Abstract
In Germany, the national routine childhood immunization schedule comprises 12 vaccinations. Primary immunizations should be completed by 24 mo of age. However, nationwide monitoring of vaccination coverage (VC) is performed only at school entry. We utilized health insurance claims data covering ~85% of the total population with the objectives to (1) assess VC of all recommended childhood vaccinations in birth-cohorts 2004–2009, (2) analyze cross-sectional (at 24 and 36 mo) and longitudinal trends, and (3) validate the method internally and externally. Counting vaccine doses in a retrospective cohort fashion, we assembled individual vaccination histories and summarized VC to nationwide figures. For most long-established vaccinations, VC at 24 mo was at moderate levels (~73–80%) and increased slightly across birth-cohorts. One dose measles VC was high (94%), but low (69%) for the second dose. VC with a full course of recently introduced varicella, pneumococcal, and meningococcal C vaccines increased across birth-cohorts from below 10% above 60%, 70%, and 80%, respectively. At 36 mo, VC had increased further by up to 15 percentage points depending on vaccination. Longitudinal analysis suggested a continued VC increase until school entry. Validation of VC figures with primary data showed an overall good agreement. In conclusion, analysis of health insurance claims data allows for the estimation of VC among children in Germany considering completeness and timeliness of vaccination series. This approach provides valid nationwide VC figures for all currently recommended pediatric vaccinations and fills the information gap between early infancy and late assessment at school entry.
Introduction
In Germany, the Standing Committee on Vaccination (STIKO) develops and endorses national vaccination recommendations. The current routine childhood immunization schedule comprises 12 vaccinations. According to this schedule, primary childhood immunizations should be completed by 24 mo of age or earlier.Citation1,Citation2 This includes four doses of diphtheria (DIP), tetanus (TET), pertussis (PER), polio (IPV), Haemophilus influenzae type b (HIB), hepatitis B (HEPB), and pneumococcal conjugate vaccine (PCV), as well as two doses of measles (MCV), mumps (MUM), rubella (RUB) vaccines preferably administered as a measles-mumps-rubella (MMR) combination vaccine, two doses of a varicella vaccine (VAR), and a single dose of meningococcal C vaccine (MENC). In cases of missing vaccinations, individual catch-up is recommended for children beyond the second year of life with the exception of PCV. Whereas most vaccinations are part of the schedule for decades (including routine childhood HEPB vaccination, which was introduced in the mid-1990s), PCV and MENC were adopted in 2006 and single-dose VAR in 2004.Citation3 A second dose of VAR is universally recommended since 2009.
Valid data on vaccination coverage (VC) and VC trends are needed to assess the performance of national immunization programs. This permits program modification and the adaption of measures to increase acceptance and vaccine uptake. VC data are also helpful for the estimation of vaccine effectiveness and interpretation of adverse events signals. Furthermore, country-level VC data are internationally collected and compared in order to assess the current state of efforts to control or eliminate vaccine-preventable diseases.Citation4
In Germany, immunization is voluntary. All administered vaccines are documented on individual vaccination cards. However, there is no central register that allows for VC analyses at national level. Various sources of VC data have previously been identified.Citation5,Citation6 These include primary data from surveys such as kindergarten entrance examinations (KEE), performed merely in a few federal states. Annual school entrance examinations (SEE) are the sole primary VC data source that is continuously and nationwide exploited. At the time of SEE, children are four to seven years of age. As a consequence, at country-level there is neither VC data on young children (e.g., at 24 mo of age) available nor data on recently introduced pediatric vaccinations.
The usefulness of health insurance claims data for the estimation of VC has previously been demonstrated.Citation7-Citation10 In an approach to complement existing nationwide VC monitoring through SEE, we analyzed data of health insurance refund claims from Associations of Statutory Health Insurance Physicians (ASHIPs) with the objectives (1) to estimate VC of all recommended childhood vaccinations at national level, and (2) to investigate VC trends by birth-cohort in cross-sections at 24 and 36 mo and in a longitudinal approach up to school-age. A further objective of this research project was (3) to validate the method internally and with primary VC monitoring data from KEE and SEE to provide evidence for or against the implementation of ASHIP data analysis for routine assessment of VC in Germany.
