Abstract
Haemophilus influenzae type b (Hib) and 7-valent pneumococcal (PCV7) vaccines both became recommended in Japan in 2010. In this study, cytokine production was investigated in peripheral blood mononuclear cells (PBMCs) cultures stimulated with diphtheria and tetanus toxoids combined with acellular pertussis vaccine (DPT), Hib, and PCV7 separately or concurrent different combinations, all as final off-the-shelf vaccines without the individual vaccine components as controls. Higher IL-1β levels were produced when cultures were stimulated with PCV than with DPT or Hib, and the concurrent stimulation including PCV7 enhanced the production of IL-1β. Although Hib induced higher levels of IL-6, no significant difference was observed in IL-6 production with the concurrent stimulation. The concurrent stimulation with Hib/PCV7 and DPT/Hib/PCV7 produced higher levels of TNF-α and human G-CSF. Cytokine profiles were examined in serum samples obtained from 61 vaccine recipients with febrile reactions and 18 recipients without febrile illness within 24 h of vaccination. No significant difference was observed in cytokine levels of IL-1β, IL-4, IL-6, IL-10, IL-12, IFN-γ, MIP-1, TNF-α, and prostaglandin E2 (PGE2) in sera between the two groups. However, significantly higher levels of human G-CSF were observed in recipients with febrile illness than in those without febrile reactions. Further investigations of the significance of elevated serum G-CSF levels are required in vaccine recipients with febrile illness.
Introduction
A long-term vaccine gap occurred in Japan from 1993 when measles mumps and rubella combined vaccine (MMR) was discontinued because of the unexpectedly high incidence of aseptic meningitis caused by mumps vaccine components.Citation1,Citation2 Thereafter, new vaccines were not introduced until 2008. However, many pediatric vaccines have been approved with the implementation of recommended immunization schedules in developed countries, which shows that vaccine preventable diseases need to be controlled.Citation3-Citation6 Haemophilus influenzae type b conjugated with tetanus toxoid (Hib) became licensed in December 2008, and 7-valent pneumococcal conjugated with recombinant diphtheria toxoid (PCV7) vaccines in February 2010, respectively. The simultaneous administration of several vaccines was recommended by the Japanese Pediatric Association, similar to the US and EU.Citation3,Citation4 PCV7 had relatively more adverse reactions of fever ≥ 38 °C, swelling, tenderness at injection site, and irritability than those receiving meningococcal vaccine having the same conjugate protein.Citation7 Combination vaccine containing diphtheria and tetanus toxoids combined with acellular pertussis vaccine (DPT), hepatitis B, and inactivated poliovirus vaccine was generally co-administered with Hib (DPT-HBV-IPV-Hib) in the EU. The incidence of fever ≥ 38.0 °C in the concomitant administration group (DPT-HBV-IPV-Hib with PCV7) was significantly higher than that reported in the separate vaccination group, but there was no significant difference in the incidence of high fever ≥ 39.0 °C.Citation8,Citation9
All effective vaccines induce acquired immunity with the development of antigen-specific antibodies and/or cell-mediated immunity, and the stimulation of innate immunity is now considered essential. Innate immunity consists of two different patterns: pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), and controls the T and B cells to regulate acquired immune responses.Citation10 The stimulation of innate immunity has been found to modulate the development of an acquired immune response through the production of cytokines.Citation11-Citation13 PAMPs consist of Toll-like receptors (TLRs) and retinoic acid inducible gene-based (RIG)-like receptors, which recognize the pattern of microbes.Citation14-Citation16 Aluminum adjuvant induces inflammation at the injection site, and endogenous products released from damaged cells (damage or danger associated signals) stimulate DAMP, activating inflammasomes.Citation17,Citation18 These have been shown to induce the production of inflammatory cytokine IL-1β from proinflammatory molecules.Citation19 DPT and PCV7 contain aluminum adjuvant and stimulate NLRP3 inflammasomes through tissue damage.Citation19 Vaccine antigens initiate innate immune response by the recognition by PAMPs at the injection site, activating dendritic cells (DCs). Antigen is processed and peptide is presented on MHC molecules (signal 1), and antigen presenting cells are migrated to the draining lymph nodes. Type I Interferon (IFN) and inflammatory cytokines enhance the expression of co-stimulatory molecules to help the recognition by T-cell (signal 2). IFN-γ, IL-4, and IL-12 modulate the differentiation toward Th1 and Th2 responses.Citation18 The mechanisms of immunogenicity induced by aluminum adjuvant regarding whether the stimulation of NLRP3 inflammasomes is necessary or not have not yet been fully understood.Citation18-Citation20 The activation of innate immunity by vaccines is indispensable for immunogenicity, and the enhanced production of inflammatory cytokines may be related to the occurrence of adverse events.Citation21 Vaccine-specific innate inflammatory responses are clearly important, and have not been sufficiently investigated regarding cytokine production using different vaccines.
In our previous report, aluminum-adjuvanted H5 whole virion inactivated vaccine (WIV) was licensed for adults in Japan but induced marked febrile reactions with significantly stronger antibody responses in children. Aluminum adjuvant alone did not induce inflammatory cytokines, and H5 WIV induced IL-6, IL-17, TNF-α, MCP-1, IFN-γ, and IFN-α in peripheral blood mononuclear cells (PBMCs) cultures. Aluminum-adjuvanted H5 WIV enhanced IL-1β production, with similar levels of other cytokines stimulated with H5 WIV.Citation21 In this report, cytokine profiling was investigated using PBMCs to evaluate cytokine production in response to the stimulation of DPT, Hib, and PCV7, separately and concurrent different combinations. Since the separate components of these final vaccines were not available, only the final formulated vaccines could be used as in-vitro stimulants. Serum cytokine levels were investigated in 61 vaccine recipients with febrile reactions and 18 recipients without febrile illness within 24 h of vaccination.
