Abstract
Developing an effective adult prophylaxis vaccine is a high priority in the global control of tuberculosis (TB), because TB remains an important public health problem and the current widely used BCG vaccine provides effective protection only for children but variable protection against adult TB. BCG priming-heterologous vaccines booster and recombinant BCG technologies have been thought as two important regimens for inducing effective protection against adult TB. Obviously, defining the protective efficacy of the two regimens would benefit more rational design of the future adult TB vaccines. In this study, a recombinant BCG strain (rBCG::685A) expressing the fusion protein of ESAT-6 and Ag85A (r685A) of Mycobacterium tuberculosis was constructed successfully and the secretion of r685A protein from rBCG strain was confirmed by western blotting with anti-ESAT-6 and anti-Ag85A polyclonal antibodies, respectively. The immune responses and protective effects in rBCG::685A vaccinated C57BL/6 mice were compared with that of our previous reported BCG prime-pcD685A booster regimen. Boosting BCG with pcD685A DNA elicited higher level of r685A protein specific IFN-γ secreted by splenocytes and a more significant increase of both TNF-α and iNOS responses in the lung, thus providing better control of bacterial growth in both lung and spleen of immunized mice challenged with virulent M. tuberculosis, compared with mice vaccinated with rBCG::685A or BCG alone. Our results have implications for development of more effective adult TB vaccines for improved control of TB.
Introduction
Tuberculosis (TB) remains a serious threat to human health with 8.7 million new cases of TB and 1.4 million TB-related deaths worldwide.Citation1 Although Mycobacterium bovis Bacille Calmette-Guerin (BCG), currently the only vaccine available against TB, has effective protection against the severe forms of TB in children,Citation2 it has unstable protective effects for adult pulmonary TB,Citation3 which accounts for more than 94% of all TB cases.Citation1 Therefore, more effective vaccines against adult TB are urgently needed.
Up to now, two regimens for developing adult TB vaccines have been generally accepted and achieved significant progress.Citation4 One is prime-boost strategyCitation5 where BCG priming followed by heterologous subunit vaccine booster has been a favored approach for providing protection against adult TB.Citation6,Citation7 To take advantage of the proven efficacy of BCG in children, BCG might first be used for neonatal vaccination as in most countries worldwide.Citation8 When the effects of BCG start to wane during adolescent, heterologous booster vaccines such as subunit vaccines,Citation9-Citation11 DNA vaccines,Citation12-Citation16 recombinant adenovirus,Citation17,Citation18 and modified vaccinia virus,Citation19-Citation22 could further be used to extend the protective efficacy of BCG priming. This strategy would benefit a significant proportion of the global population, because about 4 billion people have been immunized with BCG since 1921. The other strategy also relies on present BCG vaccination strategy and aims to replace BCG vaccine by recombinant BCG (rBCG) technology. rBCG strains could induce longer-lasting immune responses and provide stronger protection for adults than BCG after neonatal immunization, developing by different methods such as expressing immunodominant antigens of M. tuberculosis,Citation23,Citation24 deletion antigens from BCG,Citation25 cytokines, or combination,Citation26,Citation27 and enhanced CD8+ T-cell responses,Citation28 etc. Clearly, defining the protective efficacy of the two regimens would benefit more rational design of the future adult TB vaccines.
In our previous study, a candidate DNA vaccine pcD685A expressing the fusion protein of ESAT-6 and Ag85A (r685A) of M. tuberculosis was constructed and pcD685A could act as an effective BCG booster, which results in a significant reduction in bacterial load of both spleen and lung of BCG priming C57BL/6 mice challenged with virulent M. tuberculosis H37Rv, when compared with BCG or pcD685A alone.Citation15 In the present study, a recombinant BCG strain expressing the fusion protein of ESAT-6 and Ag85A (rBCG::685A) was also constructed. The immune response and protective efficacy induced separately by rBCG::685A and BCG prime-pcD685A boost were evaluated and compared in vaccinated C57BL/6 mice.