Results
Vaccination coverage estimates
Sample size
Between 2004 and 2009, an overall 4 096 643 live births were registered in Germany of which 69.1% were born in the investigated years of birth and ASHIP regions. Among those, 1 592 400 (56.3%) fulfilled the inclusion criteria for the 24 mo follow-up (range over regions and year-of-birth: 42.6–68.5%). Sample sizes of cohorts with 36 mo follow-up were comparable. Sample sizes for longitudinal analysis in two ASHIP regions were n = 47 533 in Saxony-Anhalt and n = 60 078 in Schleswig-Holstein, representing 45.9% and 43.7% of live births in these regions.
Trend analysis of birth cohorts 2004–2009
VC at national level for birth cohorts 2004–2009 is shown in . At 24 mo, VC for 4th DIP, TET, PER, IPV, and HIB were 80% on average and slightly increased from birth cohorts 2004 to 2006 but not thereafter. 4th HEPB dose VC was approximately 73% and increased between birth cohorts 2004 and 2007 but not thereafter. VC for 1st MMR was in all birth cohorts above 92%, and VC for 2nd MMR increased from 59% to 69%. Across all birth cohorts, VC of the newly introduced vaccines 4th PCV, MENC, and 2nd VAR increased from low levels (4%, 8%, and 1%) to 72%, 81%, and 64%, respectively.
Figure 1. Vaccination coverage in Germany for birth cohorts 2004 up to 2009, at the age of 24 mo and 36 mo based on health insurance claims data analysis. Upper and lower bounds of confidence intervals are omitted and were ≤0.2 percentage points above or below point estimates. DIP, diphtheria vaccine; TET, tetanus vaccine; PER, pertussis vaccine; IPV, polio vaccine; HIB, Haemophilus influenzae type b vaccine; HEPB, hepatitis B vaccine; PCV, pneumococcal conjugate vaccine; MENC, meningococcal C vaccine; MCV, measles vaccine; MUM, mumps vaccine; RUB, rubella vaccine; VAR, varicella vaccine.
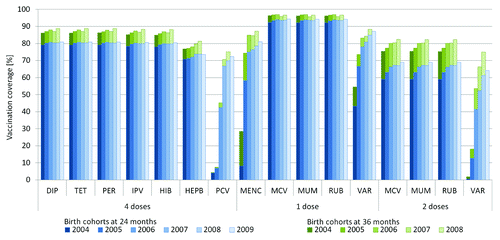
By the age of 36 mo, VC for birth cohorts 2004–2008 increased further on average by 15 percentage points (pp) (2nd MMR), 9 pp (2nd VAR), 13 pp (MENC), 7 pp (4th DIP, TET, PER, IPV, HIB, HEPB), and 3 pp (4th PCV).
VC at 24 and 36 mo by ASHIP and birth-cohort is provided as supplemental material.
Longitudinal analysis of birth cohorts 2004–2009
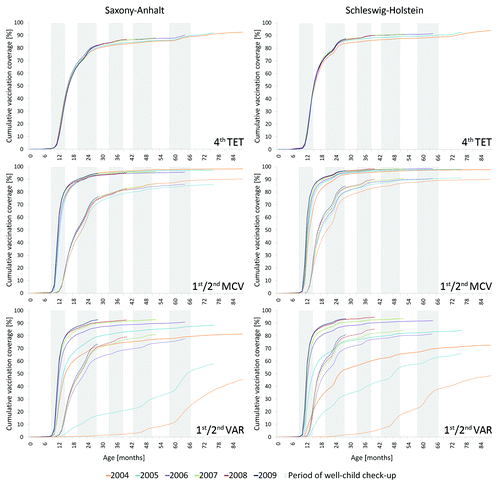
The progression of VC within and across birth cohorts as a function of age is exemplified in for selected vaccinations in the two regions of ASHIPs Saxony-Anhalt and Schleswig-Holstein. In both ASHIP regions, a strong increase starting at the recommended age was evident proceeding toward the end of the second year of life. However, the increase continued beyond 24 mo and featured distinct periods of increase that coincided with the provision of well-child check-ups in Germany. Among the well-child check-ups at two years of age and later, the period of the routine check-up performed at 20 to 27 mo featured the strongest VC alterations. Overall, we observed an increase of VC across birth cohorts; among the ones displayed, VAR vaccinations involved the strongest changes.
Figure 2. Cumulative vaccination coverage among birth cohorts 2004 to 2009 in relation to age. Depicted are figures for the Associations of Statutory Health Insurance Physicians (ASHIP) regions Saxony-Anhalt and Schleswig-Holstein based on health insurance claims data analysis. Solid lines: 1st or 4th dose, respectively; dashed lines: 2nd dose. TET, tetanus vaccine; MCV, measles vaccine; VAR, varicella vaccine.
