the cost per health state. All costs were collected in national currency in 2012 values. These were converted to 2011 values using country-specific consumer price indices from January–June 2011 and January–June 2012. Finally, for comparison, costs were converted to Euros by applying the official currency exchange rates from June 1, 2011. For each health state in the model, the main cost components were considered to be: (1) Self-management costs: (a) Drugs including over-the-counter anti-inflammatory drugs and expectorants; (2) Outpatient costs: (a) Drugs including antiviral(s), antibiotics, anti-inflammatory drugs, and expectorants; (b) Diagnostics including laboratory tests, influenza confirmation tests, and imaging diagnostics; (c) Cost of outpatient visit; (3) Hospitalization costs: (a) Total hospitalization costs for patients without ICU care; (b) Total hospitalization costs for patients with ICU care; (4) Influenza death costs: (a) The total cost of hospitalization.
Then the economic burden of influenza per country was calculated by multiplying the number of patients per health state by the cost of care per health state. Calculations, data analysis, and graphing were performed using Microsoft Excel 2010. Inbuilt regression analysis functionality was used to calculate Pearson correlations and the associated p-values. Due to the above mentioned limitations regarding epidemiological data and the potential underestimation of the cost per influenza case, the generalizability of our findings should be considered cautiously.
Discloure of Potential Conflicts of Interest
G.K. and Z.K. are employees of the Syreon Research Institute, Budapest, which received financial support from Sanofi Pasteur, Lyon, France, in order to complete this study. L.D. and M.B. are employees of Sanofi Pasteur, Lyon, France. Experts consulted by Syreon during this study, including the following authors K.J.-R., J.K., A.C., A.P., M.L., and R.K., received honorarium for their participation and time. No payments were made for authorship and contributions to the academic content of this manuscript. No other conflicts of interest are declared.
Acknowledgments
The authors would like to thank Sanofi Pasteur, Lyon, France for financial support in realizing this research project. The manuscript was developed by Rhodri Saunders and William Valentine of Ossian Health Economics and Communications, Basel, Switzerland and was funded by Sanofi Pasteur, Paris, France.
Ethical Statements
This study complied with all local laws on data collection and protection and required no approval from ethical committees because individual patient-level data were not collected.
Contributors
Prof Lidia Brydak; Dariusz Horszczaruk, MD; Sarka Hrusakova, MD; Prof emer. Andras Szalka; Rodica Popescu, MD; Odette Popovici, MD; Sorin Petrea, MD; Prof Olga Zaliska; Laurentiu Zolotusca, MD; Dr William Valentine; Dr Rhodri Saunders
References
- World Health Organization. Influenza. Fact Sheets. 2003. Available from: http://www.who.int/mediacentre/factsheets/2003/fs211/en/.
- Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med 2008; 121:258 - 64; http://dx.doi.org/10.1016/j.amjmed.2007.10.040; PMID: 18374680
- World Health Organization. Comparing deaths from pandemic and seasonal influenza. Global Alert and Response. 2009. Available from: http://www.who.int/csr/disease/swineflu/notes/briefing_20091222/en/.
- Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis 2006; 194:Suppl 2 S82 - 91; http://dx.doi.org/10.1086/507558; PMID: 17163394
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179 - 86; http://dx.doi.org/10.1001/jama.289.2.179; PMID: 12517228
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086 - 96; http://dx.doi.org/10.1016/j.vaccine.2007.03.046; PMID: 17544181
- Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004; 103:133 - 8; http://dx.doi.org/10.1016/j.virusres.2004.02.025; PMID: 15163501
- Webster RG. Immunity to influenza in the elderly. Vaccine 2000; 18:1686 - 9; http://dx.doi.org/10.1016/S0264-410X(99)00507-1; PMID: 10689149
- Lugnér AK, van Boven M, de Vries R, Postma MJ, Wallinga J. Cost effectiveness of vaccination against pandemic influenza in European countries: mathematical modelling analysis. BMJ 2012; 345:e4445; http://dx.doi.org/10.1136/bmj.e4445; PMID: 22791791
- Gasparini R, Amicizia D, Lai PL, Panatto D. Clinical and socioeconomic impact of seasonal and pandemic influenza in adults and the elderly. Hum Vaccin Immunother 2012; 8:21 - 8; http://dx.doi.org/10.4161/hv.8.1.17622; PMID: 22252007
- Brydak L, Roiz J, Faivre P, Reygrobellet C. Implementing an influenza vaccination programme for adults aged ≥65 years in Poland: a cost-effectiveness analysis. Clin Drug Investig 2012; 32:73 - 85; http://dx.doi.org/10.2165/11594030-000000000-00000; PMID: 22201294
- Jahnz-Rózyk K. [Health economic impact of viral respiratory infections and pneumonia diseases on the elderly population in Poland]. Pol Merkuriusz Lek 2010; 29:37 - 40
- Lanzieri G. The greying of the baby boomers, a century-long view of ageing in European populations. Statistics in Focus 2011; 23. Available from: http://epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-SF-11-023/EN/KS-SF-11-023-EN.PDF
- Mereckiene J, Cotter S, Weber JT, Nicoll A, Lévy-Bruhl D, Ferro A, Tridente G, Zanoni G, Berra P, Salmaso S, et al, VENICE Gatekeepers Group. Low coverage of seasonal influenza vaccination in the elderly in many European countries. Euro Surveill 2008; 13:19001; PMID: 18926107
- Rechel B, Doyle Y, Grundy E, McKee M. How can health systems respond to population ageing? World Health Organization 2009 and World Health Organization, on behalf of the European Observatory on Health Systems and Policies; 2009. Available from: http://www.euro.who.int/__data/assets/pdf_file/0004/64966/E92560.pdf
- World Health Organization. Influenza vaccines: WHO position paper. Wkly Epidemiol Rec 2005; 33:279 - 87
- Blank P, Schwenkglenks M, Szucs TD. The impact of European vaccination policies on seasonal influenza vaccination coverage rates in the elderly. Hum Vaccin Immunother 2012; 8:328 - 35; http://dx.doi.org/10.4161/hv.18629; PMID: 22327498
- World Health Organization-Europe. WHO/Europe recommendations on influenza vaccination during the 2011-2012 winter season. 2011. Available from: http://www.euro.who.int/__data/assets/pdf_file/0008/152297/EURO_2011_2012-flu-vacc-rec_V2.pdf
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128 - 32; PMID: 21866086
- The Council of the European Union. Council recommendation of 22 December 2009 on seasonal influenza vaccination (2009/1019/EU). Official Journal of the European Union. 2009. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:348:0071:0072:EN:PDF
- Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec 2012; 87:461 - 76; PMID: 23210147
- Jansen AG, Sanders EA, Nichol KL, van Loon AM, Hoes AW, Hak E. Decline in influenza-associated mortality among Dutch elderly following the introduction of a nationwide vaccination program. Vaccine 2008; 26:5567 - 74; http://dx.doi.org/10.1016/j.vaccine.2008.08.003; PMID: 18722492
- Boyle JR, Sparks RS, Keijzers GB, Crilly JL, Lind JF, Ryan LM. Prediction and surveillance of influenza epidemics. Med J Aust 2011; 194:S28 - 33; PMID: 21401485
- EuroFlu. The influenza season has begun in the WHO European Region EuroFlu Electronic Bulletin. 2012. Available from: http://www.euroflu.org/cgi-files/bulletin_v2.cgi?display=1&code=467&bulletin=467
- EuroFlu. Low and decreasing influenza activity in most countries of the WHO European Region. EuroFlu Electronic Bulletin. 2012. Available from: https://www.isirv.org/cgi-files/bulletin_v2.cgi?display=1&code=442&bulletin=442
