Abstract
The changes in lgG antibody levels to hepatitis B surface antigen (HBsAg) and in antibody to HBsAg (anti-HBs) seroconversion rates due to different dosages of hepatitis B vaccine (HepB) were compared in 2106 children. Children who had been previously vaccinated as infants with HepB were revaccinated at 5–15 y of age, after which the antibody titers were determined. After the first booster dose, the anti-HBs seroconversion rate (defined as an anti-HBs ≥10 mIU/ml) with 10 μg of HepB (93.6%) was significantly greater than the rate with 5 µg of HepB (90.3%) (P < 0.05); the anti-HBs seroconversion rate in 10–15-y-old boys vaccinated with 10 μg of HepB (90.9%) was significantly greater than the rate with 5 µg of HepB (84.3%) (P < 0.05). The anti-HBs seroconversion rates after the third booster dose with 5 or 10 μg of HepB were greater than those after the first booster dose (99.6% and 99.7%, vs. 90.3% and 93.6%, P < 0.05); as was the corresponding GMTs (658 ± 4 mIU/ml and 2599 ± 3 mIU/ml, vs. 255 ± 11 mIU/ml and 877 ± 11 mIU/ml [P < 0.05]). The immunization effects of booster vaccination with 3 doses of HepB with 5 or 10 µg are effective; a single booster dose with 10 µg of HepB for 10–15-y-old boys and with 5 or 10 µg of HepB for 5–9 y old boys and for 5–15-y-old girls are effective in generating protective antibody against HBV; however, for anti-HBs-negative children after a single dose of booster, 3 doses are needed.
Introduction
Infection with hepatitis B virus (HBV) remains a worldwide public health problem. It is estimated that more than 2 billion people have been infected with HBV, among whom 360 million have chronic liver disease, and 600 000–1 200 000 deaths result from HBV infection-related diseases annually.Citation1-Citation3 According to a 2006 national seroepidemiologic survey, the HBV infection rate in China is high, with an HBsAg carrier rate of 7.2% in people between 1 and 59 y of age. It is estimated that 9.3 million people in this group are chronic HBV carriers.Citation4,Citation5
Because the many HBV carriers do not know their infection status, they pose a significant risk of infection to a susceptible population. Especially perilous are the consequences to young children, because approximately 90% of HBV-infected newborns and 25–30% of children become chronic HBV carriers and thus are at increased risk for cirrhosis and hepatocellular carcinoma.Citation6-Citation8 In China, over 80% of the general population have anti-HAV, with the highest incidence in children.Citation9 Furthermore, chronic HBV carriers have a higher morbidity and mortality when super-infected with hepatitis A virus.Citation10 However, the universal infantile hepatitis A vaccination program began in 2007 in China, and the current hepatitis A vaccine is used on a “self-select and self-pay” basis in people >7 y of age, but does not result in 100% coverage levels in chronic HBV carriers to reduce the morbidity and mortality. Also, prevention of hepatitis B can prevent hepatitis D, as the hepatitis D virus is a defective virus that only causes hepatitis in the presence of the HBV.
While costly anti-viral treatment has limitations in resolving chronic HBV infection,Citation11,Citation12 hepatitis B vaccination is an effective and inexpensive measure against HBV infection,Citation6,Citation13 although a small number of immunization failures do occur.Citation14-Citation17 In China, infants initiated a program of 10 μg plasma-derived HepB administered at zero, 1 and 6 mo in 1992–1997, and this was modified to dosages of 5 μg of recombinant HepB in 1998. After >10 y of HepB mass vaccination, the HBV infection rate in the Chinese population, especially among children <15 y of age, has declined significantly. The prevalence of HBsAg has decreased from 9.67% in 1992 to 0.96% in 2006, and the number of HBV-infected children has decreased by nearly 80 million,Citation4,Citation5 but the prevalence of HBsAg in children between 5 and 14 y of age was 3.1–6.6% in some counties.Citation18,Citation19 It is generally observed that the antibody titers decline over time following immunization,Citation20 resulting in an increased rate of infection, especially when the antibody titers are reduced below the protective level.Citation21,Citation22 Consequently, the need for booster vaccination is becoming apparent. Some studies have reported that due to persistence of cellular immunity, booster vaccinations are unnecessary in a healthy population.Citation6,Citation23 However, Ren et al.Citation24 reported that the percentages of IFN-γ-positive and IL-4-positive in a population with an anti-HBs titers (anti-HBs < 10 mIU/mL) was significantly lower than those in a population with an anti-HBs titers (anti-HBs ≥ 10 mIU/mL) after primary immunization. This study indicates that cellular immunity to HBV may be as weak as humoral immunity in the population with an anti-HBs titers < 10 mIU/mL.