Validation of VC estimation
Comparison of VC from ASHIP data with kindergarten entrance health examinations
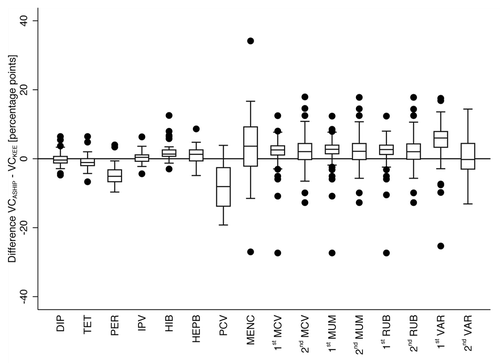
We calculated the median differences of corresponding VC point estimates from ASHIP data and KEE Schleswig-Holstein for each vaccination as depicted in . On average the median difference between ASHIP and KEE point estimates was 0.7 pp (range of median differences −6.0 pp to 8.1 pp) ().
Figure 3. Difference of vaccination coverage (VC) point estimates from data analysis of the Association of Statutory Health Insurance Physicians (ASHIP) Schleswig-Holstein and kindergarten entrance examinations (KEE) in the state of Schleswig-Holstein. The compared birth cohorts per vaccination and definitions of vaccination status are listed in . Boxes indicate lower and upper quartile, the boxes’ length the interquartile range (IQR), horizontal lines indicate medians. Whiskers span all data points within 1.5xIQR of upper or lower quartile, respectively. Dots indicate further data points. DIP, diphtheria vaccine; TET, tetanus vaccine; PER, pertussis vaccine; IPV, polio vaccine; HIB, Haemophilus influenzae type b vaccine; HEPB, hepatitis B vaccine; PCV, pneumococcal conjugate vaccine; MENC, meningococcal C vaccine; MCV, measles vaccine; MUM, mumps vaccine; RUB, rubella vaccine; VAR, varicella vaccine.
Comparison of VC from ASHIP data with school entrance health examinations
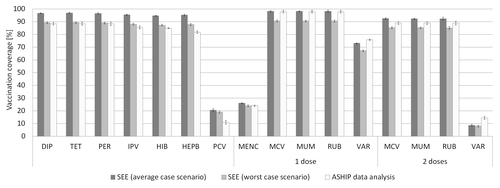
VC from ASHIP data analysis and from SEE Saxony-Anhalt is shown in . The ASHIP-estimate for 2nd MMR fitted well within the range of SEE average case and worst case scenario. VC of DIP, TET, PER, and MENC from ASHIP data analysis were closer to the SEE worst case and 1st MMR was closer to the SEE average case (overlapping confidence intervals [CIs]). VC of IPV, HIB, HEPB, and PCV were 2.5 pp, 2.3 pp, 6.1 pp, and 8.0 pp below the SEE worst case and VC of 1st and 2nd VAR was 2.7 pp and 5.7 pp above the SEE average case scenario (no overlapping CIs).
Figure 4. Vaccination coverage (VC) estimated from school entrance examinations (SEE) in the state of Saxony-Anhalt and data analysis of the Association of Statutory Health Insurance Physicians (ASHIP) Saxony-Anhalt. Only vaccinations documented on vaccination cards were considered in the examinations. VC is related to the number of children presenting a vaccination card (average case scenario) and the total number of children, irrespective of the presence of a vaccination card (worst case scenario). Definitions of vaccination status are given in . Vertical lines represent 95% confidence intervals. DIP, diphtheria vaccine; TET, tetanus vaccine; PER, pertussis vaccine; IPV, polio vaccine; HIB, Haemophilus influenzae type b vaccine; HEPB, hepatitis B vaccine; PCV, pneumococcal conjugate vaccine; MENC, meningococcal C vaccine; MCV, measles vaccine; MUM, mumps vaccine; RUB, rubella vaccine; VAR, varicella vaccine.
Re-capture of patients by screening for specific vaccination code extensions
In vaccination claims of children who had a history of receiving a complete vaccination series of two or four doses, 5.4% (range 4.3–8.2%) and 7.5% (range 7.1–8.1%) of the final doses, respectively, were falsely coded as to indicating a non-completing dose.
In claims of children with a history of receiving non-completing 1st or 3rd dose, 0.8% (range 0.2–1.1%) and 5.1% (range 2.6–11.9%) of the doses, respectively, were falsely labeled as completing.