- O’Flanagan DC, Cotter S, Mereckiene J. Vaccine European New Integrated Collaboration Effort (VENICE). Pandemic A(H1N1)2009 influenza vaccination survey, influenza season 2009/2010. VENICE. 2011. Available from: http://venice.cineca.org/Final_Report_VENICE_Pandemic_Influenza_2009.pdf
- European Centre for Disease Prevention and Control. Influenza vaccines from a public health perspective: Where are we today? Stockholm, 2012. Available from: http://www.ecdc.europa.eu/en/aboutus/organisation/director%20speeches/ecdc-director--seasonal-influenza-ivw-2012oct10.pdf
- Scuffham PA, West PA. Economic evaluation of strategies for the control and management of influenza in Europe. Vaccine 2002; 20:2562 - 78; http://dx.doi.org/10.1016/S0264-410X(02)00154-8; PMID: 12057614
- World Health Organization. Clinical management of influenza and other acute respiratory illness in resource-limited settings: learning from the influenza pandemic (H1N1) 2009. Meeting Report. Geneva, 2010. Available from: http://www.who.int/influenza/patient_care/clinical/858-WHOGIPReport_A4_WEB_FA.pdf
- Cernescu C, Ruta S-M. Distinctive features in the evolution of 2009 influenza pandemic in East European countries. Proc Rom Acad, Series B 2010; 2:99 - 106
- Ercole A, Taylor BL, Rhodes A, Menon DK. Modelling the impact of an influenza A/H1N1 pandemic on critical care demand from early pathogenicity data: the case for sentinel reporting. Anaesthesia 2009; 64:937 - 41; http://dx.doi.org/10.1111/j.1365-2044.2009.06070.x; PMID: 19645759
- van Noort SP, Muehlen M, Rebelo de Andrade H, Koppeschaar C, Lima Lourenço JM, Gomes MG. Gripenet: an internet-based system to monitor influenza-like illness uniformly across Europe. Euro Surveill 2007; 12:E5 - 6; PMID: 17991409
- Friesema IH, Koppeschaar CE, Donker GA, Dijkstra F, van Noort SP, Smallenburg R, van der Hoek W, van der Sande MA. Internet-based monitoring of influenza-like illness in the general population: experience of five influenza seasons in The Netherlands. Vaccine 2009; 27:6353 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.05.042; PMID: 19840672
- Tilston NL, Eames KT, Paolotti D, Ealden T, Edmunds WJ. Internet-based surveillance of Influenza-like-illness in the UK during the 2009 H1N1 influenza pandemic. BMC Public Health 2010; 10:650; http://dx.doi.org/10.1186/1471-2458-10-650; PMID: 20979640
- Marquet RL, Bartelds AI, van Noort SP, Koppeschaar CE, Paget J, Schellevis FG, van der Zee J. Internet-based monitoring of influenza-like illness (ILI) in the general population of the Netherlands during the 2003-2004 influenza season. BMC Public Health 2006; 6:242; http://dx.doi.org/10.1186/1471-2458-6-242; PMID: 17018161
- U.S. Department of Labor. Bureau of Labor Statistics. Illness-related work absences during flu season. Issues in Labor Statistics. 2010. Available from: http://www.bls.gov/opub/ils/pdf/opbils83.pdf
- Shellenback K. Child care & parent productivity: making the business case. Linking economic development and child care research project. Ithaca (NY): Cornell University, Cornell Cooperative extension department of city and regional planning. 2004. Available from: http://annazavaritt.blog.ilsole24ore.com/files/making-the-business-case-2.pdf
- Jefferson T. Influenza vaccination: policy versus evidence. BMJ 2006; 333:912 - 5; http://dx.doi.org/10.1136/bmj.38995.531701.80; PMID: 17068038
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2010; CD004876; PMID: 20166072
- Kissling E, Valenciano M, Falcao J, Larrauri A, Widgren K, Pitigoi D, Oroszi B, Nunes B, Savulescu C, Mazick A, et al. “I-MOVE” towards monitoring seasonal and pandemic influenza vaccine effectiveness: lessons learnt from a pilot multi-centric case-control study in Europe, 2008-9. Euro Surveill 2009; 14:19388; PMID: 19941774
- Gasparini R, Amicizia D, Lai PL, Rossi S, Panatto D. Effectiveness of adjuvanted seasonal influenza vaccines (Inflexal V ® and Fluad ® ) in preventing hospitalization for influenza and pneumonia in the elderly: a matched case-control study. Hum Vaccin Immunother 2013; 9:144 - 52; http://dx.doi.org/10.4161/hv.22231; PMID: 23143775
- Thompson M. Updated Adjusted Estimates of 2012-13 Seasonal Influenza Vaccine Effectiveness in the United States. 2013 National adult immunization summit. Atlanta, Georgia, 2013.