Currently, little is known about the effects of booster vaccination with HepB at different dosages in a large sample study. Although the effects of booster vaccination against hepatitis B have been examined,Citation25-Citation28 these studies have been limited by small sample sizes or the lack of comparability of enzyme-linked immuno sorbent assay (ELISA) or radioimmanoassay (RIA) detection methods.
Furthermore, previous studies have shown that horizontal transmission is one of the main ways children are infected with HBV,Citation29 and booster vaccination is cost-effective.Citation30 Because children >5 y of age attend school, they have a greater chance of exposure to HBV than younger children. Therefore, in this study we determined the effects of different dosage levels of HepB as booster on children >5 y of age. Subjects were vaccinated, after which the booster immunization effect of different dosages of HepB in children with anti-HBs lower less than the protective level (<10 mIU/ml) was determined. From this study we developed recommendations for a specific program of vaccination.
Results
Characteristics of the study subjects
A total of 3818 children between 5 and 15 y of age were initially enrolled in screening, and 2229 of these children were eligible for the study. Only subjects with three negative indices (HBsAg, anti-HBs, anti-HBc) were included. Of these 2229 subjects, 123 children were lost to follow-up. Thus, there were 2106 children who participated in the entire vaccination study.
Among the 2106 children, 976 (46.3%) were vaccinated with 5 μg of HepB, and the other 1130 children (53.7%) were vaccinated with 10 μg of HepB. The children characteristics are shown in . The proportion of males and females were similar in children 5–9 y of age who were vaccinated with 5 μg of HepB and with 10 μg of HepB, or in children 10–15 y of age.
Table 1. Characteristics of the study subjects
Before the booster vaccination, in children 5–9 y of age, the proportions of anti-HBs titers < 1 mIU/ml and anti-HBs titers ≥ 1 mIU/ml and < 10 mIU/ml were 36.9% and 63.1%, respectively; in children 10–15 y of age, the proportions of anti-HBs titers < 1 mIU/ml and anti-HBs titers ≥ 1 mIU/ml and < 10 mIU/ml were 50.1% and 49.9%, respectively. The proportion of anti-HBs titers in children 5–9 y of age who were revaccinated with 5 μg of HepB is similar to that in children who were revaccinated with 10 μg of HepB, similarly, in children 10–15 y of age . Whereas the differences in the proportion of anti-HBs titers in children who were revaccinated with 5 or 10 ugHepB are statistically significant between children 5–9 y and 10–15 y of age (χ2 = 15.582 or 21.054, P < 0.05, chi-square test). The distribution of age-specific anti-HBs titers on the basis of sex stratificationis is shown in .
Table 2. Distribution of age-specific anti-HBs titers before booster vaccination
Antibody seroconversion rates and GMTs after booster vaccination with 5 or 10 μg of HepB
After the first booster dose, the anti-HBs seroconversion rates with 5 or 10 μg of HepB were 90.3% and 93.6%, respectively, and these observed differences were statistically significant (χ2 = 8.107, P < 0.05 chi-square test); the corresponding GMTs were 255 ± 11 mIU/ml and 877 ± 11 mIU/ml respectively (t = −11.755, P < 0.05, t test). While the differences in anti-HBs seroconversion rates with 5 μg of HepB and 10 μg of HepB in 10–15 y old boys were statistically significant (χ2 = 5.753, P < 0.05 chi-square test). The distribution of dosage-specific anti-HBs titers on the basis of sex stratificationis is shown in .