Results
rBCG::685A expressing the secreted r685A fusion protein
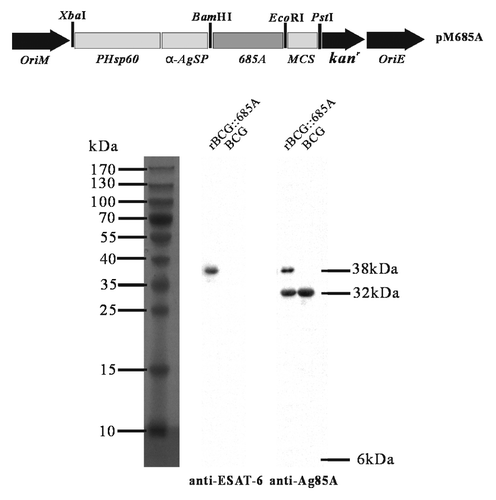
The Mycobacterium-E.coli shuttle expression plasmid pM685A () was successfully constructed and confirmed by double-digestion with the BamHI and EcoRI and DNA sequencing. The secretory expression of r685A fusion protein, with a molecular size about 38kDa, was confirmed only in the culture supernatants of rBCG::685A, whatever anti-Ag85A or anti-ESAT-6 antibodies were used (). In addition, Ag85A protein with a size of about 32kDa in the culture supernatant of both BCG and rBCG::685A was demonstrated by western blotting with anti-Ag85A antibody (). No expression of ESAT-6 protein in the culture supernatant of both BCG and rBCG::685A was demonstrated as expectedCitation29,Citation30 by western blotting with anti-ESAT-6 antibody ().
Figure 1. Recombinant Mycobacterium–E.coli shuttle plasmid pM685A and the secretory expression of r685A fusion protein from rBCG::685A. The plasmid pM685A containing the full-fragment of ESAT-6 and Ag85A was engineered to express the secreted r685A protein under the direction of Hsp60 promoter (PHsp60) and α-Ag signal peptide (α-AgSP). The expression of the r685A fusion protein in the culture supernatant from rBCG::685A was confirmed by western blotting with anti-ESAT-6 rabbit antibody or anti-Ag85A chicken polyclonal antibody. The cultural supernatant of BCG was used as negative control.
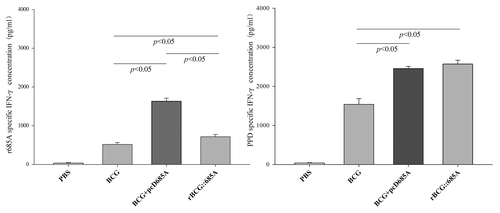
Higher levels of r685A specific IFN-γ secreted by splenocytes of mice vaccinated with BCG prime-pcD685A booster than rBCG::685A
To determine cell-mediated immune responses to vaccine candidates, splenocytes were isolated from immunized mice following stimulation with r685A and PPD, respectively. The level of IFN-γ production by splenocytes determined by ELISA was shown in . Upon stimulation with PPD or r685A, all BCG vaccinated groups produced higher levels of IFN-γ than PBS control mice (P < 0.05) and higher levels of IFN-γ were induced in both rBCG::685A and BCG prime-pcD685A booster groups than BCG (P < 0.05). Most importantly, higher levels of r685A specific IFN-γ were secreted by splenocytes of mice vaccinated with BCG prime-pcD685A booster than rBCG::685A (P < 0.05).
Figure 2. The level of IFN-γ secreted by splenocytes of different group mice detected by ELISA (n = 5). Nine weeks after immunization, splenocytes were obtained from 5 mice in each group were prepared individually and 106 cells were added into each well of 96-well microtiter plates. PPD (10 μg/ml), r685A protein (5 μg/ml) in complete RPMI-1640 medium were used as the stimulator, respectively. The results were shown as mean ± SD (pg/ml).
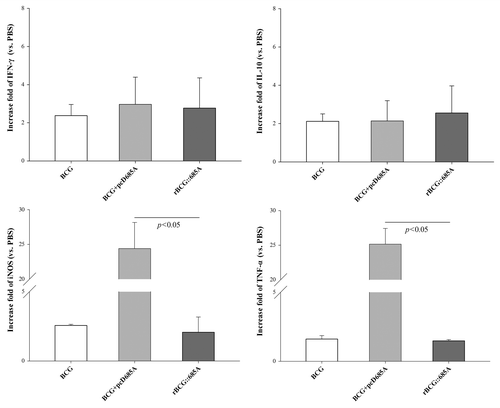
Higher mRNA levels of TNF-α and iNOS in the lung of mice vaccinated with BCG prime-pcD685A booster than rBCG::685A
As shown in , the mRNA expression level of iNOS, cytokines IFN-γ, TNF-α, and IL-10 in the lung of vaccinated mice were detected by qRT-PCR. When compared with control mice, the expression of these molecules in the different vaccinated groups increased at different degrees. Especially, all BCG based vaccination strategies increased the expression of IFN-γ and IL-10, though there was no statistics significant between these different groups. Notably, the expression of both TNF-α and iNOS in the lung of BCG prime-pcD685A booster vaccination mice increased most significantly than BCG and rBCG::685A groups (P < 0.05).
Figure 3. mRNA levels of IFN-γ, IL-10, TNF-α, and iNOS in the lung of different vaccinated mice detected by qRT-PCR (n = 5). Tested cDNAs were normalized to the endogenous RNA levels of the internal control GAPDH. The fold increase of gene expression in vaccinated group was calculated using 2−ΔΔCT method. The results were shown as mean ± SD.