Results
Cytokine production in PBMCs stimulated with the single or different combinations of vaccines
Preliminary studies of cytokine production showed that cytokines began to be produced 6 h after the stimulation and increased until 24 h, showing the same level afterward, similar to the previous report of aluminum-adjuvanted H5N1 pandemic vacine.Citation22 Cell viability of non-stimulation was approximately 85–90%, 70–75% for non-adjuvanted vaccines, 50–60% for aluminum-adjuvanted vaccines 24 h after stimulation. PBMCs were stimulated with marketed vaccines, and culture supernatant was collected 24 h after the stimulation. Seventeen cytokine profiles were examined in PBMCs cultures obtained from 29 subjects by stimulation of single or different combinations of DPT, Hib, PCV7, DPT/Hib, DPT/PCV7, Hib/PCV7, and DPT/Hib/PCV7. IL-8, MCP-1, and MIP-1β were produced in the control culture and showed no change with the stimulation. No significant difference was observed in the levels of IL-2, IL-4, IL-5, IL-7, IL-10, IL-12, IL-13, IL-17, GM-CSF, or IFN-γ in response to the single or concurrent stimulation with different combinations of vaccines. Higher levels of IL-1β, IL-6, G-CSF, and TNF-α were produced with the concurrent stimulations than with the single stimulation, and the mean values are shown with 95% confidence intervals (CI) in . DPT and Hib induced similar levels of IL-1β, 72.9 pg/ml (95% CI: 37.2–108.5 pg/ml) and 106.9 pg/ml (95% CI: 44.0–169.9 pg/ml), respectively, and 0.34 pg/ml (95% CI: 0.11–0.58 pg/ml) was detected in the control culture. PCV7 induced higher levels of IL-1β, 178.5 pg/ml (95% CI: 42.1–314.9 pg/ml). DPT/Hib and DPT/PCV7 generated similar levels of IL-1β, 212.2 pg/ml (95% CI: 124.5–299.9 pg/ml) and 289.3 pg/ml (95% CI: 158.4–429.2 pg/ml), respectively. Hib/PCV7 and DPT/Hib/PCV7 produced significantly higher levels, 433.7 pg/ml (95% CI: 226.1–641.3 pg/ml) and 510.9 (95% CI: 270.0–751.9 pg/ml), respectively. The concurrent stimulation with PCV7 induced slightly higher levels of IL-1β.
Figure 1. IL-1β, IL-6, TNF-α, and G-CSF production in PBMCs cultures stimulated with DPT, Hib, PCV7, DPT/Hib, DPT/PCV7, Hib/PCV7, and DPT/Hib/PCV7. PBMCs were obtained from 29 individuals and culture fluids were harvested 24 h after stimulation. Cytokine concentrations were measured using BioPlex 17 cytokine panel. Each bar represents the mean concentration (•) with 95% CI.
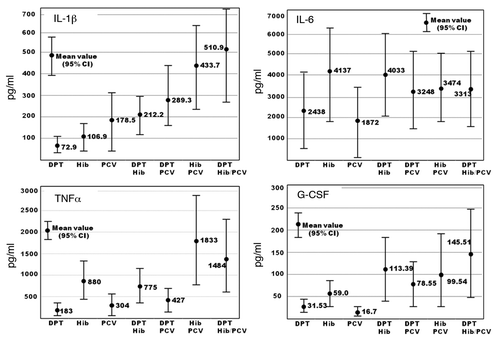
A mean of 4.56 pg/ml (95% CI: 1.3–7.8 pg/ml) of IL-6 was produced in the control cultures. The stimulation with Hib induced higher levels of IL-6 (4136.7 pg/ml, 95% CI: 1883.5–6389.9 pg/ml), while there was no significant difference in the production of IL-6 in response to the stimulation with DPT and PCV7, which showed a mean level of 2438 and 1872 pg/ml, respectively. The concurrent stimulation induced similar levels of IL-6, 3248–4033 pg/ml. No significant difference was observed in IL-6 production with the single or concurrent stimulation.
A mean of 3.53 pg/ml (95% CI: 1.85–5.21pg/ml) of TNF-α was produced in control cultures. Hib induced higher levels of TNF-α in PBMCs, 880.0 pg/ml (95% CI: 406.7–1353.4 pg/ml), than DPT (mean: 183.2 pg/ml, 95% CI: 77.0–289.3 pg/ml) or PCV7 (mean: 304.5 pg/ml, 95% CI: 51.8–557.3 pg/ml). Hib/PCV7 and DPT/Hib/PCV7 produced significantly higher levels, 1833.4 pg/ml (95% CI: 788.9–2877.9 pg/ml) and 1484.3 pg/ml (95% CI: 583.3–2385.4 pg/ml), respectively.
The results of the production of G-CSF are shown. Hib induced higher levels of G-CSF than DPT or PCV7. The concurrent stimulation with DPT/Hib, DPT/PCV7, Hib/PC V7 and DPT/Hib/PCV7 induced similar levels of G-CSF, 78.55–145.51 pg/ml.