Table 3. Antibody seroconversion rates and GMTs after the different-dosage hepatitis B vaccine booster vaccination
After the third booster dose, the anti-HBs seroconversion rates with 5 or 10 μg of HepB were higher than those after the first booster dose (all P < 0.05); the anti-HBs GMTs in 5- to 9-y-old girls vaccinated with 5 μg of HepB were similar after the third and first booster dose, whereas the differences in the other corresponding GMTs are statistically significant (all P < 0.05; ) .
After the first booster dose, the age-specific anti-HBs seroconversion rates with 5 or 10 μg of HepB in boys were similar to that in girls.
After the first booster dose, the difference in anti-HBs seroconversion rate for revaccination both with 5 or 10 μg of HepB was statistically significant in children 5–9 y and 10–15 y of age (χ2 = 16.164 or 13.934, P < 0.05 chi-square test), whereas after the third booster dose, the anti-HBs seroconversion rates were similar.
Discussion
This study’s results showed that the post–third dose anti-HBs seroconversion rates and GMTs for booster vaccination with 5 and 10 μg HepB were at a high level in children 5–15 y of age. The results of this study are similar to the results of a study involving booster vaccination in non-and-low responsers reported by Wu.Citation28Specifically, a three-dose booster vaccination regimen with 10 or 5 μg of HepB is effective.
It is generally believed that individuals whose anti-HBs antibody titers ≥10 mIU/ml after vaccination with HepB will resist HBV infection.Citation31Although the anti-HBs seroconversion rates with a 3-dose booster vaccination were greater than those with a 1-dose booster vaccination, the post-single dose anti-HBs seroconversion rates for booster vaccination with 5 or 10 μg HepB were at high levels (>88%) in 5- to 15-y-old girls and 5- to 9-y-old boys, thus a single booster dose with 5 or 10 µg of HepB for the majority of such children can prevent HBV infection. In contrast, the rate for booster vaccination with 5 μg HepB was at lower levels(<85%) in 10- to 15-y-old boys, and it may be correlated with that the vaccinees, 10–15-y-old boys, were at the upper end of the age group for which 5 μg HepB is recommended in China and that the larger body mass index than the same age girls affected the response to the first hepatitis B booster; whereas the post-dose-one anti-HBs seroconversion rate for booster vaccination with 10 μg of HepB was at a high level (>90%) in 10- to 15-y-old boys, and was higher than that reported in Sprading PR et al. study,Citation32 which indicates one dose of 5 μg HepB is insufficient for 10- to 15-y-old boys, whereas a single booster dose with 10 µg of HepB for 10- to 15-y-old boys is ideal.
In addition, this study’s results also show the post-single dose anti-HBs GMTs for booster vaccination with 10 μg of HepB were more than twice those with 3-dose 5 μg of HepB in children 5–9 y of age and were very similar to the anti-HBs GMTs with 3-dose 5 μg of HepB in children 10–15 y of age. The results of this study were higher than other reported results.Citation25,Citation33 A possible explanation for this difference was the use of different testing methods, and the serum anti-HBs antibody titers of the latter studies were measured using an ELISA or RIA.
Although the post-3 dose anti-HBs seroconversion rates and GMTs for vaccination with 10 or 5 μg of HepB were higher than the post-single dose rates and GMTs in children 5–15 y of age, a booster vaccination with one dose can reduce the number of needles. The small percentage of children (<8%) with anti-HBs titers less than protective levels after the first dose can be given an additional booster dose to improve their anti-HBs titers.
This study also showed that the proportion of anti-HBs titers (1–10 mIU/ml) in children aged 5- to 9-y-old who have anti-HBs titers less than protective levels was higher than that in children aged 10- to 15-y-old after primary immunization. The previous studies showed the immunization effect of booster vaccination was correlated with the pro-vaccination anti-HBs titers,Citation33,Citation34 and the duration of protection may be evaluated indirectly by measuring the anamnestic immune response to a booster dose of vaccine. This study showed that the same age and different sex children had similar anti-HBs seroconversion rates after the first booster dose and have an equal duration of protection, but the post-single dose anti-HBs seroconversion rates for children aged 5- to 9-y-old who were booster vaccinated with 5 or 10 μg of HepB were higher than those in children aged 10- to 15-y-old, which indicates that a shorter interval between primary immunization and booster vaccination gives a better response. The results of this study were similar to other reported studies.Citation35-Citation38Thus, the anti-HBs titer should be monitored regularly to screen for anti-HBs-negative children at the time of the students’ annual examinations. Booster vaccination can then be given to increase the antibody levels for these children.