- Puig-Barberà J, Díez-Domingo J, Arnedo-Pena A, Ruiz-García M, Pérez-Vilar S, Micó-Esparza JL, Belenguer-Varea A, Carratalá-Munuera C, Gil-Guillén V, Schwarz-Chavarri H. Effectiveness of the 2010-2011 seasonal influenza vaccine in preventing confirmed influenza hospitalizations in adults: a case-case comparison, case-control study. Vaccine 2012; 30:5714 - 20; http://dx.doi.org/10.1016/j.vaccine.2012.07.006; PMID: 22819720
- Antunes JL, Waldman EA, Borrell C, Paiva TM. Effectiveness of influenza vaccination and its impact on health inequalities. Int J Epidemiol 2007; 36:1319 - 26; http://dx.doi.org/10.1093/ije/dym208; PMID: 17977871
- Health Protection Agency. Royal College of General Practitioners Weekly Returns Service. 2013.
- Fleming DM, van der Velden J, Paget WJ. The evolution of influenza surveillance in Europe and prospects for the next 10 years. Vaccine 2003; 21:1749 - 53; http://dx.doi.org/10.1016/S0264-410X(03)00066-5; PMID: 12686088
- Nielen MMJ, Spreeuwenberg P, Paget JW, Donker GA, Meijer A, Schellevis FG, Fleming D. The age-sepcofoc impact of influenza on hospital admissions and mortality in five countries in Europe. 2010. Utrecht, NIVEL.
- Ryan J, Zoellner Y, Gradl B, Palache B, Medema J. Establishing the health and economic impact of influenza vaccination within the European Union 25 countries. Vaccine 2006; 24:6812 - 22; http://dx.doi.org/10.1016/j.vaccine.2006.07.042; PMID: 17034909
- Kardas P, Zasowska A, Dec J, Stachurska M. Reasons for low influenza vaccination coverage: cross-sectional survey in Poland. Croat Med J 2011; 52:126 - 33; http://dx.doi.org/10.3325/cmj.2011.52.126; PMID: 21495194
- Kanitz EE, Wu LA, Giambi C, Strikas RA, Levy-Bruhl D, Stefanoff P, Mereckiene J, Appelgren E, D’Ancona F, VENICE (Vaccine European New Integrated Collaboration Effort) National Gatekeepers, Contact Points. Variation in adult vaccination policies across Europe: an overview from VENICE network on vaccine recommendations, funding and coverage. Vaccine 2012; 30:5222 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.06.012; PMID: 22721901
- Groenewold M, Baron S, Tak S, Allred N.. Influenza vaccination coverage among US nursing home nursing assistants: the role of working conditions. J Am Med Dir Asso 2012; 13:85.e17 - 23; http://dx.doi.org/10.1016/j.jamda.2011.02.008
- Kardas P, Zasowska A, Dec J, Stachurska M. Reasons for low influenza vaccination coverage: cross-sectional survey in Poland. Croat Med J 2011; 52:126 - 33; http://dx.doi.org/10.3325/cmj.2011.52.126; PMID: 21495194
- Mangtani P, Breeze E, Stirling S, Hanciles S, Kovats S, Fletcher A. Cross-sectional survey of older peoples’ views related to influenza vaccine uptake. BMC Public Health 2006; 6:249; http://dx.doi.org/10.1186/1471-2458-6-249; PMID: 17034625
- Seale H, Heywood AE, McLaws ML, Ward KF, Lowbridge CP, Van D, MacIntyre CR. Why do I need it? I am not at risk! Public perceptions towards the pandemic (H1N1) 2009 vaccine. BMC Infect Dis 2010; 10:99; http://dx.doi.org/10.1186/1471-2334-10-99; PMID: 20403201