Higher levels of IL-1β were produced in PBMC cultures stimulated with PCV7 than with DPT or Hib, and Hib induced higher levels of IL-6 and TNF-α. IL-1β levels increased in PBMCs stimulated concurrently with Hib/PCV7 and DPT/Hib/PCV7, and similar patterns of TNF-α and G-CSF production were observed in PBMC cultures. No significant difference in IL-6 production was observed when cultures were stimulated separately or concurrently.
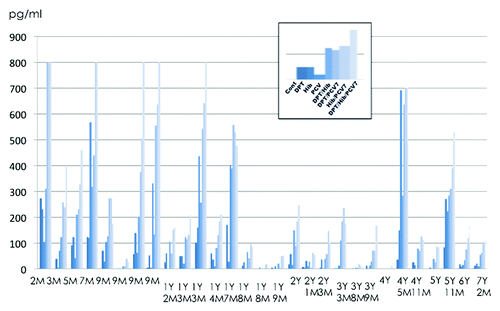
The BioPlex assay for human 17-plex shows cytokine profiles, and the actual concentrations of cytokines should be examined by quantitative EIA. IFN-α/β, IL-1β, and IL-6 were re-examined using EIA. No IFN-α/β was detected in PBMCs cultures stimulated with DPT, Hib, or PCV7, and IL-1β and IL-6 levels were similar to those obtained by the BioPlex assay. The results of IL-1β production in the 29 individuals are shown in . All subjects over 5 mo old had a DPT vaccination, whereas, very few subjects had the Hib but none had PCV7 vaccination. Higher IL-1β production was noted in young infants, but decreased at around 2 y old and or older, except for two subjects (4 y and 5 mo old and 5 y and 11 mo old) who recovered from aseptic meningitis. Scale-over values of > 800 pg/ml were observed in young infants by the stimulation with multiple stimulations of Hib/PCV7 and DPT/Hib/PCV7.
Serum cytokine profiles of vaccine recipients with or without febrile illness
Experiments with PBMCs showed that inflammatory cytokines were produced in response to the vaccine preparations, but did not reflect the situation in vivo. The next concern was whether cytokines were produced in the serum after immunization. Cytokine profiles were investigated in 61 serum samples obtained from recipients who exhibited febrile illness within 24 h of being vaccinated. Eighteen serum samples were obtained from recipients without febrile illness. These samples were taken within 48 h of vaccination in both groups. The background of their vaccination is shown in . Based upon the data of PBMCs culture, cytokine response seemed to be different according to the number of vaccine antigens. Among 61 febrile group, 30 were immunized with three or four vaccines including DPT, Hib, and PCV7, 12 with basically two bacterial vaccines, and 19 with one to three, including one bacterial vaccine. Non-febrile group was similarly categorized. Considering the results indicating that IL-1β, IL-6, G-CSF, and TNF-α were secreted in stimulated PBMC cultures, we next investigated whether the levels of inflammatory cytokines in sera of children with febrile reaction were higher than those in sera from children that did not develop fever. The results of cytokine profiles are shown in . Serum G-CSF levels were significantly higher in recipients with febrile illness than in those without febrile reactions. No detectable IL-1β was observed in sera in both febrile and non-febrile groups and no significant difference was observed in cytokine levels of IL-6 and TNF-α between the two groups. These results are summarized in . The mean serum levels of inflammatory cytokines IL-1β, IL-6, and TNF-α, were 0.68, 29.44, and 13.43 pg/ml in vaccine recipients with febrile reactions after the simultaneous injection of three (DPT/Hib/PCV) or four vaccines (DPT/Hib/PCV + other vaccine), and similar levels of inflammatory cytokines were produced in vaccine recipients with febrile reactions after immunization of one or two inactivated bacterial vaccines, also similar to those in non-febrile group. Cytokine profiles of ten normal subjects without vaccination were examined and the mean titers of cytokines are also shown in . Higher levels of IL-6, IL-10, IL-12, G-CSF, IFN-γ, and TNF-α were detected in both febrile and non-febrile groups after vaccination in comparison with those in normal subjects. No significant difference was observed in Th1 or Th2 cytokines (IL-4, IL-10, IL-12, and IFN-γ) between the two febrile and non-febrile groups. The mean G-CSF level in vaccine recipients with febrile illness was 87.24 pg/ml after three simultaneous injections, higher than those in the recipients with febrile reaction after immunization with one or two vaccines, and in the non-febrile group.
Table 1. Number of patients with or without febrile reactions after vaccination with a different combination of vaccines
Figure 3. Cytokine profiles of 61 individuals with febrile reactions within 24 h after immunization (upper panel) and those of 18 recipients without febrile illness (lower panel).
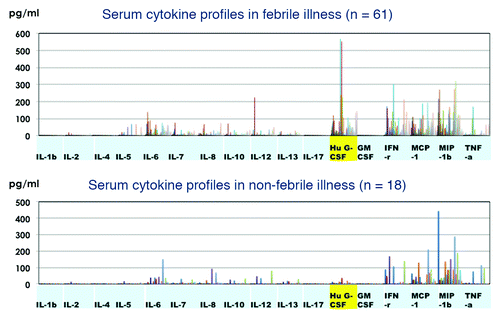
Table 2. Cytokine profiles in vaccine recipients with or without febrile reactions
As a fever-related inflammatory protein, serum PGE2 was assayed by competitive EIA, and the results are also shown in . The mean serum PGE2 concentration was 148.62 pg/ml (95%CI: 90.7–206.5 pg/ml) in the febrile group immunized three vaccines, and there was no significant difference in PGE2 concentration between febrile and non-febrile groups.