This study had some limitations. First, it was not possible to identify the reasons that some children had lower than protective levels of anti-HBs titers before booster vaccination. Possibly, this may be due to non-responsiveness to primary immunization or a decrease in the antibody titer over time. Second, we could not collect blood samples from each subject and detect antibody titer after the second booster dose because of field operation constraints, so that we could not analyze the effects of the second and the third booster doses. In Clemens R et al. study,Citation39 all non-responders developed anti-HBs levels ≥100 mIU/ml after the third booster dose, and all low-responders reached this level after the second booster dose; whereas in this study, 99.6% of anti-HBs negative children developed anti-HBs levels ≥10 mIU/ml after the third booster dose, thus this may have had an minimal impact on the results. Finally, this study used vaccines produced by different companies to compare the immunization effects of different dosages, and it is possible that the use of HepB produced by different companies caused a small effect in this study.
In conclusion, the immunization effects of booster vaccination with 3 doses of HepB with 5 or 10 µg are effective; a single booster dose with 10 µg of HepB for 10–15-y-old boys and with 5 or 10 µg of HepB for 5–9 y old boys and for 5–15-y-old girls are effective in generating protective antibody against HBV; however, for anti-HBs-negative children after a single dose of booster, 3 doses are needed.
Materials and Methods
Study participants
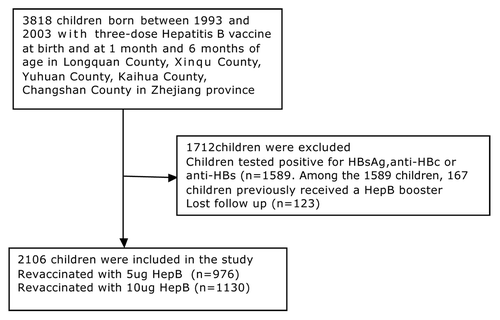
This research was performed in Longquan County, Xinqu County, Yuhuan County, Kaihua County, and Changshan County in Zhejiang province, in 2009–2010; two towns were selected in each county as research sites. Sample subjects were clustered based on school enrolment. Children were selected who were born between 1993 and 2003, and who had received three vaccinations against the hepatitis B virus: at birth, at 1 mo and at 6 mo of age (children born between 1993 and 1997 received 10 μg hepatitis B Vaccine prepared from plasma; children born between 1998 and 2003 received 5 μg recombinant hepatitis B vaccine), Children who had previously received booster vaccinations were excluded from this study, as were children who tested positive for HBsAg, anti-HBs or antibody to hepatitis B c antigen (anti-HBc). A flowchart of the participants enrolled in the study is summarized in . We ascertained whether children had received a primary hepatitis B vaccine by checking their vaccination certificates. Finally, the children’s ages were calculated on the survey date, and then rounded to the nearest whole number in years. This study was approved by the Institutional Review Board of the Zhejiang Center for Disease Control and Prevention, and written informed consent was obtained from every parent.
Figure 1. Flow chart of the participants enrolled in the study. A total of 3818 children born between 1993 and 2003 with three-dose Hepatitis B vaccine were reviewed. Of the 3818 children, 1712 children were excluded. Thus, 2106 children were included and were vaccinated with HepB in the study.
Specific inclusion criteria were as follows:
Born between May 1, 1993 and September 30, 2003 and vaccinated against HBV at 0, 1, and 6 mo;
Never received a Hepatitis B vaccine booster;
Parental willingness to participate in the follow-up study and to have their child’s blood sampled after vaccination;
Stable home address since birth;
No acute illness within the previous 7 d; no fever within the past 3 d (armpit temperature ≥38 °C); and no allergies or severe reaction to vaccination.
All information regarding the study was provided to the parents, and the consent form was signed by them.