Discussion
In Germany, STIKO recommends to complete primary childhood immunizations by the end of the second year of life. In the absence of a system for continuous and nationwide VC data collection and assessment for this age-group, we utilized health insurance claims data from ASHIPs to estimate VC, analyzed trends in birth cohorts 2004–2009 in a cross-sectional and longitudinal fashion, and validated the method.
For the external validation we compared VC estimated from ASHIP data for a number of single month-of-birth cohorts at different age with data from KEE in Schleswig-Holstein. We also compared VC estimates from ASHIP data analysis with SEE estimates in Saxony-Anhalt. In KEE Schleswig-Holstein, 89.6% of data collection forms were turned in by the physicians.Citation11 Reasons for non-response are unknown, and assumptions on the vaccination status of non-responding children are difficult to make. In contrast, the assessment of VC among school children requires average case and worst case consideration: SEE exclusively rely on presented vaccination cards, and it has been shown previously that children without vaccination cards can have lower VCCitation12 and show higher seronegativityCitation13 as compared with those presenting vaccination documents. Despite these limitations, in the absence of an immunization register both KEE and SEE constitute the best primary data sources in Germany for the validation of our estimates from ASHIP data. Still, with the exemption of the newly introduced PCV and VAR vaccines we were able to observe an overall good agreement between estimates from ASHIP data analysis and both primary sources. With a median VC difference to KEE figures of only 0.7 pp on average and with levels usually ranging between the SEE average and worst case scenario our approach of utilizing ASHIP data has demonstrated to generate comparable data.
Although we put a considerable effort on creating cohorts from claims data based on date of birth, age, and place to closely resemble cohorts from primary sources, variations in the compositions of the compared cohorts may have implications on the estimated VC. The largest VC differences between ASHIP and the primary sources used for validation concern recently introduced vaccinations (VAR and PCV). A reason for these differences might be that usage of the newly introduced vaccinations is more likely to increase quicker over time. As a result, slight cohort variations can result in a stronger effect on the corresponding VC than on estimates of vaccinations that have been recommended for a long time. Some of the sampled children may have received vaccinations in regions of different ASHIPs. Those events cannot be assigned to these patients and could artificially increase the number of children with incomplete vaccination schedules. According to our inclusion criteria, events merely in the beginning and at the end of follow-up were sufficient for a patient to be sampled. The approach only provides a high chance that just those patients are selected who have been receiving health provisions (i.e., vaccinations) exclusively within their associated ASHIP region. We therefore aimed to assess the degree of VC underestimation due to these losses to follow-up. Patients were recaptured by screening for vaccination claim code extensions that indicate doses not completing and completing vaccination series. We assumed that the proportion of falsely used code extensions coding non-completing doses in histories of completed vaccinations (5.4% and 7.5% for two and four dose schedules) can be regarded as a general failure rate in code extension usage. If vaccinations were missing in the patient’s history due to intermittent vaccinations in different ASHIP regions, code extensions for completing doses would seem prematurely used. However, the failure rate in coding non-completing doses (0.8% and 5.1% for two and four dose schedules) was even below our assumed general failure rate in code extension usage. The degree of intermittent loss to follow-up and resulting VC underestimation due to our sampling approach can therefore be regarded as negligible. It is important to mention that compliance with recommended intervals between administered doses was not part of the definition of a completed vaccination series in our analysis; the proportion of inconsistently used code extensions for completing doses could therefore be an overestimate and some doses might indeed be non-completing due to too short intervals between subsequent vaccinations.
At 24 mo of age, estimation of VC in birth cohorts 2004–2009 showed moderate coverage levels with most traditional vaccinations at 73–80% and a slightly increasing trend across cohorts. With 94%, VC of 1st MMR was relatively high, but the 2-dose MMR coverage of 69% at maximum indicates that this young age-group is still far below the 2-dose target coverage of ≥95% required for the regional elimination of measles and rubella by 2015.Citation14 We also found a strong increase of VC across birth cohorts for a full course of recently introduced vaccinations from well below 10% to moderate levels in the cases of PCV (>70%) and MENC (>80%) indicating good acceptance; however, VC of 64% for VAR is still relatively low at 24 mo of age. Universal vaccination with two doses VAR is the most recent major change in the national childhood vaccination schedule. Low coverage of 2nd VAR might therefore be an expression of this recent change and thus based on low utilization rather than general weak acceptance.