Comparison of cytokine profiles of vaccine recipients with those of patients with influenza
IL-1β, IL-6, G-CSF, and TNF-α concentrations were compared with serum levels in patients with the H1N1 2009 outbreak and 18 samples from patients admitted to the hospital with acute pneumonia and 9 from outpatients (). Levels were higher in hospitalized patients than in outpatients, but this was not significant. IL-1β was not detected in sera obtained from outpatients and no significant difference was observed in IL-6 and TNF-α levels between the influenza outpatients and immunization groups with febrile or non-febrile illness after vaccination.
The IL-1β level was < 1pg/ml and IL-6, G-CSF and TNF-α levels were < 5pg/ml in the control group with no illness.
Table 3. Comparison of cytokine profiles of acute phase sera obtained from admitted patients or outpatients with H1N1 pandemic 2009 influenza
Discussion
Currently available vaccines are categorized into live attenuated and inactivated vaccines with or without adjuvant. They induce acquired immunity: antigen-specific cytotoxic T lymphocytes (CTL) attack infected cells and antibodies prevent infections, which are modulated by innate immunity. Innate immunity consists of two different patterns, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).Citation10 The first-line of the innate immune response depends on the TLRs expressed on dendritic cells for antigen-presenting cells, polymorph nuclear neutrophils (PMNs) and monocytes, inducing cytokines in response to the invading microorganisms.Citation14,Citation23,Citation24 They recognize viral, fungal, or bacterial components, in addition to replicative or non-replicative pathogens recognized by RIG like receptor, or warning signals by some adjuvants. Recognition by innate immune receptors activates the signaling cascades of IFN-α/β, and the nuclear factor kappa B (NF-κB)-related elevated transcription of cytokines. Cellular damage and danger signals stimulate DAMP, activating inflammasomes.Citation17,Citation18 In this study, cell viability reduced to 50–60% in PBMCs stimulated with aluminum-adjuvanted vaccines, and aluminum based cellular damage may have these immunological stimulation. In our previous study, aluminum-adjuvanted H5N1 whole virion inactivated vaccine induced inflammatory cytokines, although aluminum adjuvant alone did not induce these cytokines. Inflammasomes consist of NLRP3 and apoptosis-associated speck-like protein (ASC), which is thought to be an adaptor molecule of NLRP-3, resulting in the recruitment of caspase. It induces the inflammatory cytokines, IL-1β, IL-6, and IL-18, from proinflammatory molecules.Citation17-Citation20 Type I IFN enhanced the expression of co-stimulatory molecules recognized by CD8+ CTL cells, together with MHC I molecules and inflammatory cytokines for co-stimulatory molecules for MHC II, recognized by CD4+ cells. CD4+ cells differentiate to functionally different Th1 and Th2 cells to produce different subclass antibodies through cytokines. Thus, innate immunity modulates the acquired immunity induced by vaccinations, and effective vaccines theoretically have an impact on the innate immune system by acting as the agonists of TLRs, RIG-I, and NOD-like receptors, inducing the production of cytokines and chemokines.Citation10-Citation13
Innate immune systems are not fully functional at the time of birth. Human neonatal plasma showed high levels of Th2 cytokines during the first week following birth, and neonatal APCs demonstrated skewed Th2 responses.Citation25 Caron et al.Citation26 reported that the production of regulatory Th1 and Th2 cytokines following the administration of TLR agonists was lower in cord blood than in adult blood. In contrast, TLR-stimulated pro-inflammatory cytokine (IL-1β, IL-6, and IL-8) production was markedly higher in neonates than in adults. The increased susceptibility of neonates to bacterial infections may be related to imbalanced TLR responsiveness, with enhanced pro-inflammatory cytokines and decreased regulatory cytokine production. Burl et al.Citation27 reported that most TLR agonists induced the production of TNF-α, IL-1β, IL-6, and IL-10 in cord blood. For most agonists, TLR-mediated TNF-α and IFN-γ responses increased from birth to one month of age and TLR8 agonists also induced the production of Th1-polarizing cytokines. In contrast, IL-1β, IL-6, and IL-10 responses to most agonists were robust at birth and remained stable through to 12 mo of age in Gambian infants relative to those in developed countries.
Studies of bacterial infections suggest that bacterial lipo-polysaccharides (LPS) act as TLR4 agonists, and vaccine antigens of the polysaccharides of Hib or PCV7 are considered to be TLR4 agonists.Citation28,Citation29 DPT used in Japan is an acellular formulation with 300 μg/ml of aluminum adjuvant and PCV7 consists of 250 μg/ml of aluminum adjuvant. In this study of cytokine production by PBMCs and cytokine responses after immunization, significant differences were observed in cytokine induction, particularly for IL-1β by different vaccines and stimulation of different combinations of vaccines in PBMCs. The IL-1β levels were significantly higher in response to PCV7 than to DPT and this difference depended on the antigen-aluminum formulation.Citation30 IL-1β levels with the simultaneous stimulation with DPT and Hib were the same as those induced by PCV alone, but were higher with the concurrent stimulation including of PCV7. IL-1β production did not depend on the amount of aluminum adjuvant. DPT and PCV7 contain aluminum adjuvants and the concurrent stimulation with DPT and PCV7 induced higher IL-β levels, but lower than those induced by PCV7 plus Hib. Hib induced high levels of IL-6 and no significant difference was observed in IL-6 production among the different combinations of vaccines. Hib induced higher levels of TNF-α than any other single stimulation, whereas PCV7/Hib or all three vaccines together produced higher levels than the others. In this study, there are several limitations; vaccine antigens and different backgrounds of donors’ age probably related to the immunization history. PBMCs were stimulated with final vaccine products, which contain adjuvants, preservatives, and stabilizers besides vaccine antigens. The unavailability of components of each vaccine resulted in the limitation that in-vitro stimulation profiles could not be attributable to each vaccine antigen for each vaccine. These have some possibilities to influence the cytokine production in response to aluminum antigen, but the purpose of the study is to know the response to the vaccine formulations after immunization of vaccines.