Better protective effect conferred by BCG prime-pcD685A booster than rBCG::685A
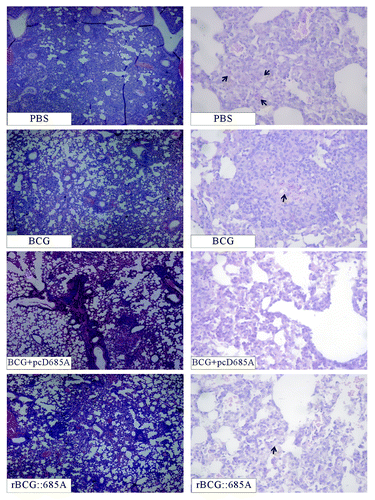
Compared with the control group, the bacterial load in both lung and spleen was significantly decreased in all BCG vaccinated groups. Among these groups, BCG prime-pcD685A booster vaccinated mice resulted in the most significant reduction of bacterial load in the lung and spleen (P < 0.05). In addition, the difference of bacterial load in both organs between rBCG::685A and BCG vaccinated groups had no statistics significance (P > 0.05) (). The hierarchy of protection based on the bacterial load in the lung was consistent with the observation under microscopy after tissue sections of the lung from different groups were stained by acid-fast staining (). Acid-fast bacilli (AFB) were present in the whole lung tissue section of control mice, and the most serious lung pathology with extensive fibrosis, perivasculitis, and alveolitis was also observed in this group. Much fewer AFB and less lung inflammation were observed in alveolar tissue from mice vaccination with BCG plus pcD685A boosting than those in rBCG::685A or BCG alone ().
Figure 4. Bacterial load per lung and spleen in C57BL/6 mice (n = 7). Nine weeks later, different vaccinated mice were challenged i.v. with 106 CFU of M. tuberculosis H37Rv. Four weeks after challenge, lungs and spleens were homogenized and cultured for bacterial load. The results were shown as the mean (± SEM) per group.
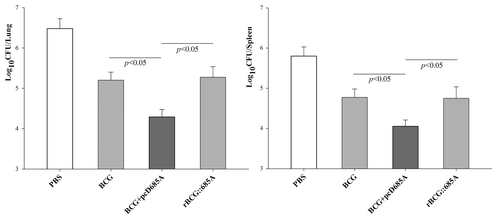
Figure 5. Representative lung pathology of different vaccinated C57BL/6 mice after infection. Four weeks post-challenge, the right lung from different vaccinated mice was fixed and embedded for HE staining (10 × 4, left row) and acid-fast staining (10 × 40, right row). Arrows indicate acid-fast staining positive bacteria.
Discussion
As a high priority in global TB control, developing more effective vaccines against adult TB than BCG has been attracting a lot of attention during last two decades.Citation4 Up to now, 14 vaccine candidates have been assessed in clinical phase and more than 200 vaccine candidates have been preclinically evaluated. However, there are no other candidates based on different platforms except BCG, which provide stronger protective efficacy than BCG so far. More than 90% of infants worldwide are vaccinated with BCG annually since the Expanded Programme on Immunization (EPI) was established by WHO in 1974Citation8 and BCG provides over 80% efficacy against childhood TB with confirmed safety. Therefore, trying to substitute BCG with other regimens might not be reasonable and practical. Correspondingly, developing a vaccine as an effective booster of BCG vaccine or designing more effective rBCG to replace BCG vaccine may be ideal, regardless of current immunization strategy. In addition, both regimens might be more effective than BCG, as evidenced in recent studies in animal models, even in clinical experiments.Citation7,Citation16,Citation20,Citation21,Citation28 Obviously, comparing the protective efficacy of two regimens and choosing the suitable type play an important role in the development of adult TB vaccines. In this study, immunogenicity and protective efficacy of two regimens were compared in vaccinated C57BL/6 mice. Our results demonstrated that the protective effects induced by BCG prime-DNA booster are better than that of the recombinant BCG expressing the same two antigens ESAT-6 and Ag85A, as evidenced by higher levels of r685A specific IFN-γ secreted by splenocytes, a more significant increase of TNF-α and iNOS levels in the lung and better control of M. tuberculosis growth in both lung and spleen of vaccinated mice. To our knowledge, this is the first report on the differential protective efficacy of both BCG prime-DNA booster and rBCG regimens based on the same antigens.