Designs and Methods
Methods
After acquiring informed consent from the parents or guardians, 3 ml blood samples were collected from each subject. Booster vaccinations of Hepatitis B vaccine (lot number: 20090309 (01–06), dosage: 10 μg HBsAg; produced by Dalianhanxin Biotechnology Co Ltd. lot number: 20071223(1–9), dosage: 5 μg HBsAg; produced by Shenzhenkangtai Biotechnology Co Ltd.) were administered by intramuscular injection in the upper arm deltoid according to the immunization procedure used at months 0, 1, and 6. One month after the first and the third dose of booster vaccine injections, 3 ml blood samples were collected from each subject and preserved for testing.
Lab testing and sample processing
Frozen separated serum samples were sent to ADICON Clinical Laboratories, Inc. in Hangzhou for quantification of HBsAg, anti-HBs, and anti-HBc by chemiluminescence immunoassay (CLIA). Samples with anti-HBs ≥ 1000 mIU/ml were diluted for further testing, while samples with anti-HBs ≥ 15 000 mIU/ml were excluded from further analysis to avoid large errors in the results.
Apparatus and reagents
An Architect-i2000 (Abbott) was used for the chemical luminescence immunoassay. The reagent lot number for the HBsAg tests was 70318HN00 (Abbott Laboratories). An signal to noise(S/N) ratio ≥ 0.05 was considered to be positive. The reagent lot number for the anti-HBs tests was 75684M100 (Abbott Laboratories), and an anti-HBs ≥ 10 mIU/ml was considered to be positive and to provide protection against HBV infection. The reagent lot number for the anti-HBc tests was 72448M100 (Abbott Laboratories), and an anti-HBc ≥ 1 mIU/ml was considered to be positive.
Data analysis
A database EpiData3.2 (EpiData) was established, and statistical analysis was performed using SPSS 18.0 and Excel 2003. We used the McNemar test for related samples enumeration data and Chi-square test or the Fisher exact test for independent samples enumeration data and the Student t test for independent sample measurement data (normal continuous variables) and Wicoxon Singed-Rank test for related samples measurement data. A two-tailed probability was used in statistical tests, with an α of 0.05 considered to be significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the children and parents who volunteered to participate in this study and to the doctors in Longqua, Xinqu, Yuhuan, Kaihua, Changshan counties Center for Disease Control and Prevention. This work was supported by the National Scientific and Technological Major Project of China (No.2008ZX10002-001) and grant from the scientific research fund of medical and health in Zhejiang province (No.2009A035 and No.2012ZHB001).
References
- Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med 2004; 350:1118 - 29; http://dx.doi.org/10.1056/NEJMra031087; PMID: 15014185
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11:97 - 107; http://dx.doi.org/10.1046/j.1365-2893.2003.00487.x; PMID: 14996343
- Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005; 34:1329 - 39; http://dx.doi.org/10.1093/ije/dyi206; PMID: 16249217
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009; 27:6550 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.08.048; PMID: 19729084
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis 2009; 200:39 - 47; http://dx.doi.org/10.1086/599332; PMID: 19469708
- World Health Organization. Hepatitis B vaccines. WklyEpidemiol Rec 2009; 84:405 - 19; PMID: 19817017
- Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology 2001; 120:1828 - 53; http://dx.doi.org/10.1053/gast.2001.24839; PMID: 11375963
- West DJ, Margolis HS. Prevention of hepatitis B virus infection in the United States: a pediatric perspective. Pediatr Infect Dis J 1992; 11:866 - 74; http://dx.doi.org/10.1097/00006454-199210000-00012; PMID: 1408488
- DaiZC, QiGM. Epidemiological serosurvey of hepatitis A in China. In: Dai ZC, ed. Viral hepatitis in China seroepidemiological survey in Chinese population. Beijing: Science and technology literature press.1997; 1-28.