- Patel MS, Davis MM. Could a federal program to promote influenza vaccination among elders be cost-effective?. Prev Med 2006; 42:240 - 6; http://dx.doi.org/10.1016/j.ypmed.2005.12.004; PMID: 16480761
- Postma MJ, Baltussen RP, Palache AM, Wilschut JC. Further evidence for favorable cost-effectiveness of elderly influenza vaccination. Expert Rev Pharmacoecon Outcomes Res 2006; 6:215 - 27; http://dx.doi.org/10.1586/14737167.6.2.215; PMID: 20528557
- Wang ST, Lee LT, Chen LS, Chen TH. Economic evaluation of vaccination against influenza in the elderly: an experience from a population-based influenza vaccination program in Taiwan. Vaccine 2005; 23:1973 - 80; http://dx.doi.org/10.1016/j.vaccine.2004.10.011; PMID: 15734070
- World Health Organization. Meeting of the strategic advisory group of experts on immunization, april 2012 – conclusions and recommendations. Wkly Epidemiol Rec 2012; 87:201 - 16
- The European Commission. Healthy Ageing – A Challenge for Europe. In: AGE European Older People’s Platform, EuroHealthNet, World Health Organization, Austrian Health Promotion Foundation, National Institute of Public Health (Czech Republic), The Health Development Agency (England), et al., eds.: The Swedish National Institute of Public Health, 2007.
- Gomez AI. European Innovation Partnership on Active and Healthy Ageing (AHAIP). EU Health Policy Forum. Brussels, 2010.
- Bryndova L, Pavlokova K, Roubal T. Czech Republic: Health system review. Health System in Transition, 2009:1-122.
- Gaál P, Szigeti S, Csere M, Gaskins M, Panteli D. Hungary health system review. Health Syst Transit 2011; 13:1 - 266; PMID: 22394651
- Katsaga A, Kulzhanov M, Karanikolos M. Kazakhstan: Health system review. Health System in Transition 2012; 14:1 - 154
- Sagan A, Panteli D, Borkowski W, Dmowski M, Domanski F, Czyzewski M, Gorynski P, Karpacka D, Kiersztyn E, Kowalska I, et al. Poland health system review. Health Syst Transit 2011; 13:1 - 193; PMID: 22551527
- Vlădescu C, Scîntee G, Olsavszky V. Romania: Health system review. Health Systems in Transition 2008; 10:1 - 172
- Lekhan V, Rudiy V, Richardson E. Ukraine: Health system review. Health Syst Transit 2010; 12:1 - 183, xiii-xiv; PMID: 21429862
- Kyncl J, Paget WJ, Havlickova M, Kriz B. Harmonisation of the acute respiratory infection reporting system in the Czech Republic with the European community networks. Euro Surveill 2005; 10:30 - 3; PMID: 15827371
- World Health Organization-Europe. Overview of sentinel systems for hospitalized severe acute respiratory infections (SARI) presented in the weekly EuroFlu surveillance bulletin. 2012. Available from: http://www.euro.who.int/__data/assets/pdf_file/0005/186863/Overview-of-SARI-Surveillance-Systems-final.pdf
- Romanowska M, Nowak I, Rybicka K, Brydak LB. The introduction of the SENTINEL influenza surveillance system in Poland--experiences and lessons learned from the first three epidemic seasons. Euro Surveill 2008; 13:8046; PMID: 18445411