ESAT-6 is a highly dominant T cell antigen with 6kDa molecular weight,Citation31 which is encoded by RD1 region of M. tuberculosis and is missing in all BCG substrains distributed around the world.Citation32 Antigen Ag85A (32kDa) has mycolyl-transferase activity and plays an important role in the biogenesis of cell wall of mycobacteria.Citation33 As promising vaccine candidates for TB, both antigens were designed as the fusion protein r685A in our previous workCitation15 and we also confirmed that the whole blood cells from TST-positive healthy household contacts stimulated with the protein resulted in higher level of antigen-specific IFN-γ release than TST-negative population.Citation15 We constructed a DNA vaccine pcD685A expressing the fusion r685A protein and confirmed its protective effect as an effective booster vaccine of BCG.Citation15 In this study, r685A protein with the same size as the product purified from recombinant E.coli, was confirmed only in the culture supernatant of rBCG::685A strain as demonstrated by western blotting, which indicated that Hsp60 promoter and α-AgSP correctly directed the expression of secreted r685A protein from the rBCG strain. Although splenocytes from rBCG::685A immunized mice secreted higher level of PPD or r685A protein specific IFN-γ than those of BCG, rBCG::685A did not provide any enhanced protection against M. tuberculosis challenge in the both lung and spleen, when compared with BCG vaccine. Several recombinant BCG strains expressing 38kDa proteinCitation34 or ESAT-6 proteinCitation30 were reported no improving protection when vaccinated mice were challenged M. tuberculosis for 4 weeks. rBCG expressing the fusion protein of ESAT-6 and one member Ag85A complex, Ag85B, also did not improve the short-time protection efficacy against TB in a mouse model.Citation35,Citation36 Meanwhile, DNA vaccine pE6/85B which consisted of ESAT-6 and Ag85B fusion gene with tpa signal sequence could provide the protection against M. tuberculosis infection in vaccinated mice and be used as an effective booster after BCG immunization,Citation16 which provided further evidence to support our conclusion. Moreover, there are some rBCG strains, such as rBCG30,Citation23 rBCG::AB,Citation24 rBCG::X,Citation37 rBCG::85B-Rv3425,Citation38 rBCG UreC: Hly,Citation28 and AERAS-rBCG,Citation39 etc, providing stronger and/or longer-lasting protective efficacy than BCG in animal models or in humans. All of these studies indicate that developing more recombinant BCG vaccine candidates mainly depend on the choice of suitable antigens. Moreover, the route and efficacy of antigen presentation could determine immune responses and protection effect induced by vaccination. BCG prime-pcD685A DNA boosted mice produced the higher levels of r685 protein specific IFN-γ secreted by splenocytes and higher mRNA expression of TNF-α and iNOS in the lung of vaccinated mice and provided the stronger protection against the challenge infection of M. tuberculosis than rBCG::685A. Though pcD685A DNA and rBCG were separately used to express the same protein r685A, differential degrees of cell-mediated immune responses were induced between rBCG and BCG prime-DNA booster vaccinated mice. These might account for the different immune protective mechanisms of DNA vaccine, rBCG, and prime-boost strategies, although exact mechanism remains unknown.
Upon aerosol exposure with M. tuberculosis, alveolar macrophages and lung DCs phagocytose M. tuberculosis. Some components of M. tuberculosis such as ManLAM would inhibit infected macrophages to produce TNF-α, which might delay the influx of APCs in the infected site and the migration of these M. tuberculosis-loaded APCs to the local lymph node.Citation40 In addition, ManLAM could promote infected DCs to secret IL-10,Citation41 which might participate in the process of immune evasion by suppressing T-cell priming in the local lymph node. Further, priming T lymphocytes were delayed to recruit to infected local site and protective immune responses were difficult to establish under this situation. However, if priming T lymphocytes are recruited to the lung, they could produce both TNF-α that might have an antagonistic effect of ManLAM, and IFN-γ that might act on new recruitment macrophages to trigger the expression of antimicrobial effectors, including iNOS, which could generate the production of nitric oxide (NO) and inhibit the growth of intracellular M. tuberculosis.Citation42 Therefore, based on the host-pathogen interaction, the expression level of the aforementioned molecules in the lung of vaccinated mice could play a vital role in determining the outcome of M. tuberculosis infection as evidenced by recent studies.Citation43,Citation44 In our study, the highest levels of r685 protein specific IFN-γ secreting by splenocytes and the most significant expression of TNF-α and iNOS in the lung were induced in BCG prime-pcD685A DNA boosted mice, which also provided the strongest protection against M. tuberculosis challenge infection among all vaccinated groups.
Boosting BCG with BCG has no proven benefit in many clinical trials and experimental animals,Citation6 and BCG revaccination had been ceased by WHO since 1995.Citation6 While MVA85A/AERAS-485 in a Phase 2b study was evaluated as a boost to BCG and was not effective, it does not negate the potential for a boost to BCG, either in infancy or adolescence/adulthood.Citation45 In the future, the rational design of adult TB vaccines will need to confirm the protective effect of combination BCG or effective enhanced rBCG strains as prime vaccine and heterogolous boost strategy to identify the best candidate for clinical testing.