The comparison of cross-sections of birth cohorts 2004–2008 at 24 and 36 mo demonstrates a further substantial VC increase until the age of three years. This increase was especially evident for those vaccinations that are recommended to be administered by the end of the second year of life and for which individual catch-up later in childhood is recommended if timely vaccination had not taken place (i.e., 2nd MMR, 2nd VAR, MENC). The substantial increase in VC between 24 and 36 mo of age has been confirmed in our longitudinal approach; similar trends have been observed previously.Citation15 It is important to note that vaccinations at around two years of age are usually given in one of several well-child check-ups in Germany. These examinations are distributed throughout the first months and years of life and take place at fixed age intervals.Citation16 Check-up “U7” can be performed at covered costs between 20 to 27 mo of age. As a consequence, a number of vaccinations due until 24 mo of age are administered with a delay into the third year of life. This is also evident from previous surveys at regional level.Citation7,Citation10 However, even beyond the third year of life there is further substantial increase of VC as demonstrated by our longitudinal analysis of birth cohort 2004. Here, VC at higher age is comparable to figures seen in SEEs.Citation17
ASHIP-specific analysis demonstrates that VC is not similar across ASHIP regions. This can be related to factors like varying time points in initial cost coverage through health insurance funds,Citation8 state level particularities in vaccination recommendations,Citation18 and historical background leading to generally higher VC in states of former East-Germany.Citation17 Since regional variations are not reflected in country-level VC estimates, subnational VC data are crucial for assessing a country’s progress toward disease elimination. Health insurance claims data permit the analysis not only on ASHIP region or federal state level, respectively, but even on district level (data not shown), thus demonstrating an additional advantage over SEE.
More than half of all children in the investigated years of birth and ASHIP regions fulfilled the inclusion criteria for VC estimation. Children born in the investigated years of birth and ASHIPs represented more than two-thirds of all live births in Germany during 2004 and 2009. These figures let assume high representativeness of VC estimates for the studied years of birth and regions as well as for the population in Germany born during this period. Moreover, validation with primary data looks promising. However, health insurance claims data provides only information on statutorily health insured individuals. It is not clear whether children of a different status differ in VC. Data on this remaining group that is mostly privately health insured is sparse. As recommended vaccinations are usually also covered by private health insurances, we assume to a large degree similar VC of children who are not contained in ASHIP data. In this regard, the authors of a bivariate and multivariate analysis in a population-based cross-sectional study found no difference in proportions of undervaccinated children from statutorily and privately health insured parents.Citation19 Our sampling method for the analysis of ASHIP data excludes individuals with potential suboptimal VC such as children who receive their first vaccination very late or children of parents who completely refuse vaccinations. In addition, mobile groups like Roma/Sinti populations that are difficult to be reached by standard immunization programsCitation20 are also possibly underrepresented in our approach resulting in a potentially positive sampling bias and a slight VC overestimation.
In conclusion, analysis of health insurance claims data from ASHIPs allows for estimation of VC in Germany. It provides information on national and state level and contains residential data for resolution down to district level. Coverage of traditional and newly recommended vaccinations based on birth cohorts can be estimated in regard to whether childhood vaccinations have been administered in time and whether series of immunizations have been completed. ASHIP data analysis fills the information gap between early infancy and late assessment at school entry thereby facilitating more timely actions in modifying and communicating national vaccination recommendations. Furthermore, data are at hand for children at 24 mo of age which is the international standard age for VC figures to be reported to the World Health Organization and the United Nations. Therefore, we conclude that ASHIP data analysis can serve to monitor compliance with childhood immunization recommendations and suggest its implementation as a countrywide routine monitoring system in Germany.
Materials and Methods
Dataflow and database
ASHIPs regularly receive insurance refund claims from all ASHIP-associated physicians for ambulatory medical services delivered to statutory health insurees (~85% of the population in Germany). The administrative regions of most of the 17 ASHIPs are organized per federal state. For our project, relevant data are extracted from the ASHIPs’ databases and anonymized by means of a hash algorithm. The algorithm includes an ASHIP-specific, non-changing password to generate a truncated patient-specific identification (hash) code. Since 2004, an updated file is quarterly transferred to the Robert Koch Institute and imported into a database which has previously been described.Citation21 In brief, the database contains vaccination data since 2004 and data on individuals’ physician consultations since 2008 (every first contact per quarter and medical specialization). Data include patient information (month/year of birth, sex, truncated zip code, and district of residence), physician information (medical specialization, district of practice), date of contact, and vaccination claim codes. Since 2008, these vaccination claim codes feature extensions that allow for the differentiation between doses not completing and completing a vaccination series.Citation22
Data protection
The Federal Commissioner for Data Protection and Freedom of Information has approved the ASHIP vaccination monitoring project, in particular the data specification and the anonymization procedure.