PBMCs obtained from young infants produced large amounts of IL-1β, and higher levels of IL-1β, TNF-α, and G-CSF were produced when stimulated with two or three combinations of inactivated bacterial vaccines. Febrile illness developed mostly 12–16 h after vaccination and disappeared within 24–48 h. Sixty-one serum samples were obtained from febrile group and 18 from non-febrile group, and the detection of higher amounts of inflammatory cytokines was suspected. However, no significant difference was observed in cytokine profiles irrespective of febrile illness within 24 h of vaccination and no IL-1β was detected. Influenza is a common infectious disease with an abrupt onset of febrile illness and is a potent inducer of cytokines.Citation15,Citation31 Compared with the acute phase of an influenza infection, cytokine profiles after vaccination were similar to those in mild-moderate outpatients infected with the 2009 pandemic strain. Higher IL-1β levels were observed in sera obtained from seriously ill patients that had been hospitalized, but no significant difference was noted. All effective vaccines induce the production of cytokines or chemokines, which modulate immunogenicity and are also involved in inducing adverse events, such as systemic febrile illness and immunotoxicity.Citation21,Citation32,Citation33 In this standpoints, IL-6, IL-10, IL-12, G-CSF, IFN-γ, and TNF-α were detected in both febrile and non-febrile groups after vaccination in comparison with those in normal subjects. Some cytokines might be associated with febrile adverse events, and others to immunogenicity, although this is not yet determined. Kamgang et al.Citation34 suggested IL-1β as a biomarker of vaccine immunotoxicity. When a vaccine is administered through an intramuscular or subcutaneous route, the antigen is transported from the muscle tissue to the regional lymph nodes, where immune responses occur. Since the vaccine antigen does not appear directly in blood, an experiment in which PBMCs were stimulated with vaccine antigen did not necessarily reflect the in vivo responses following vaccination. Although higher levels of cytokines were expected in the sera of patients with febrile reactions, the inflammatory cytokine profiles of febrile recipients were not different from those of recipients without febrile illness. IL-1β is known to be a strong stimulant of oxidative stress, resulting in COX-2 stimulation and prostaglandin E2 (PGE2) production. These have been clearly related to acute or chronic inflammatory conditions. Subsequent responsiveness to cytokines may be involved in febrile illness, such as PGE2 or cytokine receptors.Citation35,Citation36 In this study, cytokine profiles were also investigated in patients with influenza between hospitalized and outpatients groups. However, no significant difference was observed between the groups, because extremely serious patients were not included in the hospitalized patient group. Inflammatory cytokine profiles after vaccination were similar to the outpatient group infected with the influenza virus.
It was very hard to obtain the sera especially from non-febrile group (n = 18) within 24–48 h after immunization. From the results of cytokine production by PBMCs () when stimulated with single or different combinations, 61 subjects with febrile reactions were categorized into three subgroups: 30 were basically immunized with three vaccines DPT/Hib/PCV7, 12 with basically two bacterial vaccines (PCV7/Hib, and PCV7/DPT) and 19 with including one bacterial vaccine. Non-febrile group was similarly categorized. Therefore, the limitation of the study was too small number of the subjects to make relevant statistical comparisons. Several individuals had an additional vaccine (IPV, Rota, BCG, influenza, or MR) besides three inactivated bacterial vaccines. These additional vaccines might affect the cytokine production. But, these live viral vaccines rarely cause febrile reaction within 24 h after vaccination. Cytokine production was examined in PBMCs culture stimulated with IPV, influenza, and MR vaccines and very low levels of inflammatory cytokines were produced (data not shown). Therefore, additional simultaneous immunization supposed to have little influence on cytokine induction in sera.
In vaccine recipients, only human G-CSF was higher in vaccine recipients with febrile reactions and was also produced in PBMCs stimulated concurrently with two or three inactivated bacterial vaccines. G-CSF acts to mobilize and recruit neutrophils to the site of inflammation from the marginal pool.Citation37 The initial response at the injected site was the migration of neutrophils and monocytes with increased local cytokine production of G-CSF and IL-5 in experimental mouse model.Citation38 Neutrophils migrated to the injection site of the aluminum-containing vaccine and caused neutrophil extracellular traps, resulting in the degranulation of neutrophil substances.Citation39 Aluminum adjuvants induced reactive oxygen species (ROX), which caused increased the production of prostaglandin.Citation40,Citation41 But, in this study, there was no significant difference in PGE2 concentrations in sera obtained from febrile and non-febrile groups.
A recent concept in vaccine development is the vaccine immune-network because so many genes are involved in the immunogenicity of vaccines: immune effector genes, cytokine and cytokine receptor genes, and the interaction of their transcripts.Citation42-Citation44 Individual immunogenicity, low responders to some vaccines, may depend on a dysfunction in the immune regulatory network and, in a reflection of immunotoxicity, racial and individual differences are suspected in clinical adverse reactions. Further investigations of the significance of elevated serum G-CSF levels are required in vaccine recipients with febrile illness.