Materials and Methods
Bacterial strains and culture conditions
M. tuberculosis H37Rv, M. bovis BCG China, and rBCG strains were separately grown in either Middlebrook 7H9 medium (Difco Laboratories) or on Middlebrook 7H11 agar (Difco Laboratories), supplemented with 10% ADC, 0.5% glycerol, and 0.05% Tween 80. Escherichia coli strains DH5α and BL21 (DE3) were grown in Luria-Bertani medium and used for cloning and expression in E. coli, respectively. When required, a final concentration of 25 µg/ml kanamycin or 100 µg/ml ampicillin was used for agar plate or liquid culture medium.
Construction of recombinant BCG strain
The gene fragment from the Mycobacterium-E.coli shuttle plasmid pDE22 digested with XbaI and PstI, containing the DNA sequences of Hsp60 promoter (PHsp60), α-Ag signal peptide of BCG (α-AgSP), and multi-clone sites (MCS), was first ligated into the corresponding sites of pMV261 to construct the pMS261. Then, the full-length gene encoding the fusion protein of ESAT-6 and Ag85A of M. tuberculosis was isolated from pcD685A by digesting the plasmid with BamHI and EcoRI and ligated into BamHI/EcoRI-digested pMS261 vector, yielding the recombinant plasmid pM685A. The anticipated sequence of the insert was confirmed by DNA sequencing and enzyme digestion. Finally, pM685A was introduced into BCG China strain by electroporation and the recombinant BCG strain (rBCG::685A) was screened on kanamycin resistance plates. The recombinant plasmid construct rBCG::685A was engineered to express the recombinant fusion protein r685A under the direction of PHsp60 and α-AgSP.
Culture supernatants of rBCG::685A and BCG from 7H9 broth were prepared as previously described.Citation24 About 40 µg protein of each sample was separated by SDS-PAGE on a 12% gel and then analyzed by western blotting. The expression of r685A protein was detected using anti-ESAT-6 rabbit antibody (diluted 1/2000; ab45073, Abcam) or anti-Ag85A chicken polyclonal antibody (diluted 1/2000; ab14073, Abcam). The immunoblots were developed with a pro-light ECL kit according to the manufacture’s protocol (Tiangen) and were imaged by Bio-Rad Universal Hood II Gel Imager.
Mice and immunization protocol
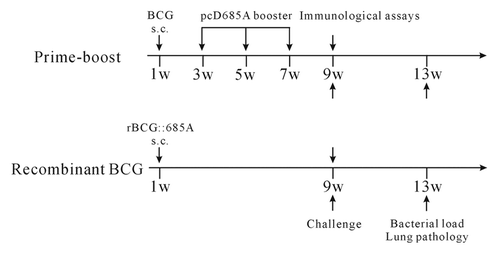
Specific pathogen-free, female C57BL/6 mice at 6 weeks of age (Beijing Vital River) were used in this study. Mice were fed commercial mouse chow and water ad libitum. Mice were immunized s.c. once at the base of the tail with 1 × 106 CFU of either BCG or rBCG::685A in a volume of 100 μl of PBS. pcD685A was used to boost thrice as described previouslyCitation15 and the protocol was performed as shown in . A control group was treated with 100 μl of PBS. Animal experiments were repeated twice. Animal experiments were performed in accordance with the guidelines of Chinese Council on Animal Care. The research protocol was approved by Tongji Medical School Committee on Biosafety.
Figure 6. Immunization scheme for 2 regimens. For prime-boost regimen, C57BL/6 mice were first immunized s.c. with a dose of 106 CFU of BCG, then 100 μg pcD685A DNA was injected intramuscularly 2 weeks later and repeated twice with 2-week intervals. For recombinant BCG regimen, mice were vaccinated once with 106 CFU of rBCG::685A. PBS was used as negative control. Nine weeks after the immunization, 7 mice were challenged with 106 CFU M. tuberculosis H37Rv and 5 mice were used for the immunological assays. All experiments were repeated twice.
Antigen specific IFN-γ secreted by splenocytes detected by ELISA
Nine weeks after C57BL/6 mice were vaccinated, spleen cells from five mice per group were prepared individually and resuspended in complete RPMI-1640 medium (Invitrogen) with 10% fetal calf serum. The concentration of cell suspension was adjusted to 107 cells /ml and 100 μl of cells were added into each well in 96-well microtiter plates. One hundred microlitres of PPD (10 μg/ml, Statens Serum Institute) and r685A protein (5 μg/ml) in complete RPMI-1640 medium were used as the stimulator, respectively. RPMI-1640 medium was used as negative control. The plates were incubated at 37 °C in a humidified CO2 incubator for 72h. Supernatants were collected from each well and the concentration of IFN-γ in the supernatants was measured in duplicate by the mouse IFN-γ ELISA kit (Dakewei Biotech) with the detection limit of 8 pg/ml. The results were shown as mean ± SD (pg/ml) of five mice per group.