Sampling
As the patients’ identification code is generated from an ASHIP-specific algorithm, data can be assigned to a single patient only within his/her associated ASHIP but not across ASHIPs. Therefore, we sampled only those insurees who were seeking health services exclusively within their associated ASHIP by the following inclusion criteria: (1) Any vaccination soon after birth (0–4 mo of age) and a physician contact within a three month age-range at the end of the follow-up time are documented in the same ASHIP (e.g., at 24–26 mo for a VC assessment of up to 24 mo of age); in any case, we did not apply the three month age-range procedure before 2008, since data on physician consultations were unavailable for that period; and (2) residency in the administrative region of this ASHIP at the time of these health service provisions.
Estimation of vaccination coverage and coverage trends
Data was aggregated using online analytical processing technology (OLAP) and prepared for analysis using ASHIP-specific OLAP cubes connected via Excel 2010 (Microsoft). Histories regarding the vaccinations of interest were assembled for the sampled patients by counting vaccine doses on an individual level in a retrospective cohort approach. Samples were selected based on year of birth and ASHIP region or based on resembling cohorts (month/year of birth and age) of primary data sources for validation, respectively. We estimated VC for incomplete vaccination series (1st MMR and VAR) and for complete vaccination series defined as four doses of DIP, TET, PER, IPV, HIB, HEPB, and PCV, two doses of MMR and VAR, and a single dose of MENC, respectively. For external validation, these definitions varied ().
Table 1. Characteristics of primary data sources kindergarten (KEE) and school (SEE) entrance examinations and corresponding data sets of the Associations of Statutory Health Insurance Physicians (ASHIPs) for validation of vaccination coverage (VC) estimates
Analysis was performed in Stata 12 (StataCorp). VC was calculated as proportion of vaccinated individuals within the sample. Results at ASHIP level were weighted by the number of live births in the ASHIP region using population statisticsCitation23,Citation24 and summarized to nationwide VC. Intervals of 95% confidence were calculated.
Data from 10 to 15 ASHIPs were available for trend analyses in cross-sections of birth cohorts from 2004 to 2009 at 24 mo and from 2004 to 2008 at 36 mo (). For the longitudinal evaluation from birth to school entry, data from ASHIPs Saxony-Anhalt and Schleswig-Holstein were exemplarily analyzed.
Table 2. Availability of health insurance claims data for trend analyses of vaccination coverage (VC) in cross-sections of birth cohorts from 2004 up to 2009 at 24 and 36 mo by federal state and Association of Statutory Health Insurance Physicians (ASHIP)
Validation of VC estimation
Comparison of VC with primary data sources
VC data from KEE 2009 in the federal state of Schleswig-Holstein and SEE 2010 in the federal state of Saxony-Anhalt were used to externally validate VC estimates derived from ASHIP data analysis. provides an overview on the sample characteristics and definitions of the vaccination status.
In KEE, physicians who collect the relevant data can assess the children’s vaccination status by consultation of vaccination cards, the physician’s patient documentation system, and by interviewing the guardians. VC is calculated as number of children having received a specified vaccine dose among all examined children of the same age of KEE. KEE data include age but not the date of examination. Therefore, in order to back-reference to month and year of birth, we assumed the month of routine school entry as examination date (August 2009), since many kindergarten places become available in this month, and subtracted age at KEE. Month-of-birth cohorts were selected from ASHIP data that reached the respective age at KEE in August 2009. Subsequently, we compared VC of month-of-birth cohort pairs from KEE and claims data analysis at the age of KEE. Where necessary, VC from ASHIP data was weighted according to the different vaccination status definitions in KEE deriving from different types of data collection forms. Median VC differences and quartiles for box plot display were calculated. The analysis was limited to those cohorts who had reached or exceeded the earliest recommended age for the according vaccination in cases where KEE data collection forms required indicating the number of administered doses (MUM, MCV, RUB, VAR, MENC); for other vaccinations the analysis was limited to cohorts at the age of ≥24 mo. PCV vaccination for children was recommended since July 2006 and without catch-up vaccinations beyond the second year of life. Therefore, PCV analysis was limited to children born July 2006 or afterwards.
VC data from SEE in Saxony-Anhalt was analyzed for children born in 2004. In SEE, only vaccinations documented on vaccination cards are considered, and the number of children who present vaccination cards is used as denominator (average case scenario). For our external validation of ASHIP-estimates we also calculated for each vaccination a worst case scenario for VC using the total number of children at SEE as denominator, assuming that children with missing vaccination documents lack the dose of vaccination investigated.