Materials and Methods
Study design and subjects
A total of 29 healthy children without any immunological disorders were enrolled in this study of cytokine production in PBMCs cultures (n = 29; 15 males and 14 females). They were admitted to Tokyo Medical College Hospital due to minor respiratory infections or clinical tests of liver or kidney biopsy (average 34 mo of age ranged from 2 mo to 7 y and 2 mo), and blood samples were collected just before discharge after the recovery of illness. Informed consent was obtained from their parents. PBMCs were obtained by centrifugation (Ficoll-PaqueTM Plus #17–5442–02, GE Healthcare Bio-science), which was subjected within three hours after taking heparinized venous blood. PBMCs were adjusted to 5 × 105 cells in 500 μl of RPMI 1640 medium supplemented with 5% FBS and adequate antibiotics in a 48-well plate. Cultures were stimulated with 50 μl of vaccine preparations and the culture supernatant was harvested 24 h later. Samples were stocked at −80 °C until Bio-Plex cytokine assay. This study protocol was reviewed and approved by the Ethics Committee of Tokyo Medical University, Tokyo, Japan.
Vaccine antigens
DPT (Kitasato), Hib (Sanofi Pasteur), and PCV7 (Pfizer) were purchased commercially. A volume of 50 µl was used for the stimulation of a single vaccine or different combinations of DPT/Hib, DPT/PCV7, Hib/PCV7, and DTP/Hib/PCV7. DPT and PCV7 have aluminum adjuvant at the concentration of 300 ug/ml and 250 ug/ml, respectively, and Hib does not contain aluminum.
Serum samples
Serum samples were obtained from 61 vaccine recipients who had febrile illness >37.5 °C within 24–48 h after a single- or simultaneous multi-vaccine administration and the details of the immunization are shown in : DPT/Hib/PCV7 (22 cases), DPT/Hib/PCV/IPV (4 cases), DPT/Hib/PCV7/Rota (3 cases), DPT/Hib/PCV7/BCG (1 case), PCV7/Hib (7 cases), PCV7/Hib/Rota (4 cases), PCV7/DPT (1 case), PCV7 (9 cases), PCV7/MR (3 cases), Hib (3 cases), and the remaining 4 cases were of a different combination of PCV7 and others. Febrile reactions were observed mainly after immunization with PCV7 and concurrent immunization including PCV7. Serum samples were also obtained within 24–48 h from 18 recipients without febrile reactions: DPT/Hib/PCV7 (4 cases), DPT/Hib/PCV7/Rota (1 case), PCV7/Hib (6 cases), DPT/Hib (1 case), PCV7 (4 cases), DPT (2 cases). For the control subjects without vaccination, serum samples were obtained from ten normal healthy subjects aged < 1–3 y old. Serum cytokine profiles were examined by Bio-Plex and the experimental protocol was approved by the Ethics Committee of Kitasato Institute. Serum samples were collected after obtaining informed consent.
Cytokine assay
Culture supernatants and serum samples were subjected to Bio-Plex ProTM Human Cytokine Assay 17-plex, using Bio-Plex 200 (GI17plex panel #M50-00031YV, Bio-Rad,). IFN-α, IL-1β, and IL-6 concentrations were measured using EIA kits, (Verikine human IFN-α/β serum sample ELISA kit #46100, pbl interferon source), (Quantikine human IL-1β EIA kit#DLB50, R&D Systems), and (Quantikine IL-6 EIA kit #D6050, R&D Systems), following the instruction manuals. Prostaglandin E2 was measured by competitive EIA (Prostaglandin E2 EIA Kit #KGE004B, R&D Systems).
Statistical analysis
Differences between groups were analyzed using the Mann-Whitney U-test or chi-square test, and a significant difference was defined as P < 0.05, using Statcel software (OMS, Saitama, Japan).
| Abbreviations: | ||
| ASC | = | apoptosis-associated speck-like protein |
| BCG | = | Bacille de Calmette et Guérin |
| CTL | = | cytotoxic T lymphocytes |
| DPT | = | diphtheria and tetanus toxoids combined with acellular pertussis vaccine |
| G-CSF | = | granulocyte-colony stimulating factor |
| Hib | = | Haemophilus influenzae type b vaccine |
| IFN | = | interferon |
| IL | = | interleukin |
| IPV | = | inactivated polio vaccine |
| JEV | = | Japanese encephalitis vaccine |
| LPS | = | lipo-polysaccharides |
| MIP-1 | = | macrophage inflammatory protein-1 |
| MMR | = | measles mumps and rubella combined vaccine |
| MR | = | measles and rubella combined vaccine |
| NF-kB | = | nuclear factor kappa B |
| NLRP-3 | = | NOD-like-receptor-family member (NLRP)-3 |
| PBMCs | = | peripheral blood mononuclear cells |
| PCV7 | = | 7-valent pneumococcal vaccine |
| PGE2 | = | prostaglandin E2 |
| PMNs | = | polymorph nuclear neutrophils |
| RIG-I | = | retinoic acid inducible gene-based-like receptors |
| ROX | = | reactive oxygen species |
| TLRs | = | Toll-like receptors |
| TNF-α | = | tissue necrotic factor-α |
Disclosure of Potential Conflicts of Interest
All authors have no conflict of interest regarding this study.
Acknowledgments
This study was supported by Research on Regulatory Science of Pharmaceuticals and Medical Devices Grants, and funding for Research on the Accumulation of Evidence for Effective Vaccine Use and Vaccine Policy, from the Ministry of Health, Labour and Welfare. All authors have no conflict of interest regarding this study.