Cytokines and iNOS mRNA expression in the lung detected by qRT-PCR
DNA-free RNA samples from lung tissue of five vaccinated mice were extracted with TRIzol reagent (Invitrogen), respectively. qRT-PCR analysis was performed using SYBR Green kit (Roche) and Applied Rotor-Gene 3000 Real-Time system (Gene) with different specific primers listed in , including IFN-γ, IL-10, TNF-α, and iNOS. Tested cDNAs were normalized to the endogenous RNA levels of the internal control GAPDH. The fold increase of gene expression in vaccinated group was calculated using 2−ΔΔCT method.
Table 1. Primers and qRT-PCR cycling parameters for murine cytokines and iNOS
Efficacy comparison and histopathological analysis
Nine weeks after immunization, mice in different groups were challenged with a dose of 106 CFU M. tuberculosis in 100 μl PBS via tail vein. Four weeks post-challenge, 7 C57BL/6 mice per group were sacrificed for efficacy comparison and histopathological analysis. The spleens and lungs were removed aseptically, homogenized, and cultured for CFU of M. tuberculosis on Middlebrook 7H11 agar containing 2 μg/ml of 2-thiophenecarboxylic acid hydrazide (TCH, Beijing Luqiao Corp.) to selectively inhibit the growth of the residual BCG. Right lung lobes from different vaccinated C57BL/6 mice were fixed in 10% formalin in PBS and embedded in paraffin. Both Hematoxylin and Eosin (H&E) staining and acid-fast staining were used to record the pathological changes in the lung.
Data analysis
The Student t test was used to compare the difference between groups and the difference was considered statistically significant when P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants of the National Mega-Projects of Science Research for the 12th Five-Year Plan (2012ZX10003008-005) and 863 program (2012AA02A401).
References
- World Health Organization. Global tuberculosis report 2012. Available from: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994; 271:698 - 702; http://dx.doi.org/10.1001/jama.1994.03510330076038; PMID: 8309034
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995; 346:1339 - 45; http://dx.doi.org/10.1016/S0140-6736(95)92348-9; PMID: 7475776
- Kaufmann SH, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet 2010; 375:2110 - 9; http://dx.doi.org/10.1016/S0140-6736(10)60393-5; PMID: 20488515
- Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go?. PLoS Pathog 2012; 8:e1002607; http://dx.doi.org/10.1371/journal.ppat.1002607; PMID: 22589713
- Chen Z, Fan X. Research progress in BCG prime-boost vaccination strategy against tuberculosis. Chinese Journal of Antituberculosis 2012; 3:188 - 91
- Brennan MJ, Clagett B, Fitzgerald H, Chen V, Williams A, Izzo AA, Barker LF. Preclinical evidence for implementing a prime-boost vaccine strategy for tuberculosis. Vaccine 2012; 30:2811 - 23; http://dx.doi.org/10.1016/j.vaccine.2012.02.036; PMID: 22387630
- WHO. . http://apps.who.int/vaccines/globalsummary/immunization/timeseries/tswucoveragebcg.htm. 2013.