Re-capture of patients by screening for specific claim code extensions
The degree of VC underestimation due to intermittent loss to follow-up was assessed, i.e., an individual receiving intermittent vaccinations by physicians of different ASHIPs. We therefore analyzed histories of vaccinations that were administered to a cohort born between July and December 2008 over a 24 mo period in selected ASHIP regions. Code extensions for doses not completing vaccination series on the one hand and completing the series on the other were analyzed from five ASHIPs that applied them from July 2008 or earlier: Among the vaccinations of children who had a history of receiving two doses of MCV, MUM, RUB, or VAR or four doses of DIP, TET, PER, IPV, HIB, HEPB, or PCV, respectively, we calculated the proportion of doses falsely labeled as non-completing. Among the vaccinations of children who had a history of receiving 1st MCV, MUM, RUB, or VAR or 3rd DIP, TET, PER, IPV, HIB, HEPB, or PCV, respectively, we also calculated the proportion of doses falsely labeled as completing. Assuming similar failure rates coding non-completing and completing doses in health insurance claims data, intermittent loss to follow-up and VC underestimation in the samples should be of concern if our calculated failure rate for coding non-completing doses were higher than the failure rate for coding completing doses. This is because administered vaccinations missing in a patient’s history would mimic premature coding of a completing dose.
| Abbreviations: | ||
| ASHIP | = | Association of Statutory Health Insurance Physicians |
| CI | = | confidence interval |
| DIP | = | diphtheria vaccine |
| HEPB | = | hepatitis B vaccine |
| HIB | = | Haemophilus influenzae type b vaccine |
| IQR | = | interquartile range |
| IPV | = | polio vaccine |
| KEE | = | kindergarten entrance examinations |
| MCV | = | measles vaccine |
| MENC | = | meningococcal C vaccine |
| MMR | = | measles, mumps, rubella vaccine |
| MUM | = | mumps vaccine |
| OLAP | = | online analytical processing technology |
| PCV | = | pneumococcal conjugate vaccine |
| PER | = | pertussis vaccine |
| pp | = | percentage points |
| RUB | = | rubella vaccine |
| SEE | = | school entrance examinations |
| STIKO | = | Standing Committee on Vaccination |
| TET | = | tetanus vaccine |
| U7 | = | routine well-child check-up performed at 20-27 months |
| VAR | = | varicella vaccine |
| VC | = | vaccination coverage |
Additional material
Download Zip (61 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study is part of a research project financed by the German Ministry of Health. We thank all Associations of Statutory Health Insurance Physicians for providing claims data; both the working group Children and Adolescent Medical Services Schleswig-Holstein as well as the Department for Hygiene at the State Office for Consumer Protection Saxony-Anhalt for providing primary vaccination coverage data; Matthias an der Heiden (Robert Koch Institute) for statistical support.
Supplemental Materials
Supplemental materials may be found here: http://www.landesbioscience.com/journals/vaccines/article/26986/
References
- Robert Koch Institute. Mitteilung der Ständigen Impfkommission am Robert Koch-Institut (RKI). Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut / Stand: Juli 2012. Epid Bulletin 2012; 30:283 - 310
- German Standing Committee on Vaccination. Vaccination schedule (English). 2012.