References
- Ueda K, Miyazaki C, Hidaka Y, Okada K, Kusuhara K, Kadoya R. Aseptic meningitis caused by measles-mumps-rubella vaccine in Japan. Lancet 1995; 346:701 - 2; http://dx.doi.org/10.1016/S0140-6736(95)92311-X; PMID: 7658837
- Sugiura A, Yamada A. Aseptic meningitis as a complication of mumps vaccination. Pediatr Infect Dis J 1991; 10:209 - 13; http://dx.doi.org/10.1097/00006454-199103000-00008; PMID: 2041668
- Centers for Disease Control and Prevention (CDC). Global routine vaccination coverage, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1520 - 2; PMID: 22071590
- Duclos P, Okwo-Bele JM, Gacic-Dobo M, Cherian T. Global immunization: status, progress, challenges and future. BMC Int Health Hum Rights 2009; 9:Suppl 1 S2; http://dx.doi.org/10.1186/1472-698X-9-S1-S2; PMID: 19828060
- Dennehy PH. Active immunization in the United States: developments over the past decade. Clin Microbiol Rev 2001; 14:872 - 908; http://dx.doi.org/10.1128/CMR.14.4.872-908.2001; PMID: 11585789
- Plotkin SA. Vaccines: the fourth century. Clin Vaccine Immunol 2009; 16:1709 - 19; http://dx.doi.org/10.1128/CVI.00290-09; PMID: 19793898
- Black S, Shinefield H. Safety and efficacy of the seven-valent pneumococcal conjugate vaccine: evidence from Northern California. Eur J Pediatr 2002; 161:Suppl 2 S127 - 31; http://dx.doi.org/10.1007/s00431-002-1064-z; PMID: 12494258
- Olivier C, Belohradsky BH, Stojanov S, Bonnet E, Petersen G, Liese JG. Immunogenicity, reactogenicity, and safety of a seven-valent pneumococcal conjugate vaccine (PCV7) concurrently administered with a fully liquid DTPa-IPV-HBV-Hib combination vaccine in healthy infants. Vaccine 2008; 26:3142 - 52; http://dx.doi.org/10.1016/j.vaccine.2007.11.096; PMID: 18502545
- Pichichero ME, Bernstein H, Blatter MM, Schuerman L, Cheuvart B, Holmes SJ, 085 Study Investigators. Immunogenicity and safety of a combination diphtheria, tetanus toxoid, acellular pertussis, hepatitis B, and inactivated poliovirus vaccine coadministered with a 7-valent pneumococcal conjugate vaccine and a Haemophilus influenzae type b conjugate vaccine. J Pediatr 2007; 151:43 - 9, e1-2; http://dx.doi.org/10.1016/j.jpeds.2007.02.013; PMID: 17586189
- Siegrist CA. Vaccine immunology. Vaccines 6th edition, edited by Plotkin SA, Orenstein WA, Offit PA, Philadelphia, Elsevier Saunders, 2013, pp14-32.
- Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res 2009; 65:98R - 105R; http://dx.doi.org/10.1203/PDR.0b013e31819f195d; PMID: 19918215
- Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010; 33:464 - 78; http://dx.doi.org/10.1016/j.immuni.2010.10.007; PMID: 21029958
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011; 470:543 - 7; http://dx.doi.org/10.1038/nature09737; PMID: 21350488
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006; 124:783 - 801; http://dx.doi.org/10.1016/j.cell.2006.02.015; PMID: 16497588
- Ichinohe T. Respective roles of TLR, RIG-I and NLRP3 in influenza virus infection and immunity: impact on vaccine design. Expert Rev Vaccines 2010; 9:1315 - 24; http://dx.doi.org/10.1586/erv.10.118; PMID: 21087109
- Ichinohe T, Iwasaki A, Hasegawa H. Innate sensors of influenza virus: clues to developing better intranasal vaccines. Expert Rev Vaccines 2008; 7:1435 - 45; http://dx.doi.org/10.1586/14760584.7.9.1435; PMID: 18980544
- Schroder K, Tschopp J. The inflammasomes. Cell 2010; 140:821 - 32; http://dx.doi.org/10.1016/j.cell.2010.01.040; PMID: 20303873
- Saïd-Sadier N, Ojcius DM. Alarmins, inflammasomes and immunity. Biomed J 2012; 35:437 - 49; http://dx.doi.org/10.4103/2319-4170.104408; PMID: 23442356
- Kool M, Soullié T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med 2008; 205:869 - 82; http://dx.doi.org/10.1084/jem.20071087; PMID: 18362170
- Spreafico R, Ricciardi-Castagnoli P, Mortellaro A. The controversial relationship between NLRP3, alum, danger signals and the next-generation adjuvants. Eur J Immunol 2010; 40:638 - 42; http://dx.doi.org/10.1002/eji.200940039; PMID: 20201020
- Batista-Duharte A, Lindblad EB, Oviedo-Orta E. Progress in understanding adjuvant immunotoxicity mechanisms. Toxicol Lett 2011; 203:97 - 105; http://dx.doi.org/10.1016/j.toxlet.2011.03.001; PMID: 21392560
- Nakayama T, Kashiwagi Y, Kawashima H, Kumagai T, Ishii KJ, Ihara T. Alum-adjuvanted H5N1 whole virion inactivated vaccine (WIV) enhanced inflammatory cytokine productions. Vaccine 2012; 30:3885 - 90; http://dx.doi.org/10.1016/j.vaccine.2012.04.004; PMID: 22507655
- Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev 2009; 227:221 - 33; http://dx.doi.org/10.1111/j.1600-065X.2008.00731.x; PMID: 19120487
- Aoshi T, Koyama S, Kobiyama K, Akira S, Ishii KJ. Innate and adaptive immune responses to viral infection and vaccination. Curr Opin Virol 2011; 1:226 - 32; http://dx.doi.org/10.1016/j.coviro.2011.07.002; PMID: 22440781
- Saito S. Cytokine network at the feto-maternal interface. J Reprod Immunol 2000; 47:87 - 103; http://dx.doi.org/10.1016/S0165-0378(00)00060-7; PMID: 10924744
- Caron JE, La Pine TR, Augustine NH, Martins TB, Hill HR. Multiplex analysis of toll-like receptor-stimulated neonatal cytokine response. Neonatology 2010; 97:266 - 73; http://dx.doi.org/10.1159/000255165; PMID: 19955831
- Burl S, Townend J, Njie-Jobe J, Cox M, Adetifa UJ, Touray E, Philbin VJ, Mancuso C, Kampmann B, Whittle H, et al. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS One 2011; 6:e18185; http://dx.doi.org/10.1371/journal.pone.0018185; PMID: 21533209
- Mogensen TH, Paludan SR, Kilian M, Østergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J Leukoc Biol 2006; 80:267 - 77; http://dx.doi.org/10.1189/jlb.1105626; PMID: 16731773
- Latz E, Franko J, Golenbock DT, Schreiber JR. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J Immunol 2004; 172:2431 - 8; PMID: 14764714
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity 2010; 33:492 - 503; http://dx.doi.org/10.1016/j.immuni.2010.10.002; PMID: 21029960
- Song BM, Kang YM, Kim HS, Seo SH. Induction of inflammatory cytokines and toll-like receptors in human normal respiratory epithelial cells infected with seasonal H1N1, 2009 pandemic H1N1, seasonal H3N2, and highly pathogenic H5N1 influenza virus. Viral Immunol 2011; 24:179 - 87; http://dx.doi.org/10.1089/vim.2010.0125; PMID: 21668359
- Gupta RK, Relyveld EH, Lindblad EB, Bizzini B, Ben-Efraim S, Gupta CK. Adjuvants--a balance between toxicity and adjuvanticity. Vaccine 1993; 11:293 - 306; http://dx.doi.org/10.1016/0264-410X(93)90190-9; PMID: 8447157
- Gribble EJ, Sivakumar PV, Ponce RA, Hughes SD. Toxicity as a result of immunostimulation by biologics. Expert Opin Drug Metab Toxicol 2007; 3:209 - 34; http://dx.doi.org/10.1517/17425255.3.2.209; PMID: 17428152
- Kamgang RK, Ramos I, Rodrigues Duarte L, Ghielmetti M, Freudenberg M, Dahinden C, Padovan E. Using distinct molecular signatures of human monocytes and dendritic cells to predict adjuvant activity and pyrogenicity of TLR agonists. Med Microbiol Immunol 2008; 197:369 - 79; http://dx.doi.org/10.1007/s00430-008-0081-6; PMID: 18283493
- Bartfai T, Conti B. Fever. ScientificWorldJournal 2010; 10:490 - 503; http://dx.doi.org/10.1100/tsw.2010.50; PMID: 20305990
- Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res 2004; 10:201 - 22; PMID: 15373964
- Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology 2008; 125:281 - 8; http://dx.doi.org/10.1111/j.1365-2567.2008.02950.x; PMID: 19128361
- Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O’Hagan DT, De Gregorio E, Seubert A, Wack A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 2011; 29:1812 - 23; http://dx.doi.org/10.1016/j.vaccine.2010.12.090; PMID: 21215831
- Munks MW, McKee AS, Macleod MK, Powell RL, Degen JL, Reisdorph NA, Kappler JW, Marrack P. Aluminum adjuvants elicit fibrin-dependent extracellular traps in vivo. Blood 2010; 116:5191 - 9; http://dx.doi.org/10.1182/blood-2010-03-275529; PMID: 20876456
- Pang T, Wang J, Benicky J, Sánchez-Lemus E, Saavedra JM. Telmisartan directly ameliorates the neuronal inflammatory response to IL-1β partly through the JNK/c-Jun and NADPH oxidase pathways. J Neuroinflammation 2012; 9:102; http://dx.doi.org/10.1186/1742-2094-9-102; PMID: 22642771
- Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, Akira S, Aritake K, Urade Y, Morimoto Y. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity 2011; 34:514 - 26; http://dx.doi.org/10.1016/j.immuni.2011.03.019; PMID: 21497116
- Poland GA, Ovsyannikova IG, Jacobson RM. Vaccine immunogenetics: bedside to bench to population. Vaccine 2008; 26:6183 - 8; http://dx.doi.org/10.1016/j.vaccine.2008.06.057; PMID: 18598732
- Poland GA, Kennedy RB, Ovsyannikova IG. Vaccinomics and personalized vaccinology: is science leading us toward a new path of directed vaccine development and discovery?. PLoS Pathog 2011; 7:e1002344; http://dx.doi.org/10.1371/journal.ppat.1002344; PMID: 22241978
- Buonaguro L, Pulendran B. Immunogenomics and systems biology of vaccines. Immunol Rev 2011; 239:197 - 208; http://dx.doi.org/10.1111/j.1600-065X.2010.00971.x; PMID: 21198673