- Rouanet C, Debrie AS, Lecher S, Locht C. Subcutaneous boosting with heparin binding haemagglutinin increases BCG-induced protection against tuberculosis. Microbes Infect 2009; 11:995 - 1001; http://dx.doi.org/10.1016/j.micinf.2009.07.005; PMID: 19635582
- Brandt L, Skeiky YA, Alderson MR, Lobet Y, Dalemans W, Turner OC, Basaraba RJ, Izzo AA, Lasco TM, Chapman PL, et al. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect Immun 2004; 72:6622 - 32; http://dx.doi.org/10.1128/IAI.72.11.6622-6632.2004; PMID: 15501795
- Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, Andersen P. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol 2006; 177:6353 - 60; PMID: 17056566
- Mollenkopf HJ, Grode L, Mattow J, Stein M, Mann P, Knapp B, Ulmer J, Kaufmann SH. Application of mycobacterial proteomics to vaccine design: improved protection by Mycobacterium bovis BCG prime-Rv3407 DNA boost vaccination against tuberculosis. Infect Immun 2004; 72:6471 - 9; http://dx.doi.org/10.1128/IAI.72.11.6471-6479.2004; PMID: 15501778
- Fan X, Gao Q, Fu R. DNA vaccine encoding ESAT-6 enhances the protective efficacy of BCG against Mycobacterium tuberculosis infection in mice. Scand J Immunol 2007; 66:523 - 8; http://dx.doi.org/10.1111/j.1365-3083.2007.02006.x; PMID: 17916110
- Wang C, Chen Z, Fu R, Zhang Y, Chen L, Huang L, Li J, Shi C, Fan X. A DNA vaccine expressing CFP21 and MPT64 fusion protein enhances BCG-induced protective immunity against Mycobacterium tuberculosis infection in mice. Med Microbiol Immunol 2011; 200:165 - 75; http://dx.doi.org/10.1007/s00430-011-0188-z; PMID: 21340709
- Lu J, Wang C, Zhou Z, Zhang Y, Cao T, Shi C, Chen Z, Chen L, Cai C, Fan X. Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein. Clin Dev Immunol 2011; 2011:617892; http://dx.doi.org/10.1155/2011/617892; PMID: 21461375
- Derrick SC, Yang AL, Morris SL. A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection with Mycobacterium tuberculosis and boosts BCG-induced protective immunity. Vaccine 2004; 23:780 - 8; http://dx.doi.org/10.1016/j.vaccine.2004.07.036; PMID: 15542202
- Vordermeier HM, Villarreal-Ramos B, Cockle PJ, McAulay M, Rhodes SG, Thacker T, Gilbert SC, McShane H, Hill AV, Xing Z, et al. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect Immun 2009; 77:3364 - 73; http://dx.doi.org/10.1128/IAI.00287-09; PMID: 19487476
- Xing Z, McFarland CT, Sallenave JM, Izzo A, Wang J, McMurray DN. Intranasal mucosal boosting with an adenovirus-vectored vaccine markedly enhances the protection of BCG-primed guinea pigs against pulmonary tuberculosis. PLoS One 2009; 4:e5856; http://dx.doi.org/10.1371/journal.pone.0005856; PMID: 19516906
- Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guérin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol 2003; 171:1602 - 9; PMID: 12874255
- Hawkridge T, Scriba TJ, Gelderbloem S, Smit E, Tameris M, Moyo S, Lang T, Veldsman A, Hatherill M, Merwe Lv, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis 2008; 198:544 - 52; http://dx.doi.org/10.1086/590185; PMID: 18582195
- Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Mauff K, Hughes EJ, Moyo S, Brittain N, Lawrie A, et al. Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J Infect Dis 2011; 203:1832 - 43; http://dx.doi.org/10.1093/infdis/jir195; PMID: 21606542
- Pathan AA, Sander CR, Fletcher HA, Poulton I, Alder NC, Beveridge NE, Whelan KT, Hill AV, McShane H. Boosting BCG with recombinant modified vaccinia ankara expressing antigen 85A: different boosting intervals and implications for efficacy trials. PLoS One 2007; 2:e1052; http://dx.doi.org/10.1371/journal.pone.0001052; PMID: 17957238
- Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic’ S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A 2000; 97:13853 - 8; http://dx.doi.org/10.1073/pnas.250480397; PMID: 11095745
- Wang C, Fu R, Chen Z, Tan K, Chen L, Teng X, Lu J, Shi C, Fan X. Immunogenicity and protective efficacy of a novel recombinant BCG strain overexpressing antigens Ag85A and Ag85B. Clin Dev Immunol 2012; 2012:563838; http://dx.doi.org/10.1155/2012/563838; PMID: 22570667
- Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 2003; 9:533 - 9; http://dx.doi.org/10.1038/nm859; PMID: 12692540
- Tang C, Yamada H, Shibata K, Maeda N, Yoshida S, Wajjwalku W, Ohara N, Yamada T, Kinoshita T, Yoshikai Y. Efficacy of recombinant bacille Calmette-Guérin vaccine secreting interleukin-15/antigen 85B fusion protein in providing protection against Mycobacterium tuberculosis. J Infect Dis 2008; 197:1263 - 74; http://dx.doi.org/10.1086/586902; PMID: 18422438