- Hofmann F. [The permanent commission on vaccinations at robert koch-institut]. Gesundheitswesen 2012; 74:49 - 58; http://dx.doi.org/10.1055/s-0031-1276901; PMID: 22006226
- World Health Organization. WHO vaccine-preventable diseases: monitoring system: 2010 global summary. 2010. Available from: http://whqlibdoc.who.int/hq/2010/WHO_IVB_2010_eng.pdf
- Poggensee G, Reuss A, Reiter S, Siedler A. [Overview and assessment of available data sources to determine incidence of vaccine preventable diseases, vaccination coverage, and immune status in Germany]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2009; 11:1019 - 28; http://dx.doi.org/10.1007/s00103-009-0952-z
- Siedler AR, Rieck T, Reuss A, Walter D, Poggensee G, Poethko-Muller C, Reiter S. Estimating vaccination coverage in the absence of immunisation registers--the German experience. Euro Surveill 2012; 17:20152; PMID: 22551497
- Kalies H, Redel R, Varga R, Tauscher M, von Kries R. Vaccination coverage in children can be estimated from health insurance data. BMC Public Health 2008; 8:82; http://dx.doi.org/10.1186/1471-2458-8-82; PMID: 18312683
- Reuss AM, Feig M, Kappelmayer L, Siedler A, Eckmanns T, Poggensee G. Varicella vaccination coverage of children under two years of age in Germany. BMC Public Health 2010; 10:502; http://dx.doi.org/10.1186/1471-2458-10-502; PMID: 20723217
- Reuss AM, Walter D, Feig M, Kappelmayer L, Buchholz U, Eckmanns T, Poggensee G. Influenza vaccination coverage in the 2004/05, 2005/06, and 2006/07 seasons: a secondary data analysis based on billing data of the German associations of statutory health insurance physicians. Dtsch Arztebl Int 2010; 107:845 - 50; PMID: 21173931
- Robert Koch Institute. Zu Impfungen bei Kindern im Alter bis zu 24 Monaten. Eine Erhebung in Schleswig-Holstein nach Abrechnungsziffern der Kassenärztlichen Vereinigung für 2003 bis 2005. Epid Bulletin 2007; 34:316 - 21
- Robert Koch Institute. Zum Impfschutz bei Aufnahme in den Kindergarten in Schleswig-Holstein im Jahr 2009. Epid Bulletin 2011; 7:49 - 53
- Wichmann O, Hellenbrand W, Sagebiel D, Santibanez S, Ahlemeyer G, Vogt G, Siedler A, van Treeck U. Large measles outbreak at a German public school, 2006. Pediatr Infect Dis J 2007; 26:782 - 6; http://dx.doi.org/10.1097/INF.0b013e318060aca1; PMID: 17721371
- Poethko-Müller C, Mankertz A. Sero-epidemiology of measles-specific IgG antibodies and predictive factors for low or missing titres in a German population-based cross-sectional study in children and adolescents (KiGGS). Vaccine 2011; 29:7949 - 59; http://dx.doi.org/10.1016/j.vaccine.2011.08.081; PMID: 21872633
- World Health Organization. Resolution. Renewed commitment to elimination of measles and rubella and prevention of congenital rubella syndrome by 2010 and sustained support for polio-free status in the WHO European Region. Moscow, Russia, WHO Regional Office for Europe. 2010.
- Poethko-Müller C, Kuhnert R, Schlaud M. [Vaccination coverage and predictors for vaccination level. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007; 50:851 - 62; PMID: 17514471
- Federal Committee of Medical Practitioners and Health Insurance. Richtlinien des Bundesausschusses der Ärzte und Krankenkassen über die Früherkennung von Krankheiten bei Kindern bis zur Vollendung des 6. Lebensjahres („Kinder-Richtlinien“) in der Fassung vom 26. April 1976, zuletzt geändert am 16. Dezember 2010. Bundesanzeiger 2011; 40:1013
- Robert Koch Institute. Impfquoten bei der Schuleingangsuntersuchung in Deutschland 2010. Epid Bulletin 2012; 16:135 - 9
- The State Chamber of Physicians of Saxony. Synopsis-Impfkalender fuer Kinder, Jugendliche und Erwachsene im Freistat Sachsen, Stand: 1. Januar 2013. 2013.
- Huber J, Lampert T, Mielck A. [Inequalities in health risks, morbidity and health care of children by health insurance of their parents (statutory vs. private health insurance): results of the German KiGGS study]. Gesundheitswesen 2012; 74:627 - 38; PMID: 22275061
- Muscat M. Who gets measles in Europe?. J Infect Dis 2011; 204:Suppl 1 S353 - 65; http://dx.doi.org/10.1093/infdis/jir067; PMID: 21666185
- Reuss A, Feig M, Kappelmayer L, Eckmanns T, Poggensee G. [Determination of vaccination coverage and disease incidence using statutory health insurance data]. Gesundheitswesen 2010; 72:340 - 6; http://dx.doi.org/10.1055/s-0030-1249691; PMID: 20446216
- Federal Joint Commitee. Richtlinie des Gemeinsamen Bundesausschusses über Schutzimpfungen nach § 20 d, Abs. 1 SGB V (Schutzimpfungs-Richtlinie/SI-RL) in der Fassung vom 18. Oktober 2007, zuletzt geändert am 24. November 2011., 2012.
- German federal statistical office. Tabelle 12612-0002: Lebendgeborene: Deutschland, Monate, Geschlecht, Januar 1950-August 2012. 2013.
- State Office for Information and Technology Northrhine-Westphalia. Landesdatenbank NRW, Tabelle 12612-01i: Lebendgeborene insgesamt - Gemeinden - Jahr. 2013.