- Tsang SW, Sung JJ. Inactivated hepatitis A vaccine in Chinese patients with chronic hepatitis B infection. Aliment PharmacolTher 1999; 13:1445 - 9; http://dx.doi.org/10.1046/j.1365-2036.1999.00628.x; PMID: 10571600
- He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, et al. Major causes of death among men and women in China. N Engl J Med 2005; 353:1124 - 34; http://dx.doi.org/10.1056/NEJMsa050467; PMID: 16162883
- Zhang H. Epidemiology of hepatitis. Chin J Vaccines Immunoreaction 2004; 10:180 - 1
- Chang MH, Hadzic D, Rouassant SH, Jonas M, Kohn IJ, Negro F, Roberts E, Sibal A, Asian Pan-Pacific Society for Pediatric Gastroenterology, Hepatology and Nutrition. Acute and chronic hepatitis: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J PediatrGastroenterolNutr 2004; 39:Suppl 2 S584 - 8; http://dx.doi.org/10.1097/00005176-200406002-00002; PMID: 15184756
- Kato H, Nakata K, Hamasaki K, Hida D, Ishikawa H, Aritomi T, Nakao K, Kato Y, Yano M, Eguchi K. Long-term efficacy of immunization against hepatitis B virus in infants at high-risk analyzed by polymerase chain reaction. Vaccine 1999; 18:581 - 7; http://dx.doi.org/10.1016/S0264-410X(99)00320-5; PMID: 10547415
- Chathuranga LS, Noordeen F, Abeykoon AM. Immune response to hepatitis B vaccine in a group of health care workers in Sri Lanka. Int J Infect Dis 2013; 17:e1078 - 9; http://dx.doi.org/10.1016/j.ijid.2013.04.009; PMID: 23810225
- Elrashidy H, Elbahrawy A, El-Didamony G, Mostafa M, George NM, Elwassif A, Saeid Mohamed AG, Elmestikawy A, Morsy MH, Hashim A, et al. Antibody levels against hepatitis B virus after hepatitis B vaccination in Egyptian diabetic children and adolescents. Hum VaccinImmunother 2013; 9; Forthcoming http://dx.doi.org/10.4161/hv.25426; PMID: 23787761
- Viviani S, Jack A, Hall AJ, Maine N, Mendy M, Montesano R, Whittle HC. Hepatitis B vaccination in infancy in The Gambia: protection against carriage at 9 years of age. Vaccine 1999; 17:2946 - 50; http://dx.doi.org/10.1016/S0264-410X(99)00178-4; PMID: 10462228
- Zhao DS, Shen Y. Analysis on the Infection of Hepatitis B Vir al in Lucheng of Wenzhou. Chinese Primary Health Care 2007; 21:49 - 50
- Hua YP, Chen XY, Su MF, Li ST. HBV Infection among the community population in Yuhuan county, Zhejiang province. Dis Surveill 2008; 8:752 - 4
- ZeWY. Practice on planned immunology. In: Diao LD, ed. Planned Immunology. 2nd edition. Shanghai: Science and technology literature press.2001, 29-65.
- Hsu HY, Chang MH, Liaw SH, Ni YH, Chen HL. Changes of hepatitis B surface antigen variants in carrier children before and after universal vaccination in Taiwan. Hepatology 1999; 30:1312 - 7; http://dx.doi.org/10.1002/hep.510300511; PMID: 10534356
- Beran J. Bivalent inactivated hepatitis A and recombinant hepatitis B vaccine. Expert Rev Vaccines 2007; 6:891 - 902; http://dx.doi.org/10.1586/14760584.6.6.891; PMID: 18377352
- Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet 2000; 355:561 - 5; http://dx.doi.org/10.1016/S0140-6736(99)07239-6; PMID: 10683019
- Ren H, Li YT, Wu HY, Yu Y. Immunity status of hepatitis B vaccine immunized population and influence factors in anti-HBs declined group. Shanghai Jourmal of Preventive Medicine 2010; 22:121 - 3
- Gong ZY, Chen EF, Shi GX. Study on immunization effects of 5ug and 10ug doses and two types Genetic Engineering Hepatitis B vaccine after reinforce immunization. Chin J Publ Health 2002; 18:479 - 80
- Gilca V, De Serres G, Boulianne N, Murphy D, Ouakki M, De Wals P, Trudeau G, Massé R, Dionne M. Long-term persistence of immunity after vaccination of pre-adolescents with low doses of a recombinant hepatitis B vaccine. Hum VaccinImmunother 2013; 9:1685 - 90; http://dx.doi.org/10.4161/hv.25015; PMID: 23744506