- Xu Y, Zhu B, Wang Q, Chen J, Qie Y, Wang J, Wang H, Wang B, Wang H. Recombinant BCG coexpressing Ag85B, ESAT-6 and mouse-IFN-gamma confers effective protection against Mycobacterium tuberculosis in C57BL/6 mice. FEMS Immunol Med Microbiol 2007; 51:480 - 7; http://dx.doi.org/10.1111/j.1574-695X.2007.00322.x; PMID: 17919299
- Desel C, Dorhoi A, Bandermann S, Grode L, Eisele B, Kaufmann SH. Recombinant BCG ΔureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J Infect Dis 2011; 204:1573 - 84; http://dx.doi.org/10.1093/infdis/jir592; PMID: 21933877
- Wang LM, Shi CH, Fan XL, Xue Y, Bai YL, Xu ZK. Expression and immunogenicity of recombinant Mycobacterium bovis Bacillus Calmette-Guérin strains secreting the antigen ESAT-6 from Mycobacterium tuberculosis in mice. Chin Med J (Engl) 2007; 120:1220 - 5; PMID: 17697571
- Bao L, Chen W, Zhang H, Wang X. Virulence, immunogenicity, and protective efficacy of two recombinant Mycobacterium bovis bacillus Calmette-Guérin strains expressing the antigen ESAT-6 from Mycobacterium tuberculosis. Infect Immun 2003; 71:1656 - 61; http://dx.doi.org/10.1128/IAI.71.4.1656-1661.2003; PMID: 12654778
- Brandt L, Elhay M, Rosenkrands I, Lindblad EB, Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun 2000; 68:791 - 5; http://dx.doi.org/10.1128/IAI.68.2.791-795.2000; PMID: 10639447
- Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 1999; 284:1520 - 3; http://dx.doi.org/10.1126/science.284.5419.1520; PMID: 10348738
- Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 1997; 276:1420 - 2; http://dx.doi.org/10.1126/science.276.5317.1420; PMID: 9162010
- Castañon-Arreola M, López-Vidal Y, Espitia-Pinzón C, Hernández-Pando R. A new vaccine against tuberculosis shows greater protection in a mouse model with progressive pulmonary tuberculosis. Tuberculosis (Edinb) 2005; 85:115 - 26; http://dx.doi.org/10.1016/j.tube.2004.10.004; PMID: 15687035
- Shi C, Wang X, Zhang H, Xu Z, Li Y, Yuan L. Immune responses and protective efficacy induced by 85B antigen and early secreted antigenic target-6 kDa antigen fusion protein secreted by recombinant bacille Calmette-Guérin. Acta Biochim Biophys Sin (Shanghai) 2007; 39:290 - 6; http://dx.doi.org/10.1111/j.1745-7270.2007.00281.x; PMID: 17417685
- Palendira U, Spratt JM, Britton WJ, Triccas JA. Expanding the antigenic repertoire of BCG improves protective efficacy against aerosol Mycobacterium tuberculosis infection. Vaccine 2005; 23:1680 - 5; http://dx.doi.org/10.1016/j.vaccine.2004.10.007; PMID: 15705472
- Shi C, Chen L, Chen Z, Zhang Y, Zhou Z, Lu J, Fu R, Wang C, Fang Z, Fan X. Enhanced protection against tuberculosis by vaccination with recombinant BCG over-expressing HspX protein. Vaccine 2010; 28:5237 - 44; http://dx.doi.org/10.1016/j.vaccine.2010.05.063; PMID: 20538090
- Wang Jl, Qie Yq, Zhu Bd, Zhang Hm, Xu Y, Wang Qz, Chen Jz, Liu W, Wang Hh. Evaluation of a recombinant BCG expressing antigen Ag85B and PPE protein Rv3425 from DNA segment RD11 of Mycobacterium tuberculosis in C57BL/6 mice. Med Microbiol Immunol 2009; 198:5 - 11; http://dx.doi.org/10.1007/s00430-008-0098-x; PMID: 18491134
- Sun R, Skeiky YA, Izzo A, Dheenadhayalan V, Imam Z, Penn E, Stagliano K, Haddock S, Mueller S, Fulkerson J, et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine 2009; 27:4412 - 23; http://dx.doi.org/10.1016/j.vaccine.2009.05.048; PMID: 19500523
- Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol 2003; 171:4552 - 60; PMID: 14568928
- Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med 2003; 197:7 - 17; http://dx.doi.org/10.1084/jem.20021229; PMID: 12515809
- MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A 1997; 94:5243 - 8; http://dx.doi.org/10.1073/pnas.94.10.5243; PMID: 9144222
- Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008; 181:4955 - 64; PMID: 18802099
- Badell E, Nicolle F, Clark S, Majlessi L, Boudou F, Martino A, Castello-Branco L, Leclerc C, Lewis DJ, Marsh PD, et al. Protection against tuberculosis induced by oral prime with Mycobacterium bovis BCG and intranasal subunit boost based on the vaccine candidate Ag85B-ESAT-6 does not correlate with circulating IFN-gamma producing T-cells. Vaccine 2009; 27:28 - 37; http://dx.doi.org/10.1016/j.vaccine.2008.10.034; PMID: 18977269
- Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al, MVA85A 020 Trial Study Team. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013; 381:1021 - 8; http://dx.doi.org/10.1016/S0140-6736(13)60177-4; PMID: 23391465