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: A 20-year follow-up study in Thailand. Hum VaccinImmunother 2013; 9:1679 - 84; http://dx.doi.org/10.4161/hv.24844; PMID: 23732904
- Wu ZH, Cui FQ, Gong XH. [Effect analysis on non-and-low response infants after revaccinated hepatitis B vaccine]. Zhongguo Yi Miao He Mian Yi 2010; 16:207 - 10; PMID: 20726258
- Wang FZ, Gong XH, Cui FQ, Chen XY, Zheng H, Wu ZH. Analysis on Risk Factors of Hepatitis B Virus Surface Antigen Prevalence among People Aged 1-14 Years in China. Zhongguo Yi Miao He Mian Yi 2012; 18:118 - 22
- Qi YL, Wang FZ, Gong XH, Pan L, Zhen XJ, Li H. Evaluation of health economics on the different reginlens of booster immunization and screening scheme before hepatitis B vaccination in Beijin. Chinese Journal of Disease Control and Prevention 2004; 8:393 - 5
- Zanetti AR, Mariano A, Romanò L, D’Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, et al, Study Group. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet 2005; 366:1379 - 84; http://dx.doi.org/10.1016/S0140-6736(05)67568-X; PMID: 16226616
- Spradling PR, Xing J, Williams R, Masunu-Faleafaga Y, Dulski T, Mahamud A, Drobeniuc J, Teshale EH. Immunity to hepatitis B virus (HBV) infection two decades after implementation of universal infant HBV vaccination: association of detectable residual antibodies and response to a single HBV challenge dose. Clin Vaccine Immunol 2013; 20:559 - 61; http://dx.doi.org/10.1128/CVI.00694-12; PMID: 23408522
- Jafarzadeh A, Zarei S, Shokri F. Low dose revaccination induces robust protective anti-HBs antibody response in the majority of healthy non-responder neonates. Vaccine 2008; 26:269 - 76; http://dx.doi.org/10.1016/j.vaccine.2007.10.044; PMID: 18037544
- Yao J, Ren J, Shen L, Chen Y, Liang X, Cui F, Li Q, Jiang Z, Wang F. The effects of booster vaccination of hepatitis B vaccine on anti-HBV surface antigen negative children 11-15 years after primary vaccination. Hum Vaccin 2011; 7:1055 - 9; http://dx.doi.org/10.4161/hv.7.10.15990; PMID: 21989290
- Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, Su IJ, Kuo HS, Huang LM, Chen DS, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15-18 years after neonatal immunization. J Infect Dis 2008; 197:1419 - 26; http://dx.doi.org/10.1086/587695; PMID: 18444799
- Su FH, Cheng SH, Li CY, Chen JD, Hsiao CY, Chien CC, Yang YC, Hung HH, Chu FY. Hepatitis B seroprevalence and anamnestic response amongst Taiwanese young adults with full vaccination in infancy, 20 years subsequent to national hepatitis B vaccination. Vaccine 2007; 25:8085 - 90; http://dx.doi.org/10.1016/j.vaccine.2007.09.013; PMID: 17920732
- Chaves SS, Groeger J, Helgenberger L, Auerbach SB, Bialek SR, Hu DJ, Drobeniuc J. Improved anamnestic response among adolescents boosted with a higher dose of the hepatitis B vaccine. Vaccine 2010; 28:2860 - 4; http://dx.doi.org/10.1016/j.vaccine.2010.01.059; PMID: 20153793
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Bock HL, Leyssen M, Jacquet JM. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine 2010; 28:730 - 6; http://dx.doi.org/10.1016/j.vaccine.2009.10.074; PMID: 19892043
- Clemens R, Sänger R, Kruppenbacher J, Höbel W, Stanbury W, Bock HL, Jilg W. Booster immunization of low- and non-responders after a standard three dose hepatitis B vaccine schedule--results of a post-marketing surveillance. Vaccine 1997; 15:349 - 52; http://dx.doi.org/10.1016/S0264-410X(96)00205-8; PMID: 9141203
